Abstract
Buprenorphine is a safe and effective medication to treat opioid use disorder (OUD) in pregnant patients and is intended to be continued throughout pregnancy, delivery, and at least the one-year postpartum period. However, delivery often involves the need for acute pain management with opioid medications, such as after a cesarean section. For patients receiving buprenorphine, the provision of prescription opioids may negatively impact OUD treatment outcomes; however, not optimally managing acute pain may also impede OUD treatment benefit. Evidence is needed to disentangle the impacts of opioid prescription provision and methods of pain management in the immediate postpartum period on OUD treatment trajectories, ultimately to inform clinical guidelines tailored to the unique needs of pregnant and postpartum people receiving buprenorphine. Accordingly, this study took an initial step towards this goal to conduct a secondary analysis of a retrospective cohort of pregnant patients taking buprenorphine for OUD at the time of delivery (n = 142) to determine whether receipt of an opioid prescription at birth hospitalization discharge was associated with the time of buprenorphine discontinuation within the 12 months following delivery. Among the sample, 26% (n = 37) were prescribed an opioid at the time of birth hospitalization discharge. The number of weeks post-delivery until buprenorphine discontinuation occurred was shorter amongst patients who were prescribed an opioid (median 11 weeks) compared to patients who were not prescribed an opioid (median 39 weeks; p < 0.001 by Mann–Whitney U test). However, a Cox regression model reported that receipt of an opioid prescription following delivery did not significantly increase the hazard ratio for buprenorphine discontinuation. In other words, OUD patients not prescribed an opioid at birth hospitalization discharge continued their buprenorphine for a longer median duration after delivery compared to their counterparts who received prescription opioids; yet, this finding did not reach statistical significance when taking into account additional clinical variables. The findings indicate how further research is warranted to inform evidence-based post-delivery pain practices for postpartum OUD treatment patients.
1. Introduction
The rate of opioid use among pregnant people has increased fourfold within the past decade, resulting in the American College of Obstetricians and Gynecologists recommending that universal screening for substance use disorders and referral to treatment programs be part of comprehensive obstetric care [1]. The American College of Obstetricians and Gynecologists recommend that all pregnant individuals seeking treatment for opioid use disorder (OUD) be offered medications for opioid use disorder (MOUD) (e.g., buprenorphine) and be supported to continue these medications during pregnancy, delivery, and postpartum. Medication continuation during the postpartum period is of particular importance, as the majority of pregnancy-associated deaths in the United States happen between seven days to one year after delivery [2], and opioid overdose is the leading cause of postpartum maternal death in individuals with OUD [3]. The utilization of medications for OUD, especially when used for longer durations, is associated with 60% lower odds of opioid overdose death in the first year postpartum for individuals with OUD [3,4]. Unfortunately, reported postpartum MOUD discontinuation rates are high, between 36% [4] and 65% [5]. In one study of over 1500 birthing people diagnosed with OUD, 42.9% received buprenorphine treatment throughout their pregnancy; however, one third experienced a treatment disruption during the postpartum period [6]. Additional research is needed to identify factors that may be driving high rates of postpartum discontinuation of medication for OUD in order to develop effective strategies to increase consistent medication continuation during this vulnerable period.
The previous studies have found that factors such as incarceration status at delivery, race, and duration of maintenance on MOUD prior to delivery are associated with postpartum MOUD adherence [4]. While informative, these are not all factors that can be easily modified by the clinician. One factor that is within clinician control and might be associated with MOUD continuation is receipt of prescription opioids to manage acute pain post-delivery. For instance, studies have shown that receipt of opioid prescriptions following birth is associated with increased likelihood of serious opioid-related events (e.g., persistent opioid use, opioid overdose, opioid-related death) during the subsequent year [7]. Additionally, one study found that receipt of an opioid prescription in the three months prior to delivery was associated with an increased likelihood of MOUD discontinuation compared to without a prenatal opioid prescription [4]. Another recent study found that opioid refill rates for post-delivery pain were increased in patients with OUD or chronic pain compared to individuals without these diagnoses [8]. On the other hand, one study found that experiences of self-reported pain are associated with recurrence of non-prescribed opioid use among individuals with OUD [9]. This literature indicates that there are likely associations between opioid prescription receipt, as well as acute pain and medication for OUD treatment outcomes, during the postpartum period. However, the directionality of these relationships has not yet been determined.
