Abstract
This population-based study aimed at providing an overview of drug prescription patterns during pregnancy in the Italian region of Lombardy from 2010 to 2020. The cohort consisted of 780,075 deliveries identified from the regional healthcare utilization databases. The prevalence of drugs’ dispensed prescriptions was estimated as the proportion of pregnant women with at least one prescription out of the total deliveries over the entire pregnancy and by trimester. Drugs were classified according to the Anatomical Therapeutic Chemical code. In addition, interrupted time series analysis was conducted to investigate temporal trends of antibiotics’ use during the onset of the COVID-19 pandemic. A total of 497,515 women (63.8%) used at least a drug, including vitamins and minerals, at some point during pregnancy. Vitamins, minerals, and anti-anaemic preparations were prescribed in 20.8%, 13.3%, and 18.3% of deliveries over the trimesters of pregnancy. Folic acid was the most prescribed drug, with about one woman out of four, followed by iron preparations, progestogen, and antibiotics (prescription rate, respectively: 15.9%, 10.2%, and 9.8%). A decreasing trend in the dispensing of antibiotics emerged during the entire study period; however, a significant further decrease following the spread of the pandemic was observed. Further evidence is needed to monitor the use of drugs during pregnancy, determinants, and implications.
1. Introduction
Despite the lack of adequate studies on the safety of drugs during pregnancy, many pregnant women take medications. Pregnancy care is still a great challenge to both healthcare providers and pregnant women because several drugs cross the placental and blood–brain barrier and pass into breast milk, which may increase the level of mediators in the developing fetus and can adversely affect the fetus’s cognitive, functional, and neurological development, as well as affect the lives of the mother [1,2,3,4].
In general, the use of medications and other over-the-counter (OTC) drugs is not recommended during pregnancy. However, it may be deemed necessary due to acute and/or chronic illness or new-onset clinical conditions. Moreover, pregnant women are still considered therapeutic orphans since most available drugs were not adequately studied despite several physiological, pharmacokinetic, and pharmacodynamic changes occurring during pregnancy [1,2,3,4].
Despite the apprehension related to the topic, the use of drugs during pregnancy is widespread. Research on drug utilization during pregnancy has shown that up to 27–99% of pregnant women take at least one medication if also considering the consumption of vitamins and minerals, with a substantial number of drugs with a hazardous risk profile [5]. In Italy, automated databases have been used to investigate drug utilization in pregnancy, providing detailed prescription information collected prospectively for large cohorts of pregnant women from different regions [6,7,8,9]. In 2020, for the first time, the Italian Medicines Agency (AIFA) published a report based on the linkage record of different regional health information flows [10,11].
Understanding the rational use of drugs in pregnancy and exploring patterns of use in pregnancy is crucial. However, there is a shortage of information about drug utilization patterns in pregnant women in Italy and the data are outdated. Therefore, we conducted an observational population-based study to provide the prevalence of dispensed drugs’ prescriptions during pregnancy in the Italian region of Lombardy from 2010 to 2020. In addition, we evaluated the general impact of the onset of the COVID-19 pandemic in March 2020 on the following prescriptions of selected medications.
2. Results
Out of 780,075 women that met the inclusion criteria, 497,515 (63.8%) used at least a drug at some point during pregnancy, including vitamins and minerals: 310,233 (39.8%) during the first trimester, 243,160 (31.2%) during the second trimester, and 273,563 (35.1%) during the third trimester. When vitamins and minerals were excluded, 373,075 (47.8%) women had at least one drug prescription, and the proportions across trimesters were 31.1%, 26.0%, and 27.2%, respectively, in the first, second, and third trimester. The characteristics of the cohort and drug prescription patterns across the three trimesters of pregnancy are provided in the Supplementary Materials (Tables S1 and S2).
