Hardwoods: Anatomy and Functionality of Their Elements—A Short Review
Abstract
1. Introduction
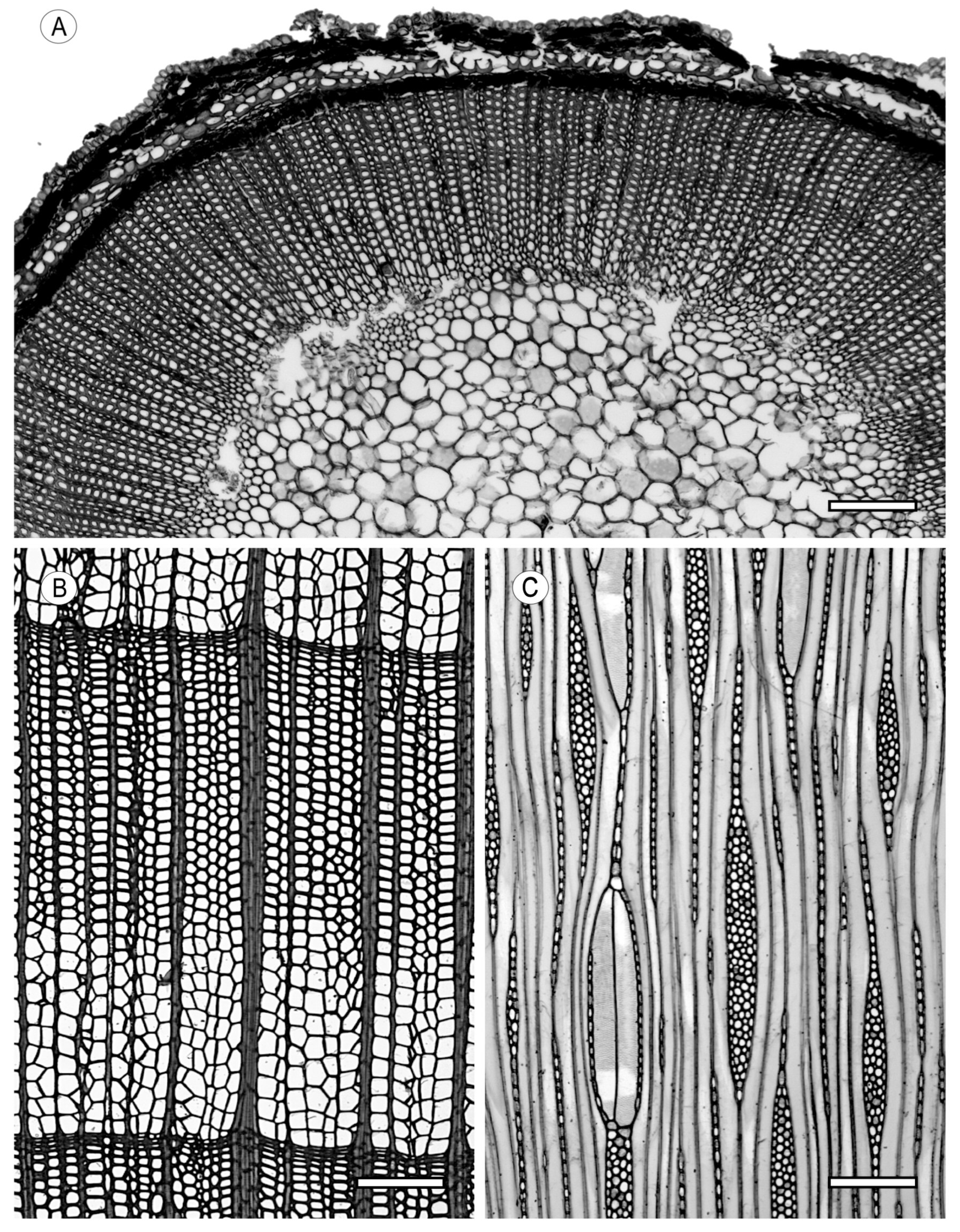
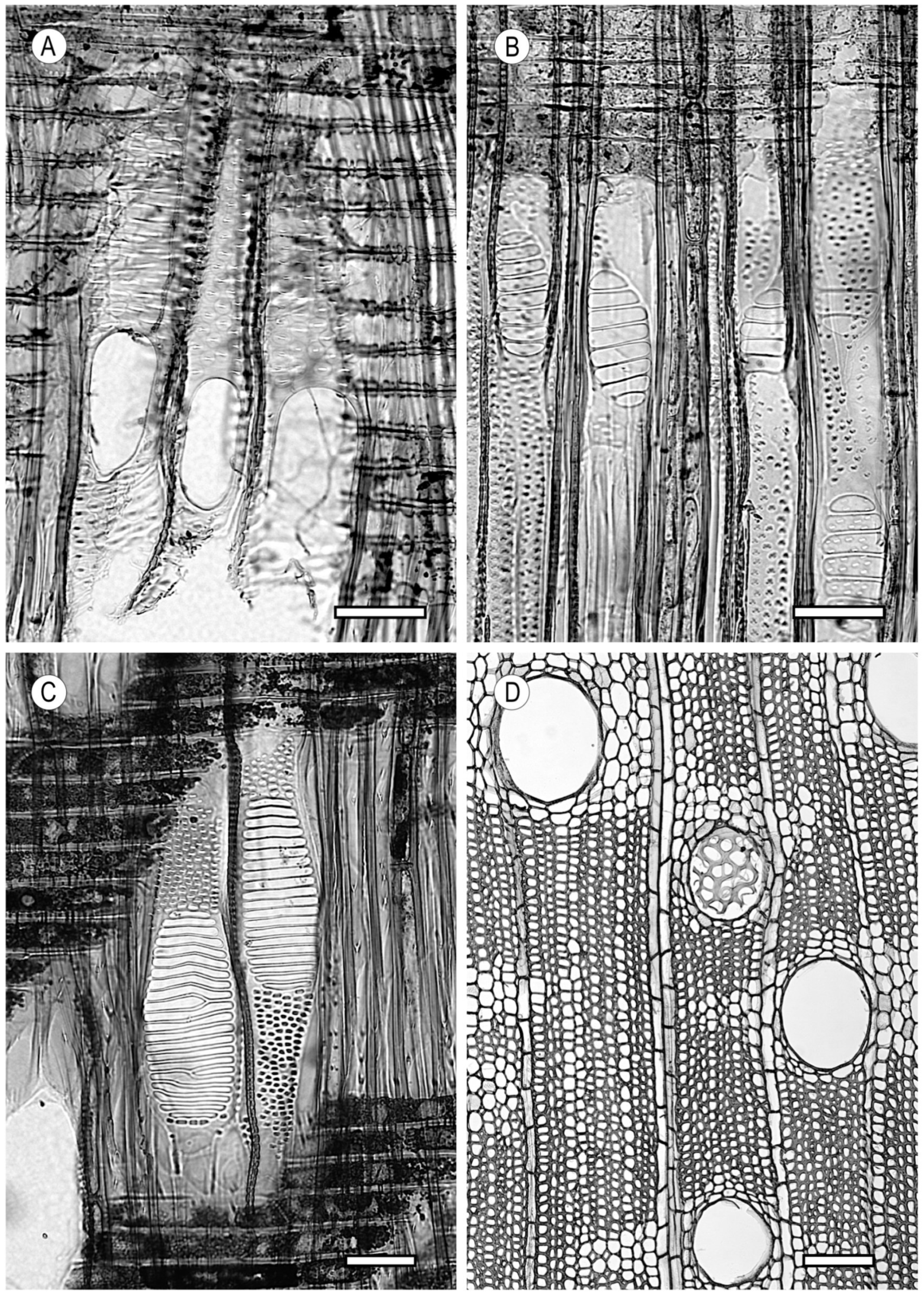
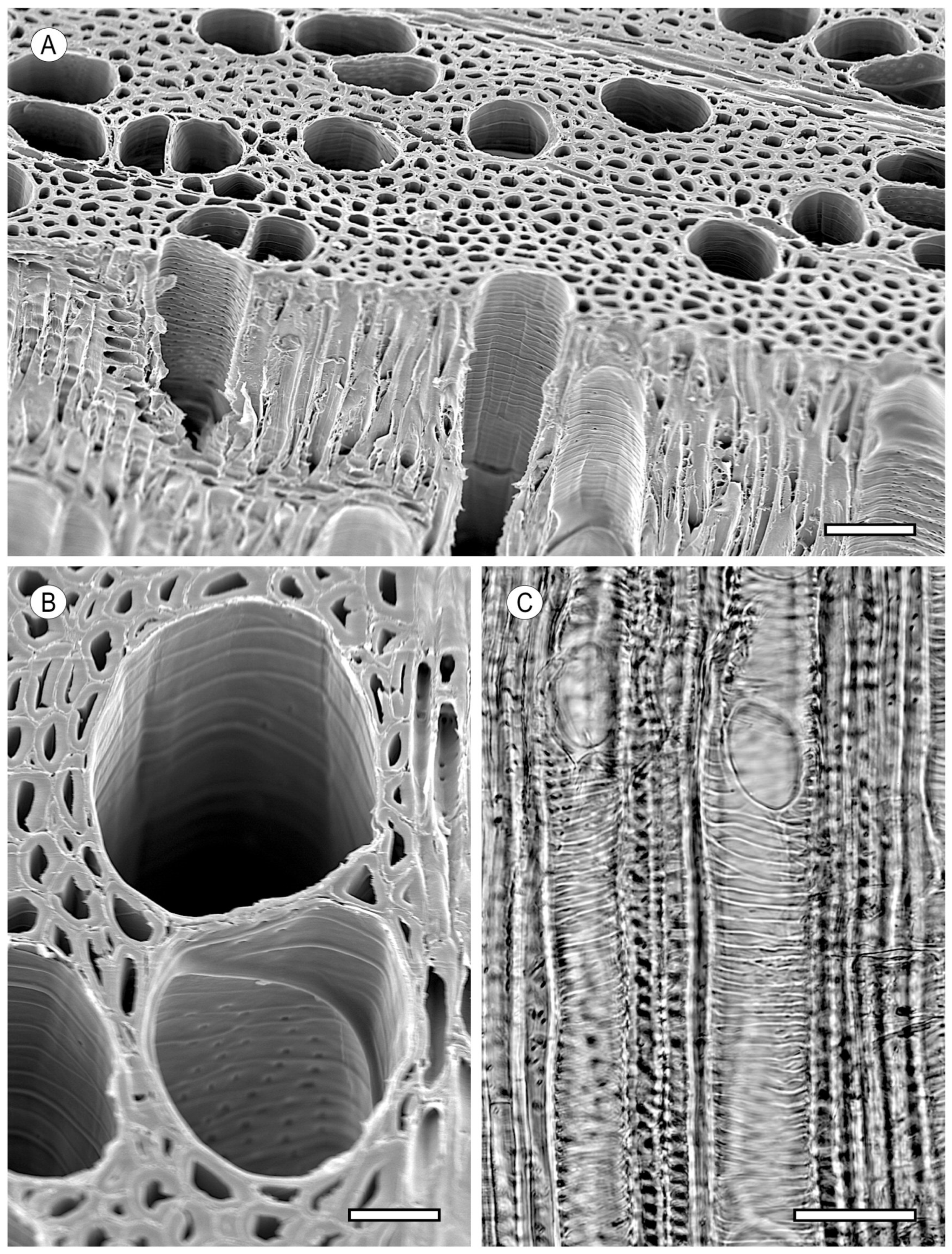
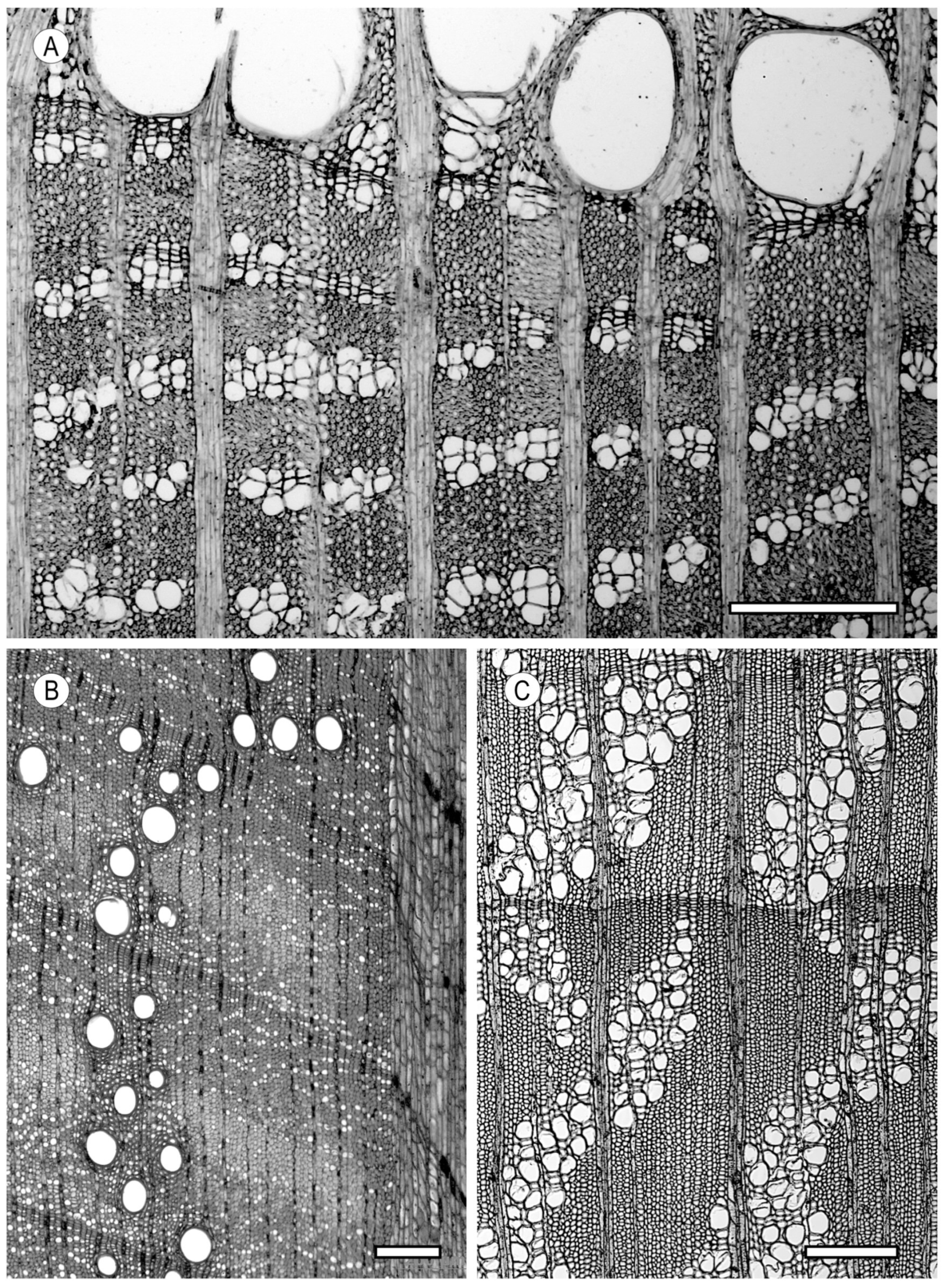
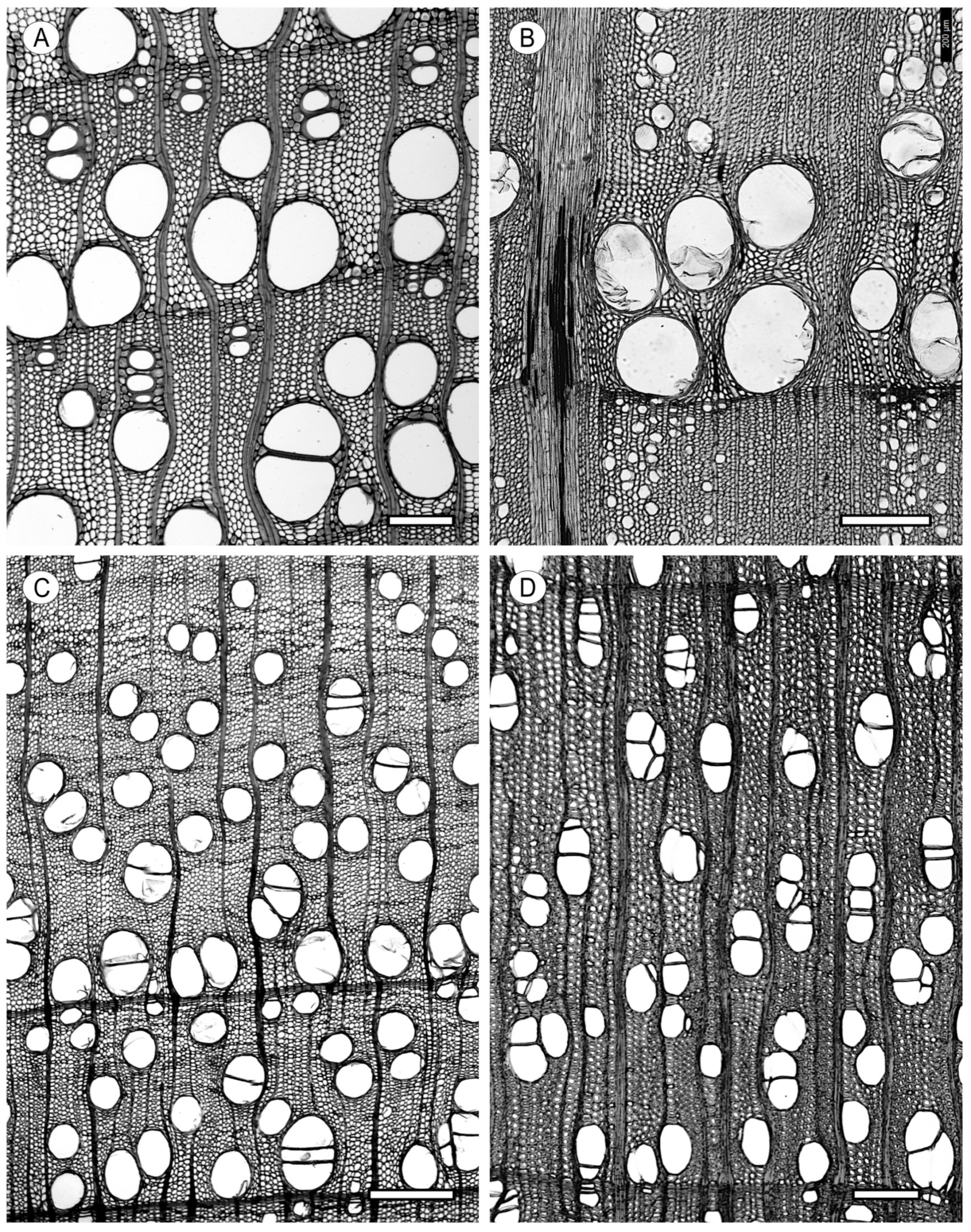
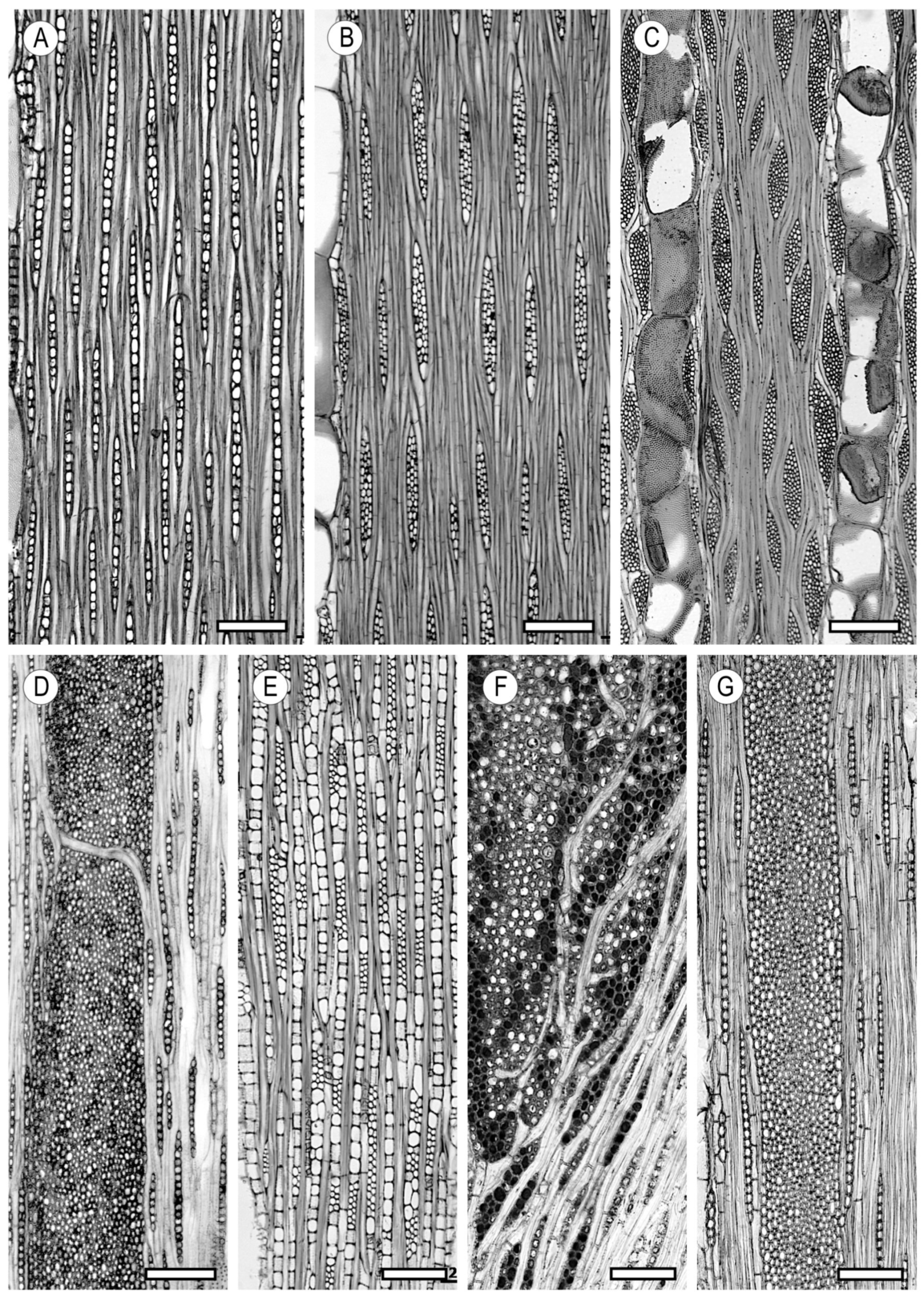
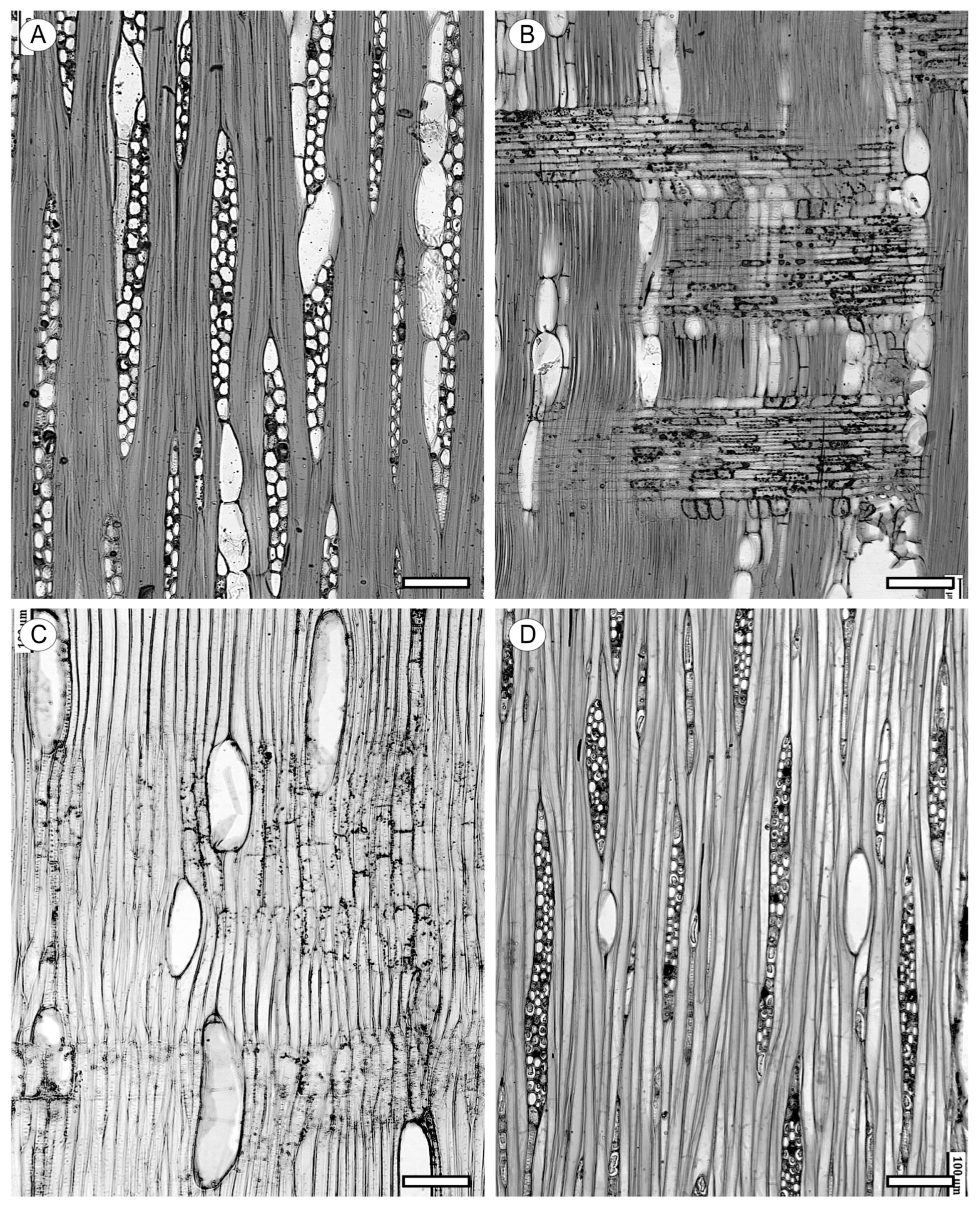
2. Vessels
2.1. Morphology
2.2. Arrangement
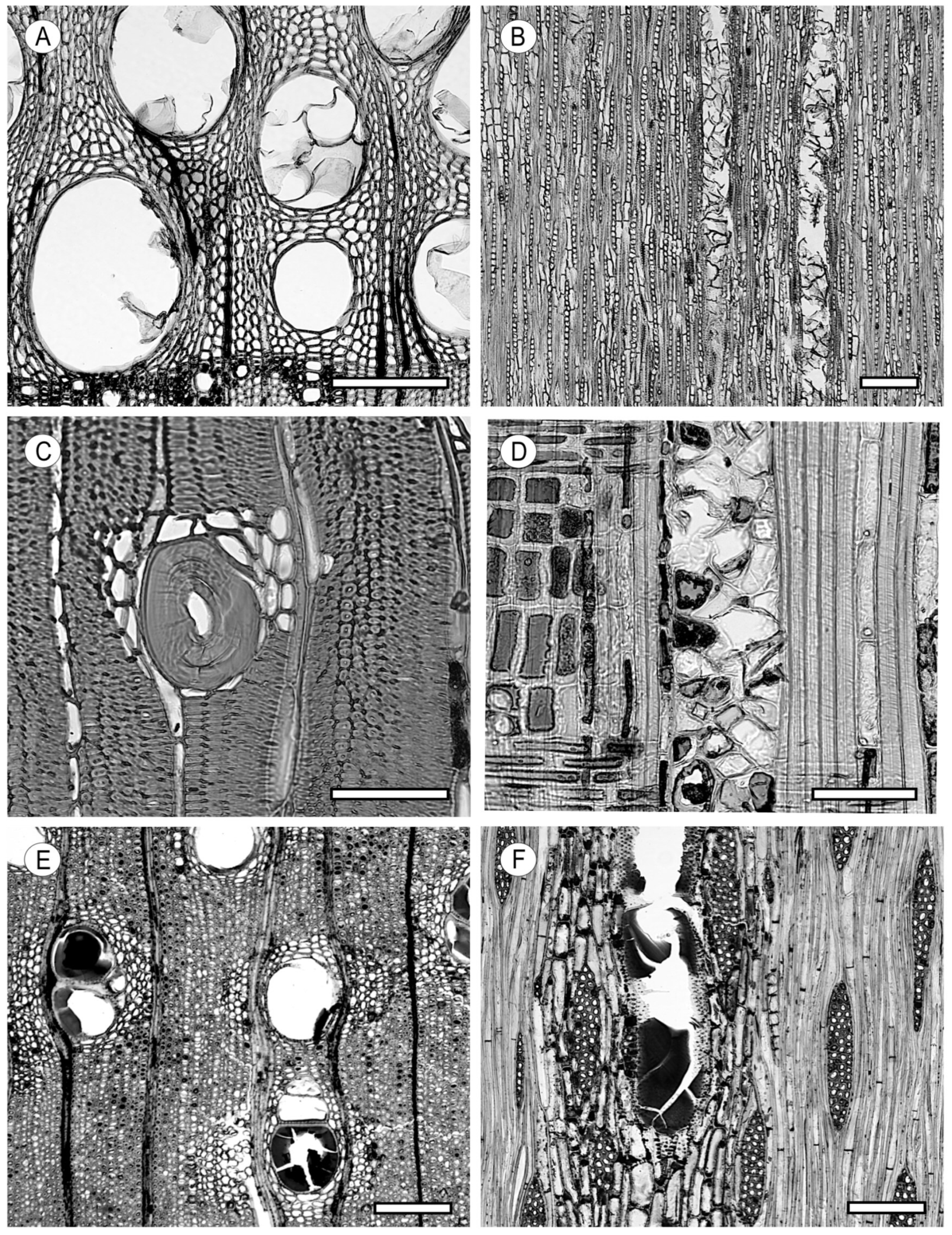
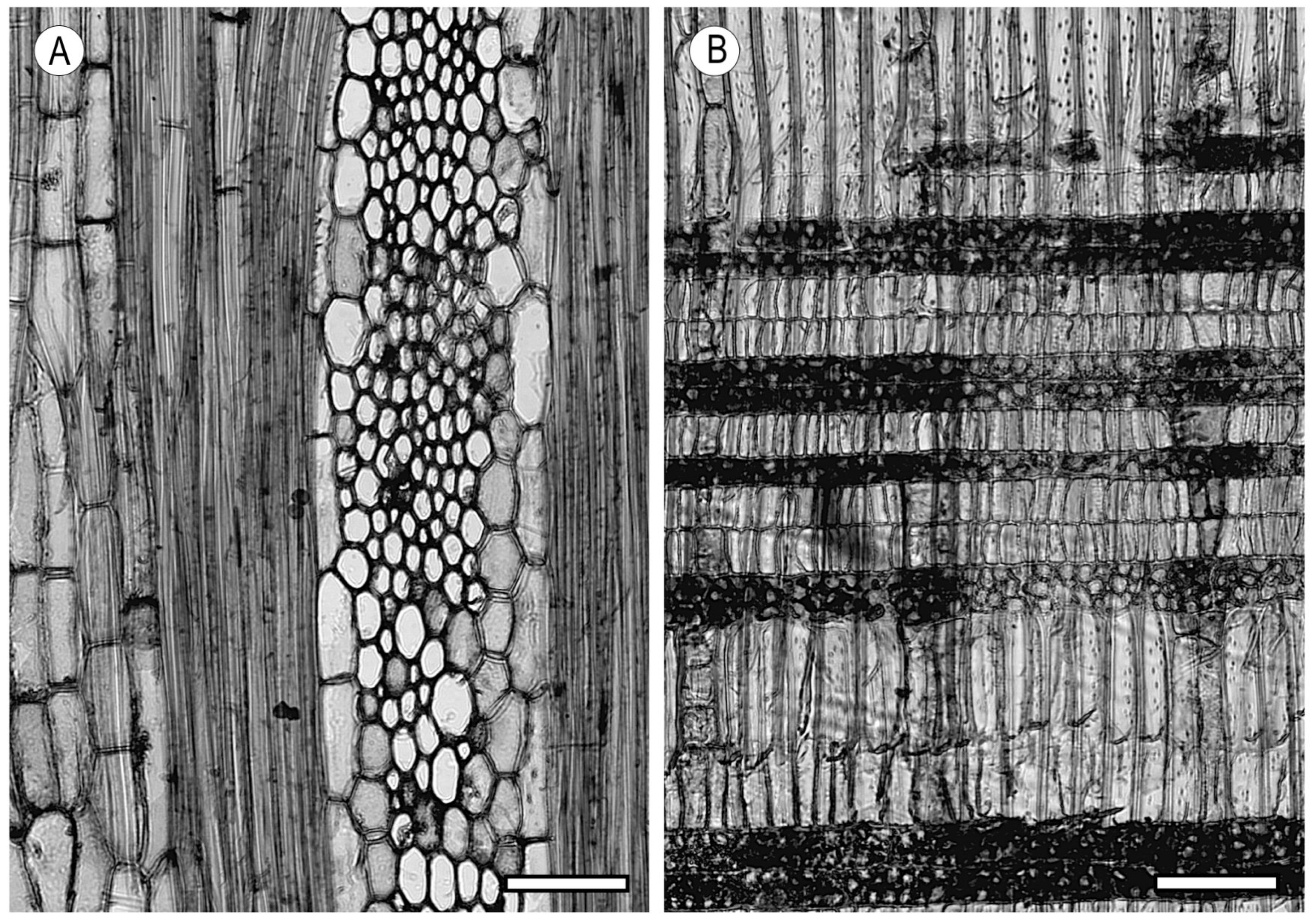
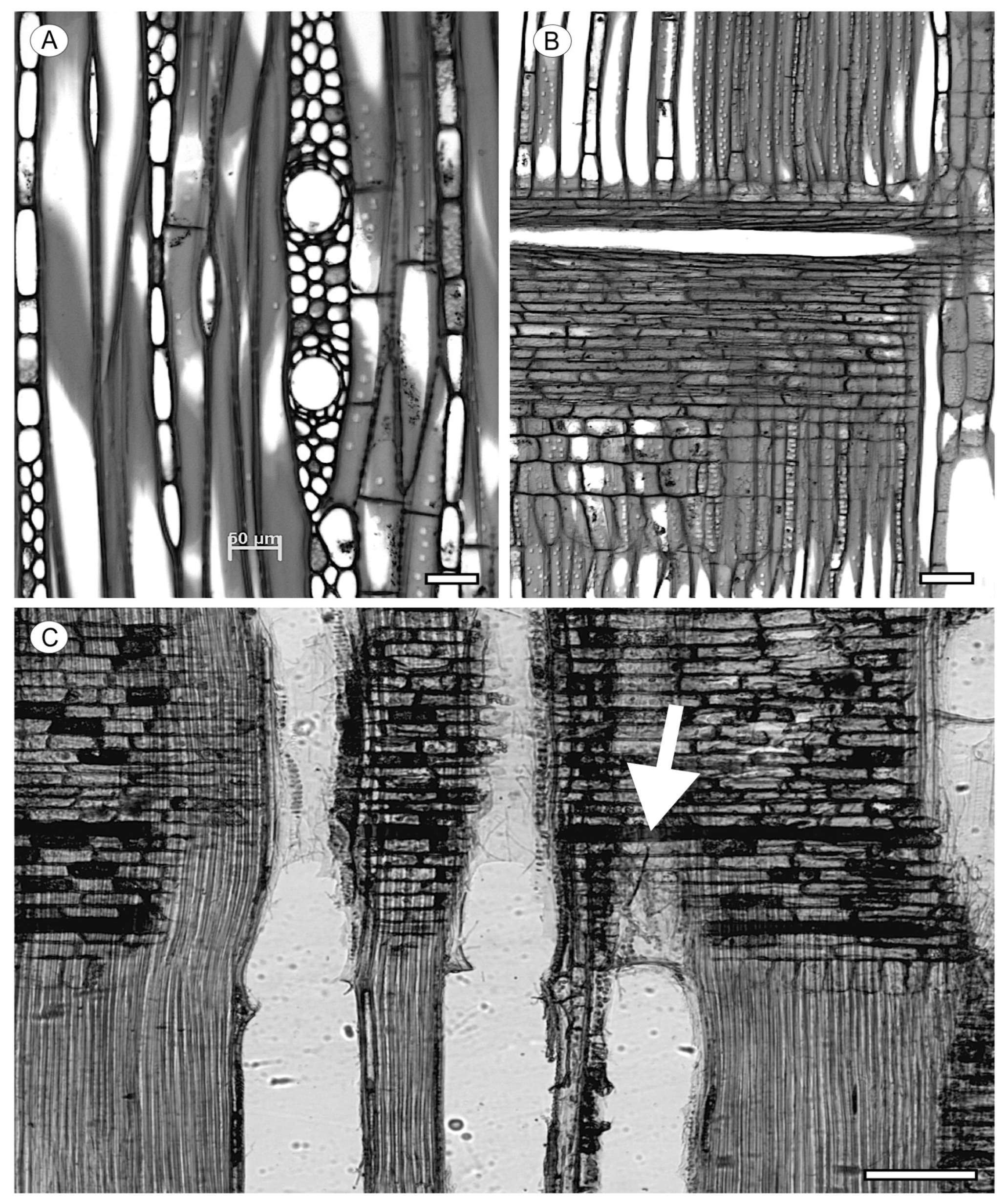
2.3. Tyloses and Deposits
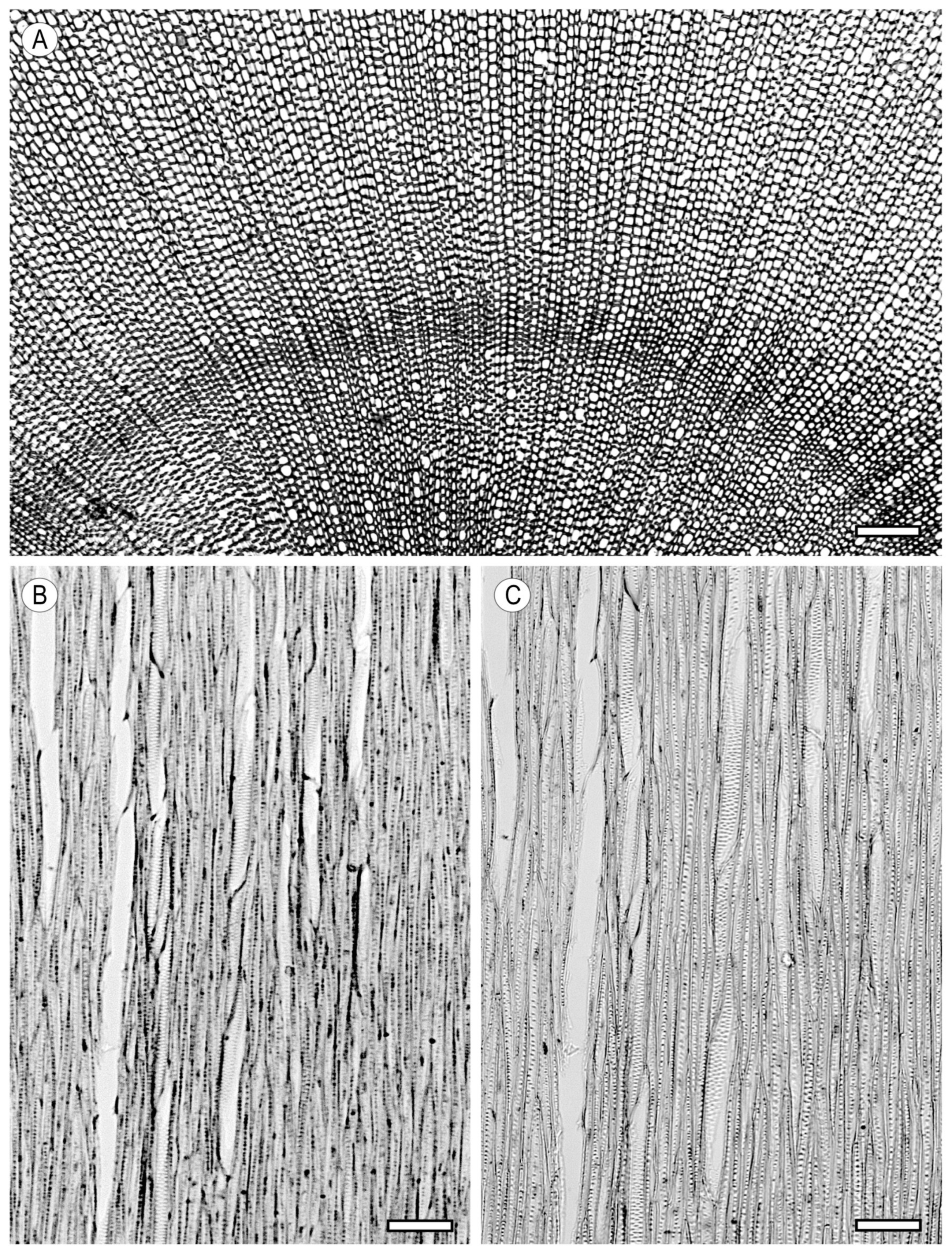
3. Tracheids and Fibres
3.1. Vascular and Vasicentric Tracheids
3.2. Fibrous Tissue
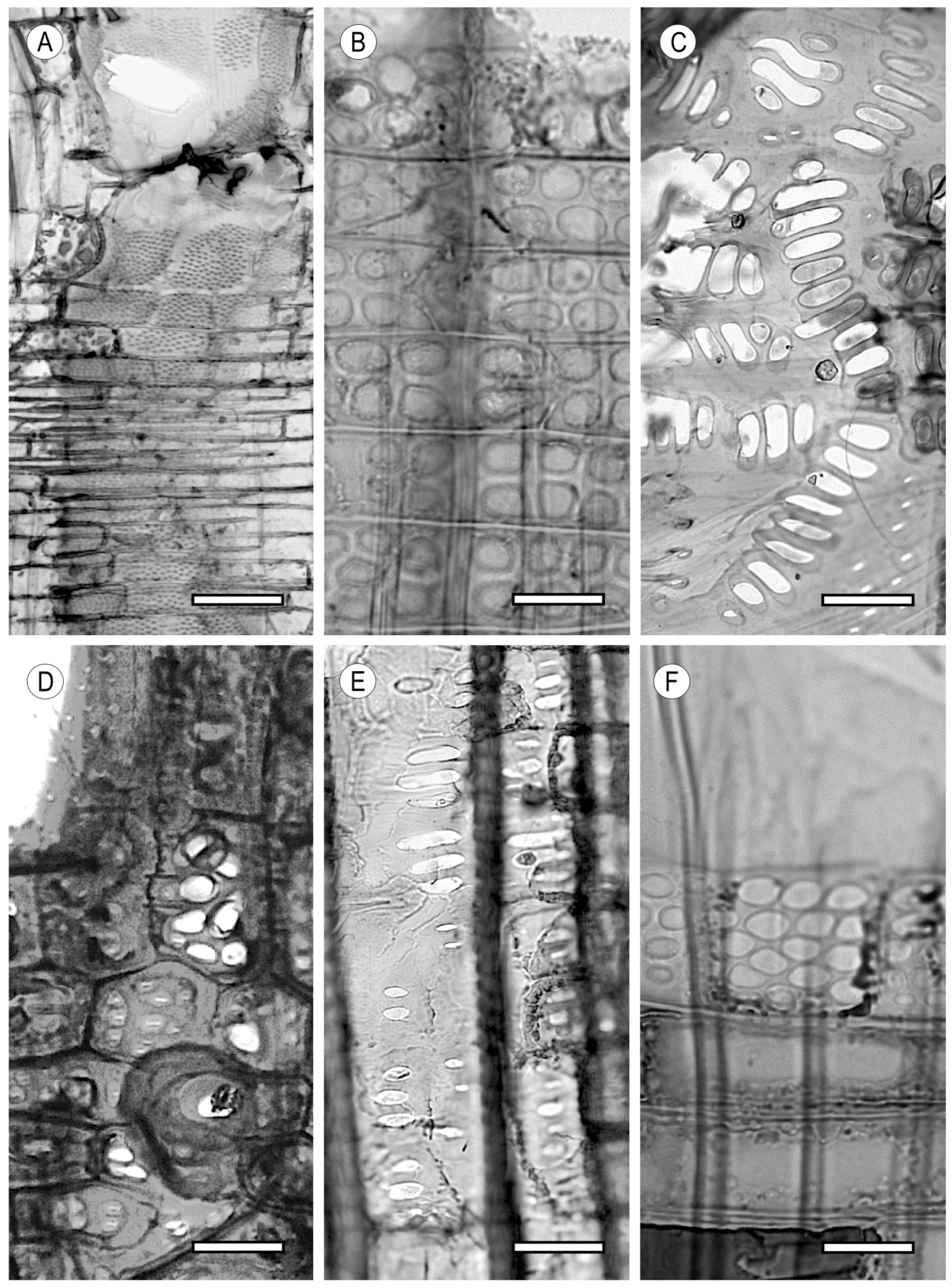
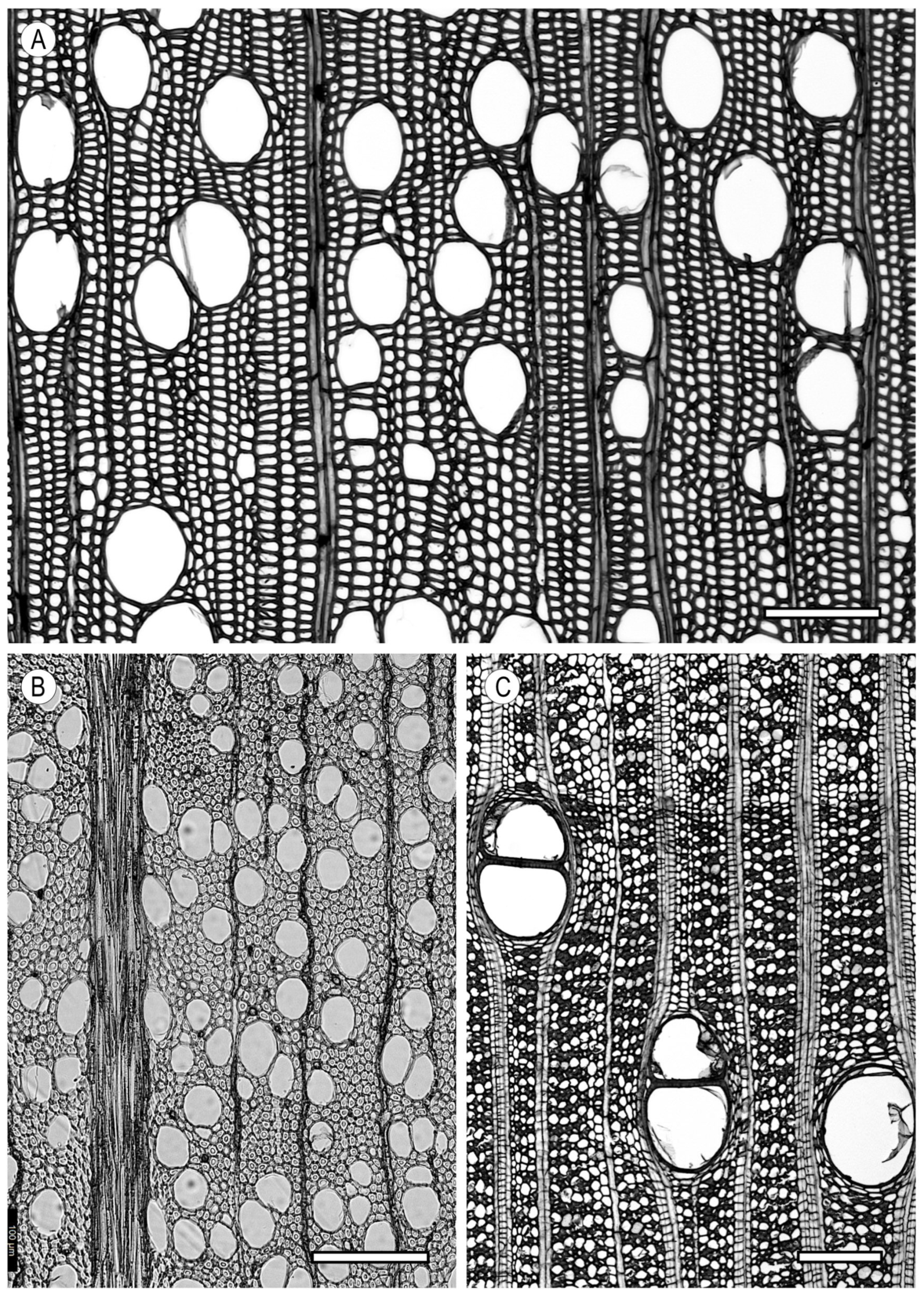
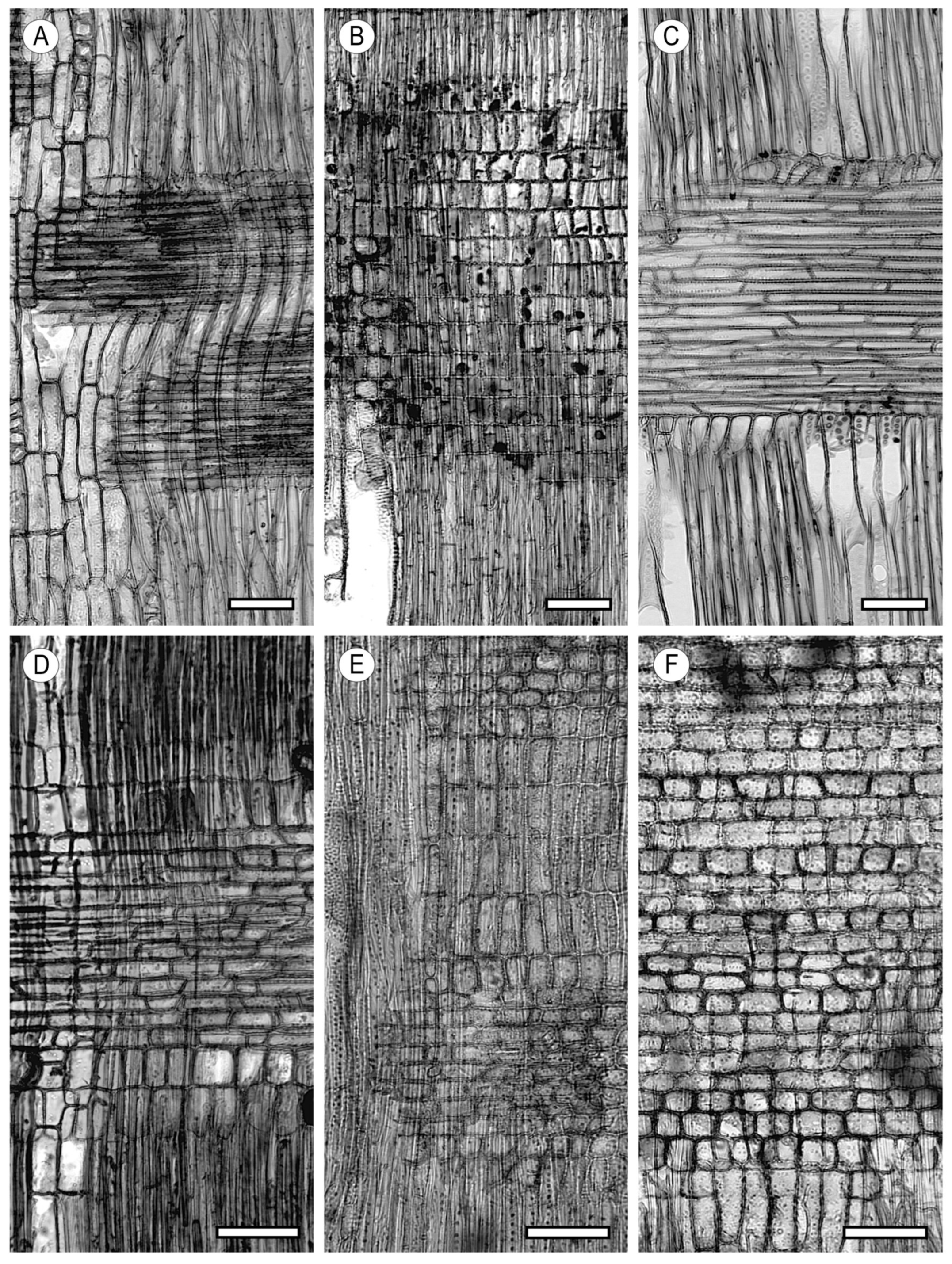
4. Parenchyma
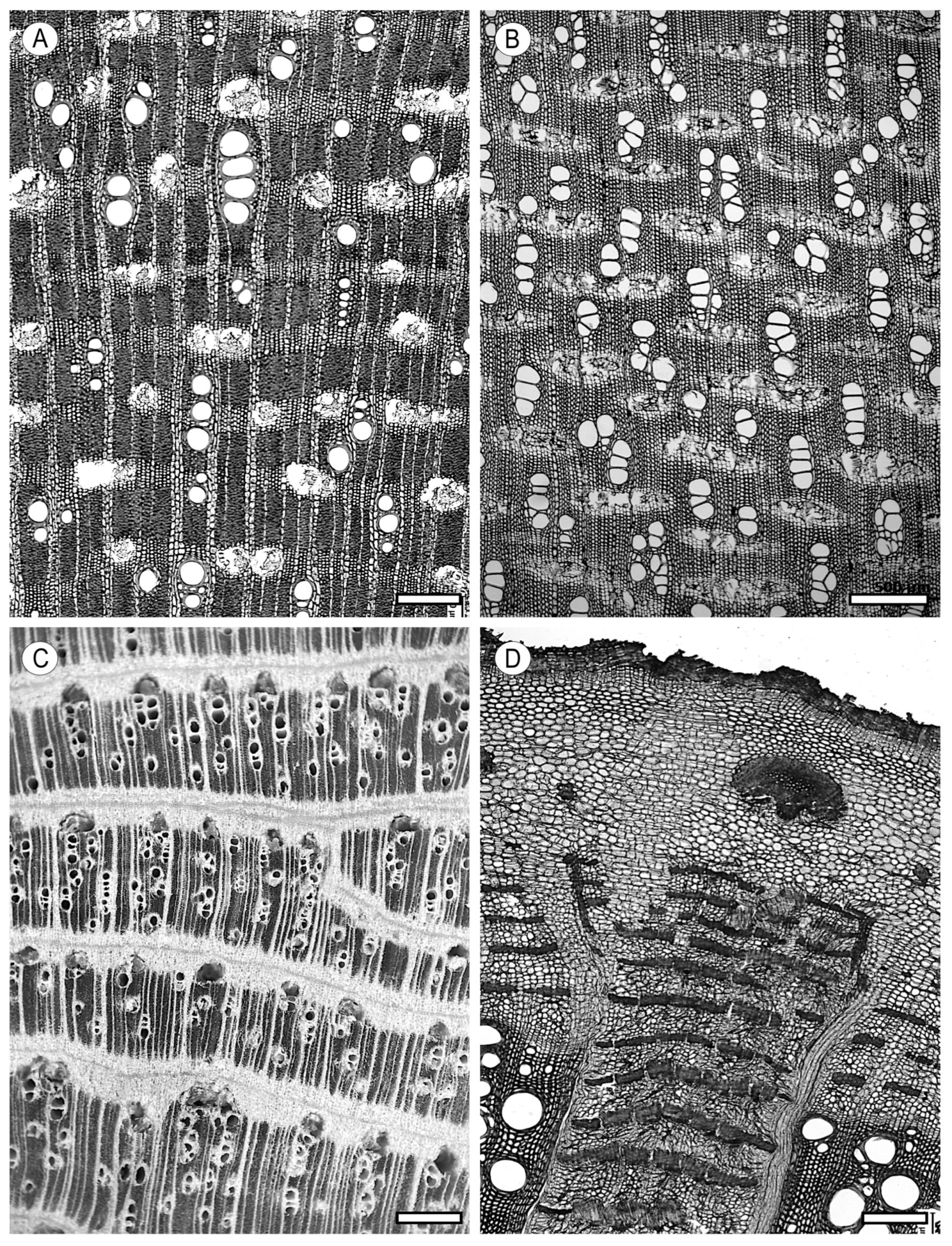
4.1. Axial Parenchyma
4.2. Ray Parenchyma
5. Storied Structures
6. Secretory Elements
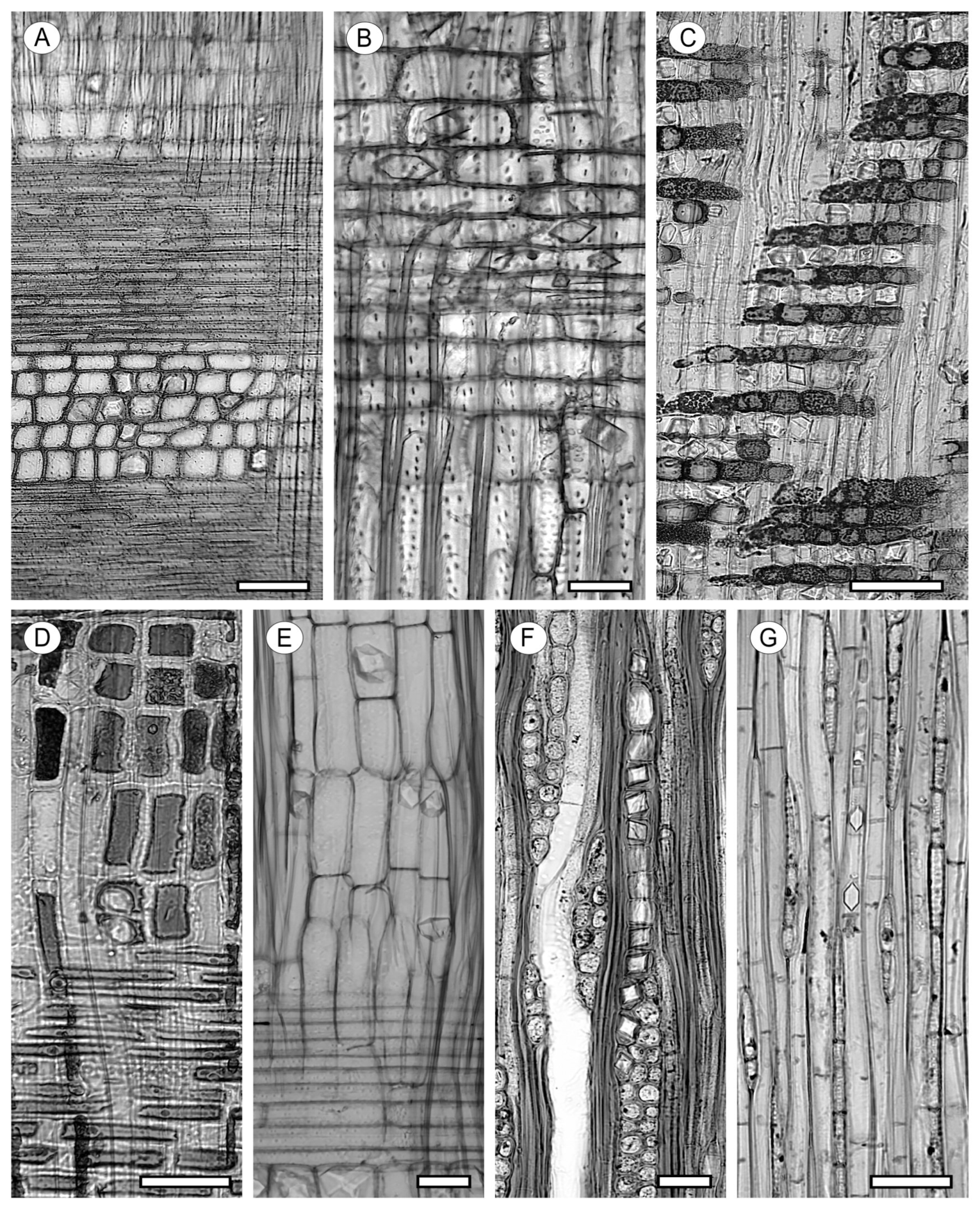
6.1. Oil Cells and Mucilage Cells
6.2. Intercellular Canals
6.3. Laticiferous and Tanniniferous Tubes
7. Included Phloem
8. Crystal Inclusions and Silica
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hudson, P.J.; Razanatsoa, J.; Feild, T.S. Early vessel evolution and the diversification of wood function: Insights from Malagasy Canellales. Am. J. Bot. 2010, 97, 80–93. [Google Scholar] [CrossRef]
- Brenner, G.; Bickoff, I.S. Palynology and age of the Lower Cretaceous basal Kurnub Group from the coastal plain to the northern Negev of Israel. Palynology 1992, 16, 137–185. [Google Scholar] [CrossRef]
- Herendeen, P.S.; Wheeler, E.A.; Baas, P. Angiosperm wood evolution and the potential contribution of paleontological data. Bot. Rev. 1999, 65, 278–300. [Google Scholar] [CrossRef]
- Baas, P.; Wheeler, E.A. Parallelism and reversibility in xylem evolution—A review. IAWA J. 1996, 17, 351–364. [Google Scholar] [CrossRef]
- Wheeler, E.A.; Baas, P. Wood evolution: Baileyan trends and functional traits in the fossil record. IAWA J. 2019, 40, 488–529. [Google Scholar] [CrossRef]
- Philippe, M.; Cuny, G.; Bashforth, A. Ecpagloxylon mathiesenii gen. nov. et sp. nov., a Jurassic wood from Greenland with several primitive angiosperm features. Plant Syst. Evol. 2010, 287, 153–165. [Google Scholar] [CrossRef]
- Baas, P.; Wheeler, E.A. Wood anatomy and climate change. In Climate Change, Ecology and Systematics; Hodkinson, T.R., Jones, M.B., Waldren, S., Parnell, J.A.N., Eds.; Cambridge University Press: Cambridge, UK, 2011; pp. 141–155. [Google Scholar] [CrossRef]
- Bailey, I.W.; Tupper, W.W. Size variation in tracheary cells. I. A comparison between the secondary xylems of vascular cryptograms, gymnosperms and angiosperms. Proc. Am. Art. Sci. Soc. 1918, 54, 149–204. [Google Scholar] [CrossRef]
- Baas, P. Ecological patterns in xylem anatomy. In On the Economy of Plant Form and Function; Givnish, T.J., Ed.; Cambridge University Press: Cambridge, UK, 1986; pp. 327–349. [Google Scholar]
- Carlquist, S. Comparative Wood Anatomy: Systematic, Ecological, and Evolutionary Aspects of Dicotyledon Wood, 2nd ed.; Springer: Heidelberg, Germany, 2001; 448p. [Google Scholar] [CrossRef]