During the birthing period, clinicians must weigh both adequate pain management and optimally supporting OUD treatment outcomes to provide the best comprehensive, long-term care for their patients. While reliance on non-opioid analgesics (e.g., non-steroidal anti-inflammatory drugs) is encouraged [10], pregnant individuals may require opioids for effective pain management, especially after a cesarean birth [7]. Individuals with a history of chronic opioid use or who are taking MOUD, such as buprenorphine, will likely require adjusted opioid dosing to address their unique pain needs [10,11]. Qualitative studies have reported that nurses and obstetric providers are unsure how to address pain management during labor and the postoperative period for individuals with OUD and patients taking MOUD [12]. This uncertainty is, in part, due to the lack of evidence-based recommendations for clinicians regarding how to safely and effectively prescribe opioids to postpartum patients with OUD.
As an initial step to address this gap in knowledge at the intersection of acute pain, opioid prescribing, and OUD treatment, this study of pregnant OUD patients maintained on buprenorphine determined the association between receipt of an opioid prescription at delivery hospitalization discharge for postpartum pain management and the time of buprenorphine discontinuation up to 52 weeks postpartum. The goal of this study is to collect evidence to inform future clinical guidelines on the best ways to achieve optimal pain management while also supporting adherence to recovery for patients on MOUD transitioning from pregnancy to postpartum.