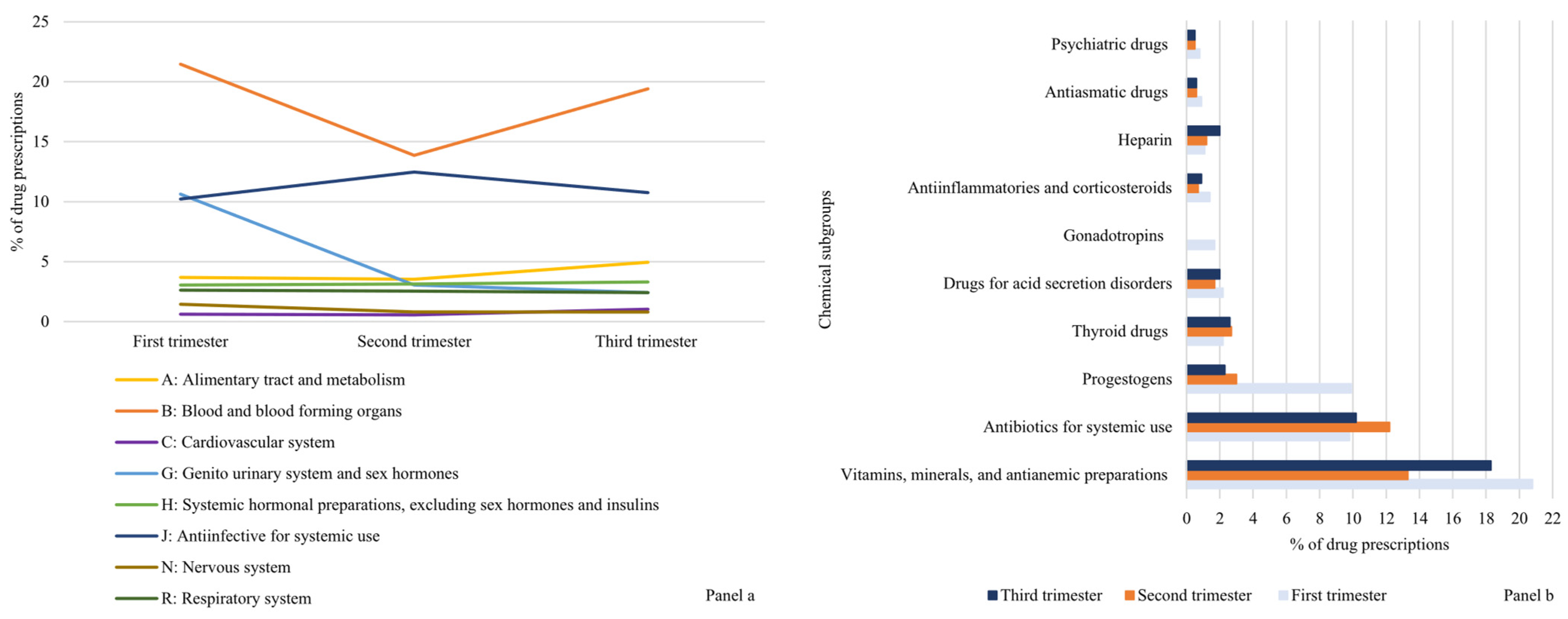
Based on anatomical group classes, drugs used for conditions of the blood and hematopoietic organs (ATC B) were observed in the highest proportion of women in all trimesters of pregnancy (21.5%, 13.9%, and 19.4 in the first, second, and third trimester, respectively). The drugs belonging to the category of antimicrobials for systemic use (ATC J) were the second most prescribed during pregnancy, with a peak of 12.5% during the second trimester. Regarding chemical classification, vitamins, minerals, and anti-anaemic preparations were prescribed in 20.8%, 13.3%, and 18.3% of deliveries over the trimesters of pregnancy. Figure 1 shows the prevalence of drug prescriptions according to selected anatomical (Panel (a)) and chemical (Panel (b)) subgroups.
Figure 1.
Prevalence of drug dispensing (%) according to selected anatomical (Panel (a)) and chemical groups (Panel (b)) across trimesters of pregnancy. Lombardy, Italy, 2010–2020.
Among the most prescribed single active agents, folic acid took first place with about one woman out of four, followed by iron supplements (about one woman out of five) and progestogen (less than one woman out of six). The proportion of deliveries with a prescription of progestogen increased from 9.2% in 2010–2012 to 14.4% in 2019–2020. The most prescribed antibiotics were amoxicillin (with or without clavulanic acid), fosfomycin, azithromycin, clarithromycin, and cefixime. Table 1 provides the prevalence of most prescribed drugs during the selected periods (i.e., 2010–2012, 2013–2015, 2016–2018, and 2019–2020).