- Jansen, S.; Baas, P.; Gasson, P.; Lens, F.; Smets, E. Variation in xylem structure from tropics to tundra: Evidence from vestured pits. Proc. Natl. Acad. Sci. USA 2004, 101, 8833–8837. [Google Scholar] [CrossRef]
- IAWA Committee. IAWA List of microscopic features for hardwood identification. IAWA Bull. 1989, 10, 219–332. [Google Scholar]
- Brown, H.P.; Panshin, A.J.; Forsaith, C.C. Textbook of Wood Technology. Volume I. Structure, Identification, Defects and Uses of the Commercial Woods of the United States, 1st ed.; McGraw-Hill: New York, NY, USA, 1949; p. 652. [Google Scholar]
- Olson, M.E.; Anfodillo, T.; Gleason, S.M.; McCulloh, K.A. Tip-to-base xylem conduit widening as an adaptation: Causes, consequences, and empirical priorities. New Phytol. 2021, 229, 1877–1893. [Google Scholar] [CrossRef]
- Baas, P.; Beeckman, H.; Cufar, K.; De Micco, V. Functional traits in wood anatomy. IAWA J. 2016, 37, 121–368. [Google Scholar] [CrossRef]
- Olson, M.E.; Pace, M.R. Tributes to Sherwin Carlquist. IAWA J. 2023, 44, 273–556. [Google Scholar] [CrossRef]
- Esteban, L.G.; Guindeo, A.; Peraza, C.; de Palacios, P. La Madera y su Anatomía; Fundación Conde del Valle de Salazar, Ed.; Mundi-Prensa y AiTiM: Madrid, Spain, 2003; 327p. [Google Scholar]
- Evert, R.F. Esau’s Plant Anatomy: Meristems, Cells, and Tissues of the Plant Body: Their Structure, Function, and Development, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; p. 601. [Google Scholar] [CrossRef]
- Wheeler, E.A.; Pearson, R.G.; LaPasha, C.A.; Zack, T.; Hatley, W. Computer-Aided Wood Identification: Reference Manual; North Carolina Agricultural Research Service Bulletin 474; NCRS: Raleigh, NC, USA, 1986. [Google Scholar]
- Esteban, L.G.; Guindeo, A. Anatomía de las maderas de frondosas españolas; AiTiM: Madrid, Spain, 1990; p. 619. [Google Scholar]
- Christman, M.A.; Sperry, J.S. Single vessel flow measurements indicate scalariform perforation plates confer higher flow resistance than previously estimated. Plant Cell Environ. 2010, 33, 431–443. [Google Scholar] [CrossRef]
- Sperry, J.S. Evolution of water transport and xylem structure. Int. J. Plant Sci. 2003, 164, 115–127. [Google Scholar] [CrossRef]
- Jansen, S.; Nardini, A. From systematic to ecological wood anatomy and finally plant hydraulics: Are we making progress in understanding xylem evolution? New Phytol. 2014, 203, 12–15. [Google Scholar] [CrossRef]
- Baas, P. Some functional and adaptive aspects of vessel member morphology. Leiden Bot. Ser. 1976, 3, 157–181. [Google Scholar]
- Feild, T.S.; Brodribb, T.; Holbrook, N.M. Hardly a relict: Freezing and the evolution of vesselless wood in Winteraceae. Evolution 2002, 56, 464–478. [Google Scholar] [CrossRef]
- Zimmermann, M.H.; Jeje, A. Vessel-length distribution in stems of some American woody plants. Can. J. Bot. 1981, 59, 1882–1892. [Google Scholar] [CrossRef]
- Ewers, F.W.; Fisher, J.B.; Chiu, S.T. A survey of vessel dimensions in stems of tropical lianas and other growth forms. Oecologia 1990, 8, 544–552. [Google Scholar] [CrossRef]
- Sperry, J.; Hacke, G.; Feild, T.S.; Sano, Y.; Sikkema, E.H. Hydraulic consequences of vessel evolution in angiosperms. Int. J. Plant Sci. 2007, 168, 1127–1139. [Google Scholar] [CrossRef]