2. Results
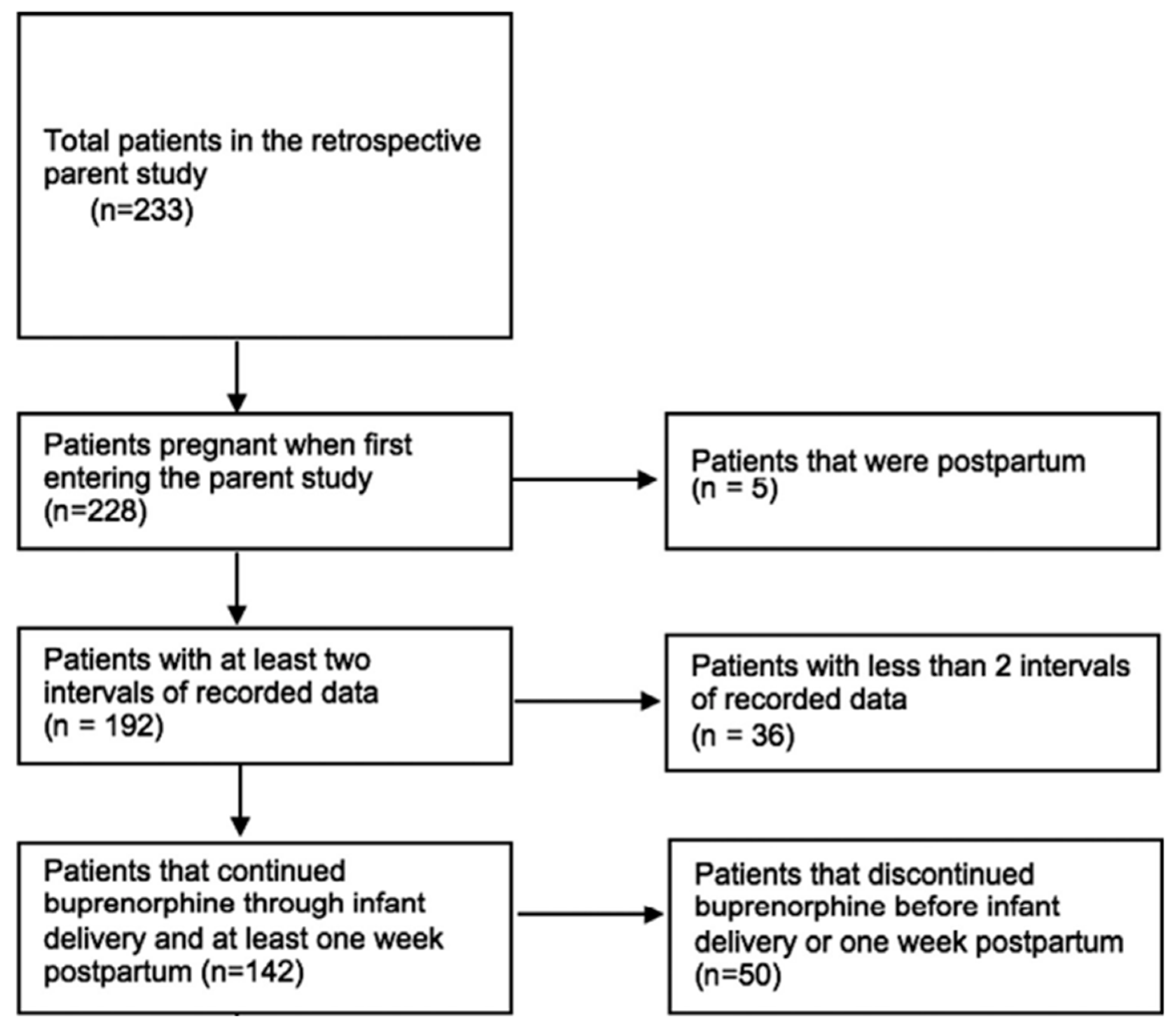
Our final sample included 142 patients (Figure 1). As shown in Table 1, the majority of patients (64%, n = 91) discontinued buprenorphine prior to 52 weeks postpartum. The majority of the sample identified as White (69.7%) or Black (28.9%), were insured (84.5%), and were not incarcerated at the time of delivery (81%). The minority of patients (23%, n = 33) received an opioid prescription at discharge following delivery. The proportion receiving an opioid prescription at birth hospitalization discharge did not differ between the group who continued (17.7%, n = 9) buprenorphine through 52 weeks postpartum and the group who discontinued (26.4%, n = 24) buprenorphine postpartum (p = 0.608). Most patients who received an opioid prescription at discharge underwent cesarean delivery (60.6%, n = 20), whereas most patients who did not receive an opioid prescription at discharge underwent a vaginal delivery (66.1%, n = 72). Notably, similar proportions of Cesarean section deliveries were noted for patients who continued buprenorphine through 52 weeks postpartum (37.2%, n = 19) versus those who discontinued (35.2%, n = 32) prior to the end of the study period (p = 0.173).
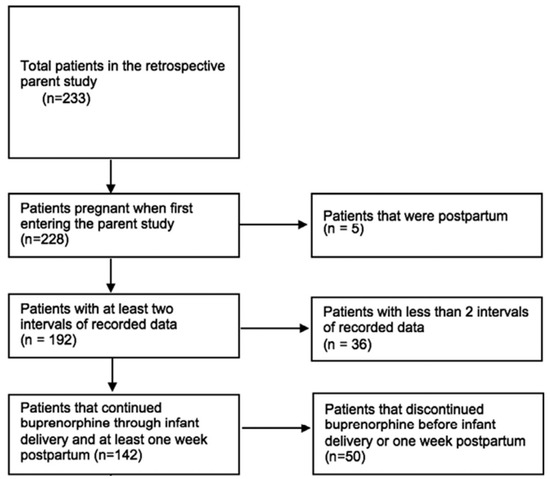
Figure 1.
Study Schema.

Table 1.
Characteristics of participants continuing and discontinuing buprenorphine treatment throughout the 52-week study period.