Table 1.
The most prescribed drugs (with a frequency of 1% or more). Lombardy, Italy, 2010–2020.
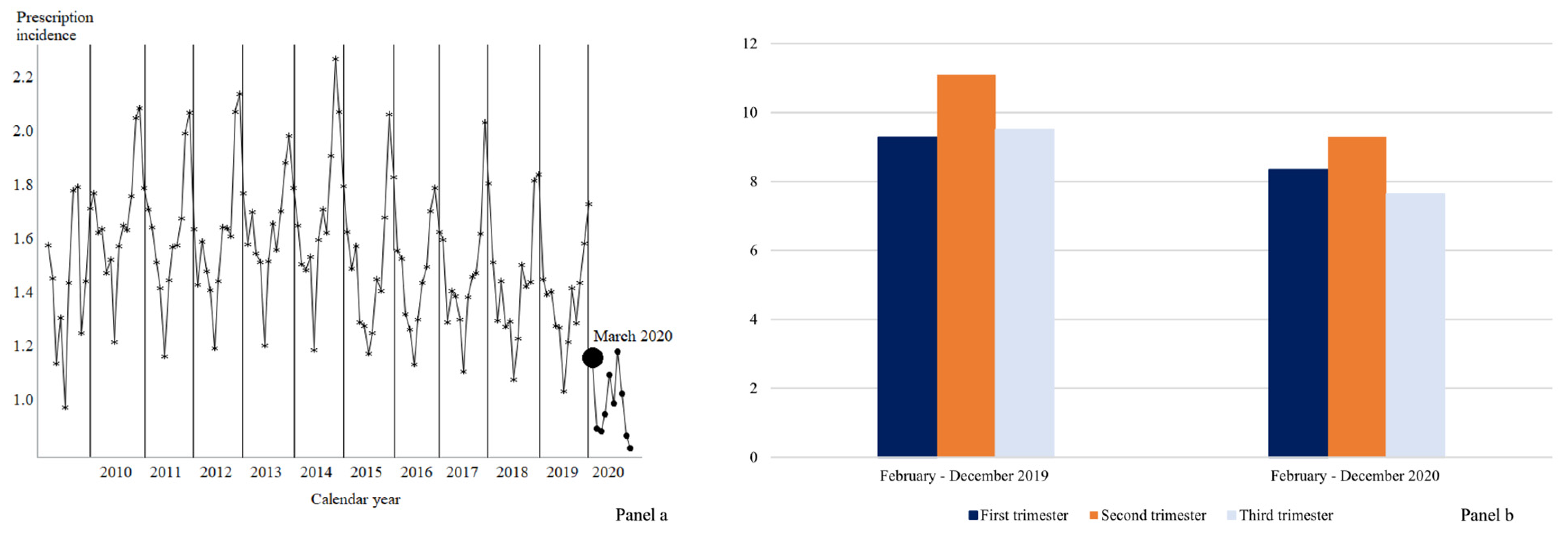
Focusing on antibiotics, during the period between February 2020 and December 2020, the first period of the spread of the COVID-19 pandemic, the prescribing rate was reduced in all three trimesters, being, respectively, 8.3%, 9.3%, and 7.6% versus 9.3%, 11.1%, and 9.5% in the same period of the previous year (Figure 2, Panel (a)). In general, a decreasing trend in the dispensing of antibiotics emerged among women who were pregnant between April 2009 and February 2020 (p-value = 0.04) (Figure 2, Panel (b)). After controlling for this trend, there was a significant (p-value < 0.01) further decrease following the onset of the COVID-19 pandemic in March 2020 by about 0.54%. However, in the following months, the trend significantly differed from the previous years (p-value < 0.01), describing an increasing trend (p-value < 0.01).
Figure 2.
Trend in antibiotics use (Panel (a)) and antibiotics use in 2019–2020 according to trimesters of pregnancy (Panel (b)). Lombardy, Italy, 2010–2020.
3. Discussion
The prevalence of prescribing medication during pregnancy in Lombardy from 2010 to 2020 was just under 65%, including vitamins and minerals. Folic acid and iron preparations were the most prescribed, followed by progestogen and antibiotics. An increasing trend in the use of progestogen and a decreasing trend in the use of antibiotics emerged during the entire study period. About 48% of women recorded at least one drug prescription during pregnancy, excluding vitamins and minerals.