- Greenidge, K.N.H. An approach to the study of vessel length in hardwood species. Am. J. Bot. 1952, 39, 570–574. [Google Scholar] [CrossRef]
- Jansen, S.; Choat, B.; Pletsers, A. Morphological variation of intervessel pit membranes and implications to xylem function in angiosperms. Am. J. Bot. 2009, 96, 409–419. [Google Scholar] [CrossRef]
- Li, S.; Lens, F.; Espino, S.; Karimi, Z.; Klepsch, M.; Schenk, H.J.; Schmitt, M.; Schuldt, B.; Jansen, S. Intervessel pit membrane thickness as a key determinant of embolism resistance in angiosperm xylem. IAWA J. 2016, 37, 152–171. [Google Scholar] [CrossRef]
- Wheeler, J.K.; Sperry, J.S.; Hacke, U.G.; Hoang, N. Intervessel pitting and cavitation in woody Rosaceae and other vesselled plants: A basis for a safety versus efficiency trade-off in xylem transport. Plant Cell Environ. 2005, 28, 800–812. [Google Scholar] [CrossRef]
- Choat, B.; Brodie, T.W.; Cobb, A.; Zwieniecki, M.A.; Holbrook, N.M. Direct measurements of intervessel pit membrane hydraulic resistance in two angiosperm tree species. Am. J. Bot. 2006, 93, 993–1000. [Google Scholar] [CrossRef]
- Hacke, U.G.; Sperry, J.S.; Wheeler, J.K.; Castro, L. Scaling of angiosperm xylem structure with safety and efficiency. Tree Physiol. 2006, 26, 619–701. [Google Scholar] [CrossRef]
- Bailey, L.W. Preliminary notes on cribriform and vestured pits. Trop. Woods 1932, 3, 46–48. [Google Scholar]
- Bailey, L.W. The cambium and its derivative tissues: No. VIII. Structure, distribution, and diagnostic significance of vestured pits in dicotyledons. J. Arnold Arbor. 1933, 14, 259–273. [Google Scholar] [CrossRef]
- Jansen, S.; Smets, E.; Baas, P. Vestures in woody plants: A review. IAWA J. 1998, 19, 347–382. [Google Scholar] [CrossRef]
- Parham, R.A.; Baird, W.M. Warts in the evolution of angiosperm wood. Wood Sci. Technol. 1974, 8, 1–10. [Google Scholar] [CrossRef]
- Carlquist, S. Wood anatomy of Onagraceae: Further species; root anatomy; significance of vestured pits and allied structures in dicotyledons. Ann. Mo. Bot. Gard. 1982, 69, 755–769. [Google Scholar] [CrossRef]
- Carlquist, S. Comparative Wood Anatomy. Systematic, Ecological, and Evolutionary Aspects of Dicotyledon Wood; Springer: Berlin/Alemania, Germany, 1988; 436p. [Google Scholar]
- Zweypfenning, R.C.V.J. A hypothesis on the function of vestured pits. IAWA Bull. 1978, 1, 13–15. [Google Scholar]
- Jansen, S.; Baas, P.; Gasson, P.; Smets, E. Vestured. Pits: Do they promote safer water transport? Int. J. Plant Sci. 2003, 164, 405–413. [Google Scholar] [CrossRef]
- Zimmerman, M.H. Xylem Structure and the Ascent of Sap; Springer: Heidelberg, Germany, 1983; p. 146. [Google Scholar] [CrossRef]
- Costa, A.; Wiedenhoeft, A.C. On the possible functions of helical thickenings in conductive cells of wood. IAWA J. 2023, 44, 381–398. [Google Scholar] [CrossRef]
- Ohtani, J.; Meylan, B.A.; Butterfield, B.G. A note on vestures on helical thickenings. IAWA Bull. 1984, 5, 9–11. [Google Scholar] [CrossRef]