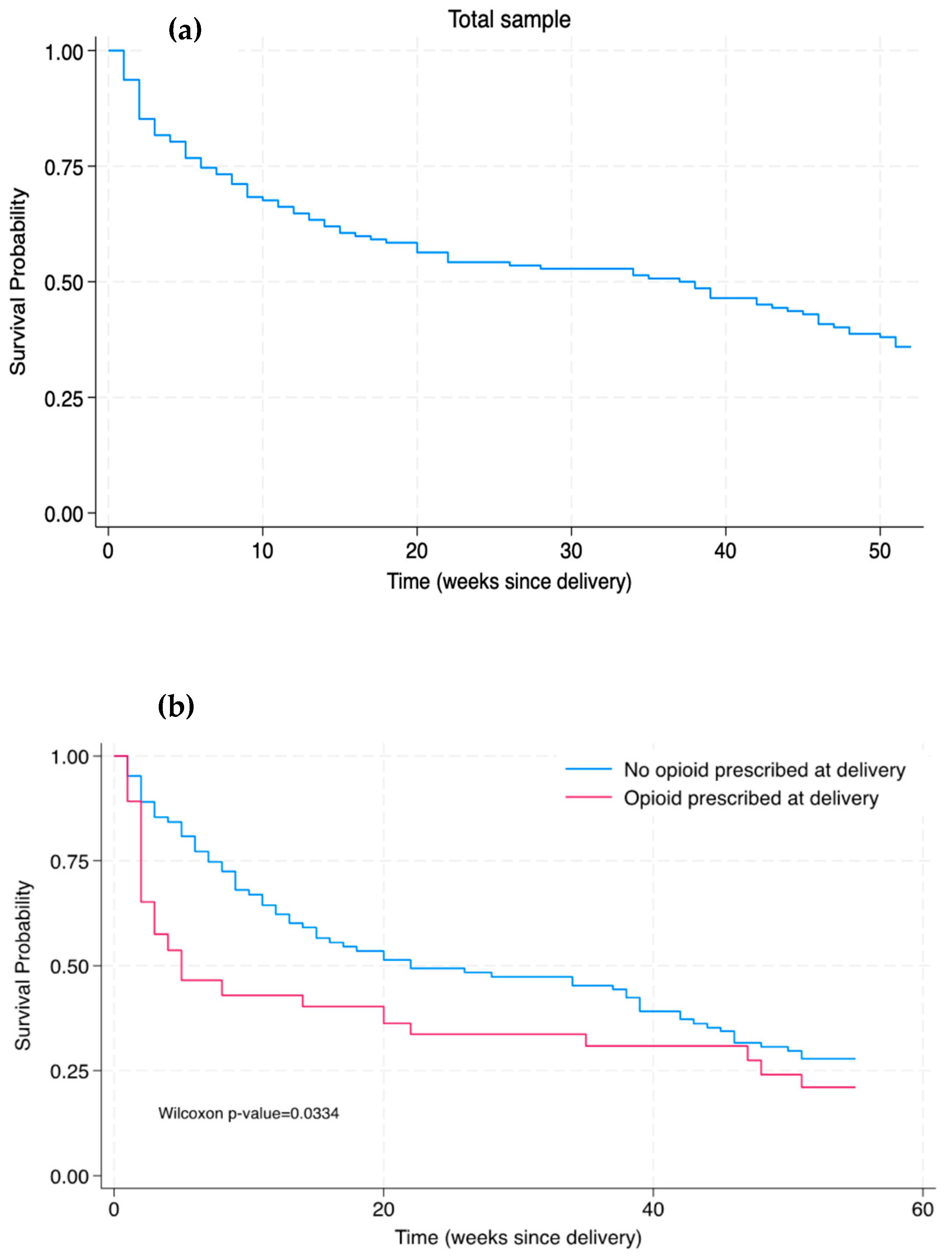
Figure 2 shows Kaplan–Meier survival curves for buprenorphine continuation in both the total sample and in participants who received or did not receive an opioid prescription at hospital discharge after delivery. In the total sample, the median time of discontinuation was 38 weeks postpartum. The number of weeks post-delivery until buprenorphine discontinuation was shorter amongst patients who were prescribed an opioid (median 11 weeks) compared to patients who were not prescribed an opioid (median 39 weeks; p < 0.001). There was a significant difference in the Kaplan–Meier survival distributions between patients with and without an opioid prescribed at delivery, as determined by the Wilcoxon–Breslow–Gehan test (Figure 2b; p = 0.0334). In other words, being prescribed an opioid at delivery was associated with a significantly decreased time to buprenorphine discontinuation compared to those who were not prescribed an opioid at delivery (without taking into consideration other clinical variables).