In high-income countries, the use of prescription medicines in pregnancy is widespread; the overall estimates range from 27% to 99% of pregnant women filling at least one prescription, including vitamins and minerals. Differences in study methods and reporting made the results of drug utilization studies challenging to compare. Considering only studies using the outpatient drug prescriptions registry, the lowest rates of prescription drug use during pregnancy were reported in Northern Europe (44–57%), while the highest rates were observed in Germany (85%) and France (93%) [5].
Our finding was in line with the available evidence from Italy. Interview-based studies provided the first results on the use of drugs during pregnancy in Italy; women who reported taking at least one drug during pregnancy ranged from 55% to 80% [12,13,14]. More recently, cohort studies using automated administrative databases were conducted in different regions of Italy [6,7,8,9].
As regards the most prescribed drugs, our observation was consistent with previous cohort studies [6,7,8,9,10]. Folic acid, ferrous sulfate, progesterone, and selected antibiotics represented the most used medication during pregnancy.
In our study, we observed an increase in the use of folic acid from about 18% in 2010–2012 to over 25% after 2016; however, the actual consumption of folic acid was probably underestimated due to the high use of OTC medications [15]. National and international guidelines on the prevention of neural tube defects and other congenital anomalies recommended a daily supplementation with 0.4 mg of folic acid in all women looking for pregnancy at least one month before conception and until 12 gestational weeks [16]. In Italy, the official recommendations were launched in April 2004, and the use of folic acid has been free of charge since 2005 under prescription. However, knowledge about the benefits of this vitamin seems to be inadequate among women who planned the pregnancy, as does knowledge about women’s access to services for preconception care [17]. Some investigations focus on folic acid supplements during the preconception period. A cross-sectional study conducted in 2012 in six Italian regions found that preconception folic acid supplement use in a sample of over 2000 women was low (less than 25% of participants) [18]. According to another Italian survey, including only women with planned pregnancies, less than half of the women interviewed (43.4%) took folic acid before becoming pregnant, reflecting a clinical practice far from the recommendation [19].
Progestogen represents a crucial tool in modern clinical practice, particularly in the field of reproductive medicine, but its use remains controversial [20]. In our cohort, we highlighted an increasing trend in the use of progestogen during pregnancy. High variability was observed across regions; in particular, in Puglia, the proportion of women with at least a prescription of progestogen was two-times higher [7]. Comparisons with other countries are made difficult by differences in databases’ composition and reimbursement modalities; however, differences were observed across European countries. For example, a study from Norway reported a prescription rate of drugs included in the class of sex hormones of about 5% [21], while in France, a four-times-higher rate was observed [22]. Nowadays, progestogen and its related molecules are largely used in assisted reproductive protocols; its use in the luteal phase is associated with higher rates of live birth or ongoing pregnancy [23]. However, there is no evidence in favor of prolonged use during pregnancy of progestogen, which seems to not significantly improve the incidence of a term birth and/or live birth rates for women with threatened or recurrent pregnancy loss [24,25].
Antibiotic treatment during pregnancy and birth is widespread, especially for genito-urinary tract and upper respiratory tract infections. Over one-third of the women are prescribed oral antibiotics during pregnancy [26,27,28]. According to this evidence, antibiotics were one of the most commonly prescribed drugs in our investigation. We evaluated the impact of the COVID-19 pandemic and observed a sharp reduction in the months just after the pandemic onset. This decrease in antibiotic utilization could reflect a decrease in infections, mainly respiratory and genito-urinary tract infections, due to the effectiveness of lockdown and preventive measures against the COVID-19 infection (e.g., enhanced hand hygiene, wearing of masks, smart-working, and social distancing). Despite this first reduction in the use of antibiotics, in the period between April and December 2020, an increased trend emerged. A recent systematic review suggested that the pandemic influenced antibiotic consumption over a long period, and a decrease in antibiotic consumption at the community level was reported [29]. From a more general perspective, the deep reorganization of healthcare delivery and the modified procedures of access to medical consultations required by the pandemic might have changed the antibiotic and other medications prescribing strategies. The COVID-19 pandemic also highlighted the crucial need to prioritize and strengthen infection prevention and control measures, especially in care facilities. This emphasizes the significance of monitoring and managing antimicrobial resistance, an emerging public health problem.