- Karam, G.N. Biomechanical Model of the Xylem Vessels in Vascular Plants. Ann. Bot. 2005, 95, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Woodworth, R.H. Fibriform vessel members in the Passifloraceae. Trop. Woods 1935, 41, 8–16. [Google Scholar]
- Bissing, D.R. Variation in qualitative anatomical features of the xylem of selected dicotyledonous woods in relation to water availability. Bull. Torrey Bot. Club. 1982, 109, 371–384. [Google Scholar] [CrossRef]
- Wheeler, E.A.; Gasson, P.E.; Baas, P. Using the InsideWood web site: Potentials and pitfalls. IAWA J. 2020, 41, 412–462. [Google Scholar] [CrossRef]
- De Micco, V.; Balzano, A.; Wheeler, E.; Baas, P. Tyloses and gums: A review of structure, function and occurrence of vessel occlusions. IAWA J. 2016, 37, 186–205. [Google Scholar] [CrossRef]
- Murmanis, L. Formation of tyloses in felled Quercus rubra L. Wood Sci. Technol. 1975, 9, 3–14. [Google Scholar] [CrossRef]
- Sun, Q.; Ros, T.L.; Reid, M.S.; Matthews, M.A. Ethylene and not embolism is required for wound-induced tylose development in stems of grapevines (Vitis vinifera L.). Plant Physiol. 2007, 145, 1629–1636. [Google Scholar] [CrossRef] [PubMed]
- Malpighi, M. Opera omnia. In Opere Scelte di Marcello Malpighi; Belloni, L., Ed.; Unione Tipografico-Editrice Torinese: Torino, Italy, 1967; pp. 75–99. [Google Scholar]
- Scheckler, S.E.; Galtier, J. Tyloses and ecophysiology of the early carboniferous progymnosperm tree Protopitys buchiana. Ann. Bot. 2003, 91, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Chattaway, M.M. The development of tyloses and secretion of gum in heartwood formation. Aust. J. Biol. Sci. 1949, 2, 227–240. [Google Scholar] [CrossRef]
- Esau, K. Plant Anatomy, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1965; p. 767. [Google Scholar]
- Pearce, R.B.; Holloway, P.J. Suberin in the sapwood of oak (Quercus robur L.): Its composition from a compartmentalization barrier and its occurrence in tyloses in undecayed wood. Physiol. Plant Pathol. 1984, 24, 71–81. [Google Scholar] [CrossRef]
- Hillis, W.E. Heartwood and Tree Exudates; Springer: Heidelberg, Germany, 1987; p. 268. [Google Scholar] [CrossRef]
- Olson, M.E. Imperforate tracheary element classification for studies of xylem structure-function relations. IAWA J. 2023, 44, 439–464. [Google Scholar] [CrossRef]
- Carlquist, S. Vasicentric tracheids as a drought survival mechanism in the woody flora of southern California and similar regions: Review of vasicentric tracheids. Aliso 1985, 11, 37–68. [Google Scholar] [CrossRef]
- Carlquist, S. How wood evolves: A new synthesis. Botany 2012, 90, 901–940. [Google Scholar] [CrossRef]
- Sano, Y.; Morris, H.; Shimada, H.; Ronse de Craene, L.P.; Jansen, S. Anatomical features associated with water transport in imperforate tracheary elements of vessel-bearing angiosperms. Ann. Bot. 2011, 107, 953–964. [Google Scholar] [CrossRef]
- Scholz, F.G.; Bucci, S.J.; Goldstein, G.; Meinzer, F.C.; Franco, A.C.; Miralles-Wilhelm, F. Biophysical properties and functional significance of stem water storage tissues in Neotropical savanna trees. Plant. Cell. Environ. 2007, 30, 236–248. [Google Scholar] [CrossRef]