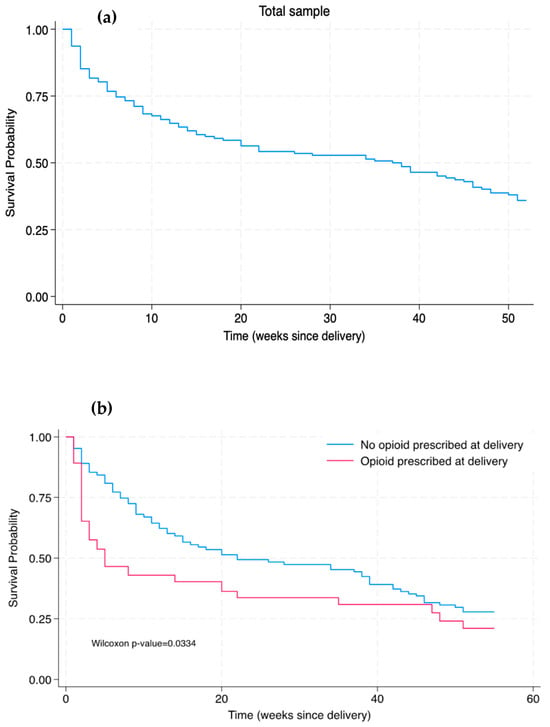
Figure 2.
Kaplan–Meier survival curves of time of buprenorphine discontinuation through 52 weeks postpartum among (a) the total sample and (b) the sub-samples of patients prescribed and not prescribed an opioid at hospital discharge for delivery.
Table 2 shows the unadjusted and adjusted Cox proportional hazard regression models. The association between opioid prescription status at delivery and time of buprenorphine discontinuation was non-significant in the unadjusted and adjusted Cox proportional hazard regression models. In the adjusted model, only psychiatric comorbidity was significantly associated with time of postpartum buprenorphine discontinuation (0.51 adjusted HR; 95% CI: 0.32, 0.80).

Table 2.
Association of opioid prescription receipt at delivery with time of buprenorphine discontinuation within 52 weeks postpartum.
3. Discussion
In this retrospective cohort study, we did not find a clear association between receipt of an opioid prescription for postpartum pain management at birth hospitalization discharge and buprenorphine discontinuation throughout one year postpartum among a cohort of pregnant patients with OUD. Overall, the results of the current study suggest that prescription opioids may be associated with buprenorphine discontinuation in the high-risk postpartum period, but it is likely not a main factor contributing to the high reported rates of buprenorphine discontinuation within the year after delivery. There are additional factors, such as engagement with other addiction and mental health care services, that are likely playing more meaningful roles in influencing this relationship. As such, a clinician’s decision to prescribe opioids to postpartum patients with OUD should take into consideration individualized patient factors. To inform future evidence-based clinical guidelines, investigations are warranted at the intersection between OUD treatment and postpartum pain management that incorporate longitudinal data and holistic patient evaluations.
While the Kaplan–Meier curves demonstrated an early significant difference in buprenorphine discontinuation between those with versus without an opioid prescription at delivery, there were no significant differences detected in both the unadjusted and adjusted Cox proportional hazards models by opioid prescription group. Notably, the Cox proportional hazards model indicated that after adjusting for opioid prescription at delivery and select other clinical variables (incarceration at delivery, receipt of buprenorphine before delivery, and chronic pain), the presence of a psychiatric comorbidity was associated with a reduced risk of buprenorphine discontinuation. This result is reported on in our previous study [13], which suggested that individuals with a psychiatric comorbidity may be at reduced risk of discontinuing buprenorphine treatment due to multiple factors such as increased engagement in OUD care and potentially more contact points with various dimensions (e.g., addiction care, mental health, recovery support services) of the integrated care model within the academic center in which this study was conducted.
Importantly, a major interpretive complication of our study is that the individual’s reason for buprenorphine discontinuation is unknown. While buprenorphine continuation is encouraged during the postpartum period, there are numerous reasons why a patient may discontinue buprenorphine postpartum. For instance, it is possible that the patient chose to manage their OUD symptoms without medication, the patient faced structural barriers to continued OUD treatment, or the patient had a return to non-prescribed substance use that precipitated treatment dropout. The data regarding management of the patient’s OUD-related symptoms (with patient-reported outcomes) following prescription opioid receipt for postpartum pain may give a more meaningful indication on how the receipt of prescription opioids for acute pain affects OUD treatment outcomes through the year after pregnancy. Overall, multi-level assessments utilizing a combination of research methodologies, such as patient surveys, analyses of large observational databases, and qualitative data collection, are needed to understand the potential intrapersonal, interpersonal, and structural factors that could influence buprenorphine discontinuation during the high-risk postpartum period.