In the framework of the COVID-19 pandemic, the Italian healthcare system was significantly influenced. During the first pandemic in Lombardy, there was a notable decrease of up to 20% in recommended healthcare for gynecologic visits during pregnancy [30], and an impact on the management of the event of birth was documented [31]. With this in mind, one could argue that it is reasonable to assume that with the advent of COVID-19, both pregnant women and clinicians would be more careful. This can be supported by several factors. Firstly, the pandemic brought significant attention to healthcare systems worldwide, prompting a heightened sense of caution among both patients and medical professionals. The potential risks associated with the virus created a general atmosphere of increased vigilance, leading pregnant women to be more conscious of their health and to seek timely medical advice. Secondly, the nature of pregnancy itself makes it a critical period, requiring careful monitoring and management. Pregnant women are often more cautious about their wellbeing, as they are aware of the potential impact their health can have on the development of their unborn child. Additionally, healthcare providers have also adapted their practices to prioritize the safety of pregnant women during the pandemic. Guidelines and protocols have been established to ensure the appropriate screening, testing, and management of COVID-19 cases among pregnant individuals. This increased emphasis on safety measures has likely influenced clinicians to be more attentive and proactive in monitoring and addressing potential risks for pregnant women.
Some limitations warrant consideration. We identified only the prescriptions of drugs that were dispensed and reimbursed by the NHS, excluding non-reimbursed and OTC medications. Thus, the number of women who use drugs during pregnancy might be underestimated. On the other hand, some drugs could be dispensed but not taken by pregnant women. In addition, we did not include pregnancies ending in abortions, which could signify an underestimation of drug prescribing with potential fetal harm. The study’s major strength is its population-based design, with a large cohort available over ten years.
The current investigation provided an overview of pregnancy prescription medication patterns in Lombardy. Monitoring drug prescription patterns during pregnancy could represent a tool of the utmost importance to evaluate the adherence to clinical practice and to recommend the consumption of supplements and medications.
4. Materials and Methods
The study cohort consisted of all births recorded in Lombardy from 1 January 2010 to 31 December 2020. Lombardy is a region in Italy with almost ten million inhabitants (16% of the country’s population). The healthcare use of all residents of Lombardy is covered by the government-funded National Health Service (NHS) and recorded in the HealthCare Utilization (HCU) databases of Lombardy, an automated system of databases in use since 1997. The HCU system records demographic and administrative data for all beneficiaries of the Regional Health Service (approximately coinciding with the entire resident population) and their healthcare use. We used information from (i) the hospital discharges registry, which reports all diagnoses released from public or private hospitals; (ii) the outpatient drug prescriptions registry, which reports all dispensations of NHS-reimbursable drugs; and (iii) the Certificates of Delivery Assistance (CeDAP), a specific form filled out by midwives at the delivery which provides detailed information on pregnancy, childbirth, and newborn wellbeing at delivery. The linking of records across HCU databases is made possible through a unique patient-identifying code included in all databases, identifying a large and unselected birth cohort and reconstructing relevant traits and care pathways of mothers and newborns.
Using the CeDAP database, we identified all deliveries of mothers who were beneficiaries of the NHS in Lombardy aged 15 to 55 years who delivered between the gestational ages of 22 and 42 weeks. To ensure the complete ascertainment of women, we required that all of them have continuous enrolment from at least one year before the delivery to at least one year after delivery (n = 83,382). We excluded pregnancies of mothers who did not have a hospital admission for delivery (ICD-9-CM diagnostic codes: V27.xx, 640.xx–676.xx; procedure codes: 72x.xx, 732.xx, 735.xx, 736.xx, 738.xx–742.xx, 744.xx, 749.9) (n = 8827) and those for which the infant could not be linked because of a missing identification code (n = 8914) (Supplementary Materials, Figure S1). The cohort selection was performed in February 2023.
4.1. Drugs Utilization Patterns
Using the outpatient drug prescriptions registry, which records information on the active agent, the date when the prescription was withdrawn, the quantity, and the cost of the medication, we classified drugs according to the World Health Organization (WHO) Anatomical Therapeutic Chemical (ATC) coding system. We considered selected anatomical subgroups, chemical subgroups, and single active agents (ATC fifth level), i.e., those for which a prescription rate equal to 1% or more was reported.