- Pfautsch, S.; Renard, J.; Tjoelker, M.G.; Salih, A. Phloem as capacitor: Radial transfer of water into xylem of tree stems occurs via symplastic transport in ray parenchyma. Plant Physiol. 2015, 167, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Loepfe, L.; Martínez-Vilalta, J.; Piñol, J.; Mencuccini, M. The relevance of xylem network structure for plant hydraulic efficiency and safety. J. Theor. Biol. 2007, 247, 788–803. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Vilalta, J.; Mencuccini, M.; Álvarez, X.; Camacho, J.; Loepfe, L.; Piñol, J. Spatial distribution and packing of xylem conduits. Am. J. Bot. 2012, 99, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Pratt, R.B.; Castro, V.; Jacobsen, A.L. The functional significance of tracheids co-occurring with vessels in xylem of Eudicots suggests a role in embolism tolerance. IAWA J. 2023, 44, 477–494. [Google Scholar] [CrossRef]
- Barotto, A.J.; Fernandez, M.E.; Gyenge, J.; Meyra, A.; Martinez-Meier, A.; Monteoliva, A. First insights into the functional role of vasicentric tracheids and parenchyma in eucalyptus species with solitary vessels: Do they contribute to xylem efficiency or safety? Tree Physiol. 2016, 36, 1485–1497. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, E.A.; Baas, P.; Rodgers, S. Variations in dicot anatomy: A global analysis based on the Inside Wood database. IAWA J. 2007, 28, 229–258. [Google Scholar] [CrossRef]
- Harrar, E.S. Notes on starch grains in septate fiber-tracheids. Trop. Woods 1946, 85, 1–9. [Google Scholar]
- Spackman, W.; Swamy, B.G.L. The nature and occurrence of septate fibers in dicotyledons. Am. J. Bot. 1949, 36, 804. [Google Scholar]
- Plavcova, L.; Olson, M.E.; Jandova, V.; Dolezal, J. Parenchyma is not the sole site of storage: Storage in living fibres. IAWA J. 2023, 44, 465–476. [Google Scholar] [CrossRef]
- Morris, H.R. The Structure and Function of Ray and Axial Parenchyma in Woody Seed Plants. Ph.D. Thesis, Universität Ulm, Ulm, Germany, 20 July 2016. [Google Scholar] [CrossRef]
- Frison, E. De Ia presence d’amidon dans le Iumen des fibres du bois. Bull. Agric. Congo Belg. 1948, 39, 869–874. [Google Scholar]
- Chattaway, M.M. Proposed standards for numerical values used in describing woods. Trop. Woods 1932, 29, 20–28. [Google Scholar]
- Carlquist, S. Further concepts in ecological wood anatomy, with comments on recent work in wood anatomy and evolution. Aliso 1980, 9, 499–553. [Google Scholar] [CrossRef]
- Carlquist, S.; Hoekman, D.A. Ecological Wood Anatomy of the Woody Southern Californian Flora. IAWA J. 1985, 6, 319–347. [Google Scholar] [CrossRef]
- Plomion, C.; Leprovost, G.; Stokes, A. Wood Formation in Trees. Plant Physiol. 2001, 127, 1513–1523. [Google Scholar] [CrossRef] [PubMed]
- Plavcová, L.; Jansen, S. The Role of Xylem Parenchyma in the Storage and Utilization of Nonstructural Carbohydrates. In Functional and Ecological Xylem Anatomy; Hacke, U., Ed.; Springer: Cham, Switzerland, 2015; pp. 209–234. [Google Scholar] [CrossRef]
- Morris, H.; Jansen, S.; Arber, A. Opinion paper—Secondary xylem parenchyma—from classical terminology to functional traits. IAWA J. 2016, 37, 1–15. [Google Scholar] [CrossRef]
- Magel, E.; Einig, W.; Hampp, R. Carbohydrates in trees. Dev. Crop Sci. 2000, 26, 317–336. [Google Scholar] [CrossRef]