There are several possibilities as to why the current study did not find a robust association between opioid prescription receipt and buprenorphine discontinuation. Two are discussed. First, receipt of an opioid prescription at hospital discharge was rare, with only 37 patients (26% of total sample) receiving a prescription. The disproportionate sample sizes between individuals who received versus did not receive an opioid prescription at discharge makes it difficult to draw conclusions about the differences between these two groups. This limitation is of particular importance given the small sample size of this study and resulting low statistical power. Second, the current study had data regarding whether the patient was prescribed an opioid medication at discharge but did not have data about whether the patient went through the necessary steps to (a) fill the prescription and (b) take the medication. Additional factors such as access to transportation, access to childcare, or socioeconomic status may have influenced whether the individual engaged in the necessary behaviors to take the prescription opioid.
This study supports the need for future research to determine optimal pain management interventions for birthing people with OUD. Specifically, non-opioid methods for pain relief during pregnancy and birth should be evaluated in the context of both effective pain management and OUD treatment outcomes, to optimize both OUD recovery and health outcomes for the parent–infant dyad. A 2022 systematic literature review [13] highlighted a gap in the research with regards to alternative analgesia methods during pregnancy and birth in an OUD population. Additionally, a recent study of patients with OUD who did not receive opioids for postpartum pain management found that 39% of patients reported poorly controlled pain in the hospital and one-week post discharge and that these levels of pain interfered with their parenting responsibilities [14]. Due to the lack of research on effective pain management strategies in pregnant individuals with OUD, many clinicians are uncertain of how to effectively care for this population of patients [12]. Overall, the results of the current study add to the existing literature calling for further patient-centered research regarding postpartum pain management for people with OUD in order to better assist clinicians in making evidence-based treatment plans that reduce maternal morbidity and mortality associated with OUD.
4. Materials and Methods
4.1. Study Design
The study consisted of a secondary analysis of a retrospective cohort study involving pregnant patients who were receiving buprenorphine treatment for OUD at the time of delivery between January 2017 and March 2020. This study was conducted at an academic medical center: Virginia Commonwealth University (VCU). VCU is equipped with an obstetric/addiction clinic, OB MOTIVATE, that specializes in providing services for pregnant and parenting people using an integrated care model [15]. Although receiving care at the clinic was not a requirement for enrollment in the study, many individuals did receive care at OB MOTIVATE. The detailed methods for the parent study have been described previously [5], and the study was conducted with IRB approval from VCU. Briefly, participants were identified using the electronic medical record and included in the study if they had received a buprenorphine prescription at any point during their pregnancy and/or through one year postpartum. Manual chart abstractions were completed and data on psychosocial variables, pregnancy and delivery outcomes, and buprenorphine continuation were collected. Buprenorphine receipt was monitored through both the Virginia Prescription Monitoring Program and chart review. Chart abstractions began at the time of initial buprenorphine receipt during pregnancy and completed in four-week increments (to mirror clinical practice) until the participant either discontinued buprenorphine for two 4-week consecutive intervals or reached 52 weeks postpartum.
The primary outcome was the week of buprenorphine discontinuation (up to 52 weeks) after delivery. The independent variable was receipt of an opioid prescription upon discharge from the hospital following delivery (yes/no). For clinical context, opioid prescriptions provided at discharge following delivery generally consist of oxycodone 5 mg tablets with amounts protocolized to be in line with CDC recommendations (e.g., 3–7 days of prescription).
4.2. Sample Criteria
The patients were included in the current analysis if the patient (a) was pregnant when entering parent study, (b) had at least two consecutive intervals of longitudinal data, (c) was receiving buprenorphine at the time of delivery, and (d) continued buprenorphine through infant delivery and at least one week postpartum. Out of the 233 patients in the parent study, 142 met the inclusion criteria and were included in our analysis (Figure 1).