4.2. Statistical Analysis
The prevalence of drugs’ dispensed prescriptions was estimated as the proportion of pregnant women with at least one prescription out of the total deliveries. Drug prevalence was analyzed over the entire pregnancy and by trimester. We inferred the last menstrual period date from the date of birth and the gestational age reported in the CeDAP, which was certified by ultrasound. We computed the start and end dates of each trimester, starting from the last menstrual period date plus three months. Exposure in each trimester of pregnancy was defined based on the presence of at least a prescription of considered drugs in the three periods of interest. In cases where at least one prescription was observed only in one trimester, but none were found in the others, we considered the exposure solely for the specific trimester.
When we considered the single active agents, we provided the prevalence of prescribing during selected periods (i.e., 2010–2012, 2013–2015, 2016–2018, and 2019–2020). In addition, regarding the period of 2016–2018, we compared it with the AIFA national report, which considered the same years.
Finally, we focused our attention on the use of antibiotics (ATC: J01). The prescription incidence rate was defined as the number of antibiotics prescriptions per 1000 person-days. The person-time was calculated from the date of conception until the date of delivery. Interrupted time series analysis was conducted to compare trends in antibiotics prescription between January 2010 and February 2020 and the period after the onset of the COVID-19 pandemic between April 2020 and December 2020. This analysis was performed by accounting for the month of prescription to consider the seasonality of infections potentially requiring antibiotic treatment.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pharma2030021/s1, Figure S1: Flowchart of inclusion and exclusion criteria in the study cohort. Lombardy Region, 2010–2020; Table S1: Selected maternal characteristics. Lombardy, Italy, 2010–2020; Table S2: Drug prescription patterns across three trimesters of pregnancy. Lombardy, Italy, 2010–2020.
Author Contributions
Conceptualization: G.E., A.C. and F.P.; methodology, software, formal analysis, and data curation: G.E., A.C. and M.F.; writing—original draft preparation: G.E. and A.C.; writing—review and editing: M.F. and F.P.; supervision: G.C. and F.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by EU funding within the NextGenerationEU-MUR PNRR Extended Partnership initiative on Emerging Infectious Diseases (Project no. PE00000007, INF-ACT).
Institutional Review Board Statement
This study followed the principles of the Declaration of Helsinki. Data used in this study were anonymized before their use. According to Italian law, studies based entirely on registry data are exempt from IRB authorization and informed consent (General Authorization for the Processing of Personal Data for Scientific Research Purposes Issued by the Italian Privacy Authority on 10 August 2018; https://www.garanteprivacy.it/web/guest/home/docweb/-/docweb-display/docweb/9124510, accessed on 10 February 2023).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the Lombardy region, but restrictions apply to the availability of these data, which were used under license for the current study. The data used in this study cannot be made available in the manuscript, the Supplementary Materials, or in a public repository due to Italian data protection laws. The anonymized datasets generated during and/or analyzed during the current study can be provided on reasonable request, from the corresponding author, after written approval from the Lombardy region.
Conflicts of Interest
Giovanni Corrao received research support from the European Community (EC), the Italian Agency of Drugs (AIFA), and the Italian Ministry for University and Research (MIUR). He took part in a variety of projects that were funded by pharmaceutical companies (i.e., Novartis, GSK, Roche, AMGEN, and BMS). He also received honoraria as a member of the advisory board to Roche. The other authors declare that they have no conflict of interest to disclose.