- Eriksson, K.E.L.; Blanchette, R.A.; Ander, P. Microbial and Enzymatic Degradation of Wood and Wood Components; Springer: Heidelberg, Germany, 1990; p. 407. [Google Scholar] [CrossRef]
- Lundquist, R.; Brunow, G. Lignins: Natural products from oxidative coupling of 4 hydroxyphenylpropanoids. Phytochem. Rev. 2004, 3, 29–60. [Google Scholar] [CrossRef]
- Ros Barceló, A. Lignification in plant cell walls. Int. Rev. Cytol. 1997, 176, 87–132. [Google Scholar] [CrossRef] [PubMed]
- Moura, J.C.; Bonine, C.A.; de Oliveira Fernandes Viana, J.; Dornelas, M.C.; Mazzafera, P. Abiotic and biotic stresses and changes in the lignin content and composition in plants. J. Integr. Plant Biol. 2010, 52, 360–376. [Google Scholar] [CrossRef]
- Spicer, R.; Holbrook, N.M. Parenchyma cell respiration and survival in secondary xylem: Does metabolic activity decline with cell age? Plant Cell Environ. 2007, 30, 934–943. [Google Scholar] [CrossRef]
- Schweingruber, F.; Hellmann, L.; Tegel, W.; Braun, S.; Nievergelt, D.; Buntgen, U. Evaluating the wood anatomical and dendroecological potential of Arctic dwarf shrub communities. IAWA J. 2013, 34, 485–497. [Google Scholar] [CrossRef]
- Esteban, L.G.; de Palacios, P.; Heinz, I.; Gasson, P.; García-Iruela, A.; García-Fernández, F. Softwood Anatomy: A Review. Forests 2023, 14, 323. [Google Scholar] [CrossRef]
- Morris, H.; Brodersen, C.; Schwarze, F.W.M.R.; Jansen, S. The parenchyma of secondary xylem and its critical role in tree defense against fungal decay in relation to the CODIT model. Front. Plant Sci. 2016, 7, 1665. [Google Scholar] [CrossRef]
- Kramer, P.J.; Kozlowski, T.T. Physiology of Woody Plants, 2nd ed.; Academic Press: New York, NY, USA, 1979; p. 811. [Google Scholar]
- Hartmann, H.; Trumbore, S. Understanding the roles of nonstructural carbohydrates in forest trees—From what we can measure to what we want to know. New Phytol. 2016, 211, 386–403. [Google Scholar] [CrossRef]
- Tomasella, M.; Petrussa, E.; Petruzzellis, F.; Nardini, A.; Casolo, V. The Possible Role of Non-Structural Carbohydrates in the Regulation of Tree Hydraulics. Int J. Mol. Sci. 2020, 21, 144. [Google Scholar] [CrossRef] [PubMed]
- Piper, F.I.; Paula, S. The Role of Nonstructural Carbohydrates Storage in Forest Resilience under Climate Change. Curr. For. Rep. 2020, 6, 1–13. [Google Scholar] [CrossRef]
- Spicer, R. Symplastic networks in secondary vascular tissues: Parenchyma distribution and activity supporting long-distance transport. J. Exp. Bot. 2014, 65, 1829–1848. [Google Scholar] [CrossRef] [PubMed]
- Morris, H.; Plavcova, L.; Cvecko, P.; Fichtler, E.; Gillingham, M.A.F.; Martinez-Cabrera, H.I.; McGlinn, D.J.; Wheeler, E.; Zheng, J.; Zieminska, K.; et al. A global analysis of parenchyma tissue fractions in secondary xylem of seed plants. New Phytol. 2016, 209, 1553–1565. [Google Scholar] [CrossRef] [PubMed]
- Fahn, A.; Leshem, B. Fibras de madera con protoplastos vivos. New Phytol. 1963, 62, 91–98. [Google Scholar] [CrossRef]
- Panshin, A.J.; de Zeeuw, C. Textbook of Wood Technology, 4th ed.; McGraw-Hill: New York, NY, USA, 1980; p. 722. [Google Scholar]