4.3. Statistical Analysis
Chi-square or t-tests were conducted to evaluate differences in various demographic, clinical, and psychosocial variables of patients who continued buprenorphine through 52 weeks postpartum versus patients who discontinued buprenorphine before 52 weeks postpartum. The Mann–Whitney U test was used to evaluate differences in median weeks until buprenorphine discontinuation between those prescribed opioids following delivery compared to those who were not. Kaplan–Meier survival curves were generated to visualize the distribution of time (in weeks) of buprenorphine continuation following delivery among patients who received versus did not receive an opioid prescription at delivery. Statistically significant differences between the curves were determined using the Wilcoxon–Breslow–Gehan test, a modified log-rank test that is sensitive to early differences. This decision to use a statistical test that is sensitive to early differences in the survival curves was informed by prior studies emphasizing that buprenorphine discontinuation at early timepoints (0–38 weeks) within the postpartum period are critical for clinicians to make timely interventions [2,5,16]. Cox proportional hazard models were used to assess associations between opioid prescription at delivery and time (in weeks) to postpartum buprenorphine discontinuation with and without adjusting for the covariates of incarceration status at delivery, psychiatric comorbidity, and the number of weeks maintained on buprenorphine before delivery; these factors were selected based on the prior literature indicating these variables’ impacts on our outcomes and their ability to provide a holistic clinical context (albeit with a small, yet appropriate, number of variables given the sample size) for the adjusted model [5,17]. We evaluated proportionality assumptions by analyzing adjusted log–log plots and Schoenfeld residuals for the outcomes. All analyses were conducted using Stata (version 18.0) and R (version 3.0) [18,19].
Author Contributions
Conceptualization, T.N.H. and C.E.M.; methodology, software, and formal analysis, D.T.Z. and H.S.; investigation, C.E.M., T.N.H. and D.T.Z.; resources, funding acquisition, C.E.M.; supervision, C.E.M. and M.M.M.; writing—original draft preparation, T.N.H., D.T.Z. and L.T.; writing—review and editing, T.N.H., D.T.Z., H.S., L.T., M.M.M., C.E.M. and A.S.; visualization, D.T.Z., T.N.H. and C.E.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by Nation Institute on Drug Abuse (NIDA) (PI: C.E. Martin; K23 DA053507), CTSA award UL1TR002649 from the National Center for Advancing Translational Sciences, and the Jeanann Dunlap Foundation.
Institutional Review Board Statement
The VCU Institutional Review Board approved this study (IRB#HM20018384).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
De-identified data are potentially available upon request to the principal investigator.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Committee on Obstetric Practice. Committee Opinion No. 711: Opioid Use and Opioid Use Disorder in Pregnancy. Obstet. Gynecol. 2017, 130, e81–e94. [Google Scholar] [CrossRef] [PubMed]
- Trost, S.; Beauregard, J.; Chandra, G.; Njie, F.; Berry, J.; Harvey, A.; Goodman, D. Pregnancy-Related Deaths: Data from Maternal Mortality Review Committees in 36 US States, 2017–2019. Education 2022, 45. Available online: https://www.cdc.gov/reproductivehealth/maternal-mortality/erase-mm/data-mmrc.html (accessed on 31 January 2024).