References
- Ayad, M.; Costantine, M.M. Epidemiology of medications use in pregnancy. Semin. Perinatol. 2015, 39, 508–511. [Google Scholar] [CrossRef] [PubMed]
- Bookstaver, P.B.; Bland, C.M.; Griffin, B.; Stover, K.R.; Eiland, L.S.; McLaughlin, M. A review of antibiotic use in pregnancy. Pharmacotherapy 2015, 35, 1052–1062. [Google Scholar] [CrossRef] [PubMed]
- Belay, M.; Kahaliw, W.; Ergetie, Z. Assessment of drug utilization pattern during pregnancy in adama riferral hospital, oromia region, ethiopia. Int. J. Pharm. Sci. Res. 2013, 4, 1905–1911. [Google Scholar]
- Yates, L.M.; Thomas, S.H. Prescribing medicines in pregnancy. Medicine 2016, 44, 438–443. [Google Scholar] [CrossRef]
- Daw, J.R.; Hanley, G.E.; Greyson, D.L.; Morgan, S.G. Prescription drug use during pregnancy in developed countries: A systematic review. Pharmacoepidem. Drug Saf. 2011, 20, 895–902. [Google Scholar] [CrossRef]
- Gagne, J.J.; Maio, V.; Berghella, V.; Louis, D.Z.; Gonnella, J.S. Prescription drug use during pregnancy: A population-based study in regione emilia-romagna, italy. Eur. J. Clin. Pharmacol. 2008, 64, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Fortinguerra, F.; Belleudi, V.; Poggi, F.R.; Bortolus, R.; Puccini, A.; Solfrini, V.; Stella, P.; Trotta, F. Medication prescriptions before, during and after pregnancy in italy: A population-based study. Ann. Ist. Super. Sanita 2021, 57, 249–258. [Google Scholar]
- D’Aloja, P.; Da Cas, R.; Belleudi, V.; Fortinguerra, F.; Poggi, F.R.; Perna, S.; Trotta, F.; Donati, S.; Mo, M.N.G. Drug prescriptions among italian and immigrant pregnant women resident in italy: A cross-sectional population-based study. Int. J. Environ. Res. Public Health 2022, 19, 4186. [Google Scholar] [CrossRef]
- Ventura, M.; Maraschini, A.; D’Aloja, P.; Kirchmayer, U.; Lega, I.; Davoli, M.; Donati, S. Drug prescribing during pregnancy in a central region of italy, 2008-2012. BMC Public Health 2018, 18, 623. [Google Scholar] [CrossRef]
- Belleudi, V.; Fortinguerra, F.; Poggi, F.R.; Perna, S.; Bortolus, R.; Donati, S.; Clavenna, A.; Locatelli, A.; Davoli, M.; Addis, A.; et al. The italian network for monitoring medication use during pregnancy (mom-net): Experience and perspectives. Front. Pharmacol. 2021, 12, 699062. [Google Scholar] [CrossRef]
- Available online: https://www.aifa.gov.it/documents/20142/1228539/osmed_uso_farmaci_in_gravidanza.pdf (accessed on 12 January 2023).
- Chianale, M.P.; Gho, E.; Rovere, F.; Ostino, G.; Borga, A.D.; Maggiorotti, P. La gravidanza: La prescrizione e il ricorso ai servizi sanitari studio epidemiologico nel territorio delle UU.SS.LL. di Torino. G. Ital. Farm. Clin. 1990, 4, 5–17. [Google Scholar]
- De Vigan, C.; De Walle, H.E.K.; Cordier, S.; Goujard, J.; Knill-Jones, R.; Ayme, S.; Calzolari, E.; Bianchi, F.; Grp, O.W. Therapeutic drug use during pregnancy: A comparison in four european countries. J. Clin. Epidemiol. 1999, 52, 977–982. [Google Scholar] [CrossRef]
- Donati, S.; Baglio, G.; Spinelli, A.; Grandolfo, M.E. Drug use in pregnancy among italian women. Eur. J. Clin. Pharmacol. 2000, 56, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Maraschini, A.; D’Aloja, P.; Lega, I.; Buoncristiano, M.; Kirchmayer, U.; Ventura, M.; Donati, S. Do italian pregnant women use periconceptional folate supplementation? Ann. Ist. Super. Sanita 2017, 53, 118–124. [Google Scholar] [PubMed]
- U.S. Preventive Services Task Force. Folic acid supplementation for the prevention of neural tube defects: Recommendation statement. Am. Fam. Physician 2017, 95. [Google Scholar]