- Chafe, S.C.; Chauret, G. Cell wall structure in the xylem parenchyma of trembling aspen. Protoplasma 1974, 80, 129–147. [Google Scholar] [CrossRef]
- Carlquist, S. Anatomy of Vine and Liana Stems: A Review and Synthesis. In The Biology of Vines; Putz, F.E., Mooney, H.A., Eds.; Cambridge University Press: Cambridge, UK, 1992; pp. 53–72. [Google Scholar] [CrossRef]
- Cocoletzi, E.; Angeles, G.; Sosa, V.; Patrón, A. The chloroplasts and unlignified parenchyma of two tropical pioneer forest tree species (Urticaceae). Bot. Sci. 2013, 91, 251–260. [Google Scholar] [CrossRef]
- Alvarado, M.; Terrazas, T. Ontogenesis and variation of wood ‘parenchymatization’ in Cochlospermum vitifolium (Bixaceae). IAWA J. 2024; published online ahead of print. [Google Scholar] [CrossRef]
- Smith, A.M. The cohesive strength of water and xylem transport. Ann. Bot. 1994, 74, 64–651. [Google Scholar] [CrossRef]
- Zimmermann, U.; Meinzer, F.; Benkert, R.; Zhu, J.J.; Schneider, H.; Goldstein, G.; Kuchenbrod, E.; Haase, A. Xylem water transport: Is the available evidence consistent with the cohesion theory? Plant Cell Environ. 1994, 17, 1169–1181. [Google Scholar] [CrossRef]
- Zwieniecki, M.A.; Melcher, P.J.; Holbrook, N.M. Hydrogel control of xylem hydraulic resistance in plants. Science 2001, 291, 1059–1062. [Google Scholar] [CrossRef] [PubMed]
- Salleo, S.; Trifilo, P.; Esposito, S.; Nardini, A.; Lo Gullo, M.A. Starch-to-sugar conversion in wood parenchyma of field-growing Laurus nobilis plants: A component of the signal pathway for embolism repair. Funct. Plant Biol. 2009, 36, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Jansen, S.; Gortan, E.; Lens, F.; Lo Gullo, M.A.; Salleo, S.; Scholz, A.; Stein, A.; Trifilò, P.; Nardini, A. Do quantitative vessel and pit characters account for ion-mediated changes in the hydraulic conductance of angiosperm xylem? New Phytol. 2011, 189, 218–228. [Google Scholar] [CrossRef]
- Brodersen, C.R.; McElrone, A.J. Maintenance of xylem network transport capacity: A review of embolism repair in vascular plants. Front. Plant Sci. 2013, 4, 1–11. [Google Scholar] [CrossRef]
- Trifilò, P.; Barbera, P.M.; Raimondo, F.; Nardini, A.; Lo Gullo, M.A. Coping with drought induced xylem cavitation: Coordination of embolism repair and ionic effects in three Mediterranean evergreens. Tree Physiol. 2014, 34, 109–122. [Google Scholar] [CrossRef]
- Salleo, S.; Trifilò, P.; Lo Gullo, M.A. Vessel wall vibrations: Trigger for embolism repair? Funct. Plant Biol. 2008, 35, 289–297. [Google Scholar] [CrossRef]
- Secchi, F.; Zwieniecki, M.A. Sensing embolism in xylem vessels: The role of sucrose as a trigger for refilling. Plant Cell Environ. 2011, 34, 564–579. [Google Scholar] [CrossRef]
- Secchi, F.; Zwieniecki, M.A. Analysis of xylem sap from functional (non-embolized) and non-functional (embolized) vessels of Populus nigra: Chemistry of refilling. Plant Physiol. 2012, 160, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Klein, G. Zur Ätiologie der Thyllen. Z. Botanik 1923, 15, 418–439. [Google Scholar]
- Zimmermann, M.H. Hydraulic architecture of some diffuse-porous trees. Can. J. Bot. 1978, 56, 2286–2295. [Google Scholar] [CrossRef]