- Suarez, E.A.; Huybrechts, K.F.; Straub, L.; Hernández-Díaz, S.; Creanga, A.A.; Connery, H.S.; Gray, K.J.; Vine, S.M.; Jones, H.E.; Bateman, B.T. Postpartum Opioid-Related Mortality in Patients With Public Insurance. Obstet. Gynecol. 2023, 141, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Schiff, D.M.; Nielsen, T.C.; Hoeppner, B.B.; Terplan, M.; Hadland, S.E.; Bernson, D.; Greenfield, S.F.; Bernstein, J.; Bharel, M.; Reddy, J.; et al. Methadone and buprenorphine discontinuation among postpartum women with opioid use disorder. Am. J. Obstet. Gynecol. 2021, 225, 424.E1–424.E12. [Google Scholar] [CrossRef] [PubMed]
- Shadowen, H.; Violante, S.; Gataric, A.; Goulding, A.N.; Martin, C.E. Psychiatric comorbidities and their treatment predict buprenorphine continuation among postpartum people with opioid use disorder. Drug Alcohol Depend. Rep. 2022, 5, 100121. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Meisel, Z.; Kellom, K.; Whitaker, J.; Strane, D.; Chatterjee, A.; Rosenquist, R.; Matone, M. Receipt and duration of buprenorphine treatment during pregnancy and postpartum periods in a national privately-insured cohort. Drug Alcohol Depend. Rep. 2023, 9, 100206. [Google Scholar] [CrossRef] [PubMed]
- Osmundson, S.S.; Min, J.Y.; Wiese, A.D.; Hawley, R.E.; Mitchel, E.; Patrick, S.W.; Samuels, L.R.; Griffin, M.R.; Grijalva, C.G. Opioid Prescribing After Childbirth and Risk for Serious Opioid-Related Events: A Cohort Study. Ann. Intern. Med. 2020, 173, 412–414. [Google Scholar] [CrossRef] [PubMed]
- Peahl, A.F.; Keer, E.; Hallway, A.; Kenney, B.; Waljee, J.F.; Townsel, C. Postpartum Opioid Prescribing in Patients with Opioid Use Prior to Birth. Am. J. Perinatol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Ellis, M.S.; Kasper, Z.; Cicero, T. Assessment of Chronic Pain Management in the Treatment of Opioid Use Disorder: Gaps in Care and Implications for Treatment Outcomes. J. Pain 2021, 22, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Gopman, S. Prenatal and postpartum care of women with substance use disorders. Obstet. Gynecol. Clin. N. Am. 2014, 41, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Holland, E.; Gibbs, L.; Spence, N.Z.; Young, M.; Werler, M.M.; Guang, Z.; Saia, K.; Bateman, B.T.; Achu, R.; Wachman, E.M. A comparison of postpartum opioid consumption and opioid discharge prescriptions among opioid-naïve patients and those with opioid use disorder. Am. J. Obstet. Gynecol. MFM 2023, 5, 101025. [Google Scholar] [CrossRef] [PubMed]
- Nowakowski, E.; Dayananda, S.; Morgan, M.; Jarvis, O.; Altamirano, V.; LaSorda, K.R.; Krans, E.; Lim, G. Obstetric pain management for pregnant women with opioid use disorder: A qualitative and quantitative comparison of patient and provider perspectives (QUEST study). Addiction 2023, 118, 1093–1104. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.; Soens, M.; Wanaselja, A.; Chyan, A.; Carvalho, B.; Landau, R.; George, R.B.; Klem, M.L.; Osmundson, S.S.; Krans, E.E.; et al. A Systematic Scoping Review of Peridelivery Pain Management for Pregnant People With Opioid Use Disorder: From the Society for Obstetric Anesthesia and Perinatology and Society for Maternal Fetal Medicine. Anesth. Analg. 2022, 135, 912–925. [Google Scholar] [CrossRef] [PubMed]
- Townsel, C.; Irani, S.; Nguyen, B.H.; Hallway, A.; Shuman, C.J.; Waljee, J.; Jaffe, K.; Peahl, A.F. Use of Opioid-Sparing Protocols and Perceived Postpartum Pain in Patients with Opioid Use Disorder and Chronic Prenatal Opioid Exposure. Matern. Child Health J. 2023, 27, 1416–1425. [Google Scholar] [CrossRef] [PubMed]
- Ellis, L.P.; Parlier-Ahmad, A.B.; Scheikl, M.; Martin, C.E. An Integrated Care Model for Pregnant and Postpartum Individuals Receiving Medication for Opioid Use Disorder. J. Addict. Med. 2022, 17, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Lo-Ciganic, W.; Donohue, J.M.; Kim, J.Y.; Krans, E.E.; Jones, B.L.; Kelley, D.; James, A.E.; Jarlenski, M.P. Adherence trajectories of buprenorphine therapy among pregnant women in a large state Medicaid program in the United States. Pharmacoepidemiol. Drug Saf. 2019, 28, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.; Shadowen, H.; Shadowen, C.; Thakkar, B.; Knittel, A.K.; Martin, C.E. Incarceration status at buprenorphine initiation and OUD treatment outcomes during pregnancy. Front. Psychiatry 2023, 14, 1157611. [Google Scholar] [CrossRef] [PubMed]
- StataCorp. Stata, Release 18. Statistical Software; StataCorp LLC: College Station, TX, USA, 2023. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).