- Bortolus, R.; Parazzini, F.; Addis, A. Folic acid for the prevention of neural tube defects. JAMA Pediatr. 2017, 171, 709–710. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, R.M.; Leoncini, E.; Gastaldi, P.; Allegri, V.; Agostino, R.; Faravelli, F.; Ferrazzoli, F.; Finale, E.; Ghirri, P.; Scarano, G.; et al. Prevalence and determinants of preconception folic acid use: An italian multicenter survey. Ital. J. Pediatr. 2016, 42, 65. [Google Scholar] [CrossRef]
- De Santis, M.; Quattrocchi, T.; Mappa, I.; Spagnuolo, T.; Licameli, A.; Chiaradia, G.; De Luca, C. Folic acid use in planned pregnancy: An italian survey. Matern. Child Health J. 2013, 17, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Di Renzo, G.C.; Tosto, V.; Tsibizova, V. Progesterone: History, facts, and artifacts. Best. Pract. Res. Clin. Obstet. Gynaecol. 2020, 69, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Engeland, A.; Bjorge, T.; Klungsoyr, K.; Hjellvik, V.; Skurtveit, S.; Furu, K. Trends in prescription drug use during pregnancy and postpartum in norway, 2005 to 2015. Pharmacoepidemiol. Drug Saf. 2018, 27, 995–1004. [Google Scholar] [CrossRef]
- Demailly, R.; Escolano, S.; Quantin, C.; Tubert-Bitter, P.; Ahmed, I. Prescription drug use during pregnancy in france: A study from the national health insurance permanent sample. Pharmacoepidemiol. Drug Saf. 2017, 26, 1126–1134. [Google Scholar] [CrossRef] [PubMed]
- van der Linden, M.; Buckingham, K.; Farquhar, C.; Kremer, J.A.M.; Metwally, M. Luteal phase support for assisted reproduction cycles. Cochrane Database Syst. Rev. 2015, 2015, CD009154. [Google Scholar] [CrossRef] [PubMed]
- Devall, A.J.; Papadopoulou, A.; Podesek, M.; Haas, D.M.; Price, M.J.; Coomarasamy, A.; Gallos, I.D. Progestogens for preventing miscarriage: A network meta-analysis. Cochrane Database Syst. Rev. 2021, 4, CD013792. [Google Scholar] [PubMed]
- Yan, Y.; Chen, Z.; Yang, Y.; Zheng, X.; Zou, M.; Cheng, G.; Yuan, Z. Efficacy of progesterone on threatened miscarriage: An updated meta-analysis of randomized trials. Arch. Gynecol. Obstet. 2021, 303, 27–36. [Google Scholar] [CrossRef]
- Stokholm, J.; Schjorring, S.; Pedersen, L.; Bischoff, A.L.; Folsgaard, N.; Carson, C.G.; Chawes, B.L.; Bonnelykke, K.; Molgaard, A.; Krogfelt, K.A.; et al. Prevalence and predictors of antibiotic administration during pregnancy and birth. PLoS ONE 2013, 8, e82932. [Google Scholar] [CrossRef]
- Broe, A.; Pottegard, A.; Lamont, R.F.; Jorgensen, J.S.; Damkier, P. Increasing use of antibiotics in pregnancy during the period 2000-2010: Prevalence, timing, category, and demographics. BJOG 2014, 121, 988–996. [Google Scholar] [CrossRef]
- Cantarutti, A.; Rea, F.; Franchi, M.; Beccalli, B.; Locatelli, A.; Corrao, G. Use of antibiotic treatment in pregnancy and the risk of several neonatal outcomes: A population-based study. Int. J. Environ. Res. Public. Health 2021, 18, 12621. [Google Scholar] [CrossRef]
- Fukushige, M.; Ngo, N.H.; Lukmanto, D.; Fukuda, S.; Ohneda, O. Effect of the COVID-19 pandemic on antibiotic consumption: A systematic review comparing 2019 and 2020 data. Front Public Health 2022, 10, 946077. [Google Scholar] [CrossRef]
- Corrao, G.; Cantarutti, A.; Monzio Compagnoni, M.; Franchi, M.; Rea, F. Change in healthcare during COVID-19 pandemic was assessed through observational designs. J. Clin. Epidemiol. 2022, 142, 45–53. [Google Scholar] [CrossRef]
- Esposito, G.; Rossi, M.; Favilli, A.; Franchi, M.; Corrao, G.; Parazzini, F.; La Vecchia, C. Impact of the First and Second Lockdown for COVID-19 Pandemic on Preterm Birth, Low Birth Weight, Stillbirth, Mode of Labor, and of Delivery in Lombardy, Italy. J. Pers. Med. 2023, 13, 499. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).