- Brodersen, C.R.; McElrone, A.J.; Choat, B.; Matthews, M.A.; Shackel, K.A. The dynamics of embolism repair in xylem: In vivo visualizations using high-resolution computed tomography. Plant Physiol. 2010, 154, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Pratt, R.B.; Jacobsen, A.L.; Ewers, F.W.; Davis, S.D. Relationships among xylem transport, biomechanics and storage in stems and roots of nine Rhamnaceae species of the California chaparral. New Phytol. 2007, 174, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Martínez-Cabrera, H.I. Wood anatomical correlates with theoretical conductivity and wood density across China: Evolutionary evidence of the functional differentiation of axial and radial parenchyma. Ann. Bot. 2013, 112, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Gregory, R.A. Cambial activity and ray cell abundance in Acer saccharum. Can. J. Bot. 1977, 55, 2559–2564. [Google Scholar] [CrossRef]
- Van Bel, A.J. Xylem-phloem exchange via the rays: The undervalued route of transport. J. Exp. Bot. 1990, 41, 631–644. [Google Scholar] [CrossRef]
- Carlquist, S. Wood anatomy of Onagraceae, with notes on alternative modes of photosynthate movement in dicotyledon woods. Ann. Mo. Bot. Gard. 1975, 62, 386–424. [Google Scholar] [CrossRef]
- Barnett, J.; Cooper, P.; Bonner, L.J. The protective layer as an extension of the apoplast. IAWA J. 1993, 14, 163–171. [Google Scholar] [CrossRef]
- Van Bel, A.J.; Van der Schoot, C. Primary function of the protective layer in contact cells. Buffer against oscillations in hydrostatic pressure in the vessels. IAWA Bull. 1988, 9, 285–288. [Google Scholar] [CrossRef]
- Carlquist, S. Types of cambial activity and wood anatomy of Stylidium (Stylidiaceae). Am. J. Bot. 1981, 68, 778–785. [Google Scholar] [CrossRef]
- Kribs, D.A. Commercial Foreign Woods on the American Market; Dover Publications: New York, NY, USA, 1968; p. 241. [Google Scholar]
- Gregory, M.; Baas, P. A survey of mucilage cells in vegetative organs of the dicotyledons. Isr. J. Bot. 1989, 38, 125–174. [Google Scholar] [CrossRef]
- de Bary, A. Comparative Anatomy of the Vegetative Organs of the Phanerogams and Ferns; Clarendon Press: Oxford, UK, 1884. [Google Scholar]
- Nissen, S.J.; Foley, M.E. No latex starch utilization in Euphorbia esula L. Plant Physiol. 1986, 81, 696–698. [Google Scholar] [CrossRef] [PubMed]
- Spilatro, S.R.; Mahlberg, P.G. Characterization of Starch Grains in the Nonarticulated Laticifer of Euphorbia pulcherrima (Poinsettia). Am. J. Bot. 1990, 77, 153––158. [Google Scholar] [CrossRef]
- Paterson-Jones, J.C.; Gilliland, M.G.; Van Staden, J. The biosynthesis of natural rubber. J. Plant Physiol. 1990, 136, 257–263. [Google Scholar] [CrossRef]
- Raven, P.H.; Evert, R.F.; Eichhorn, S.E. Biology of Plants, 7th ed.; Freeman: New York, NY, USA, 2005; p. 686. [Google Scholar]
- Homer, H.T.; Tiffany, L.H.; Cody, A.M.; Knaphu, G. Development of fungal calcium oxalate crystals associated with the basidiocarps of Geastrum minus (Lycoperdales). Scanning Electron Microscroscopy 1985, 2, 789–801. [Google Scholar]
- Finley, D.S. Patterns of calcium oxalate crystals in Young tropical leaves: A possible role as an anti-herbivory defense. Rev. Biol. Trop. 1999, 47, 27–31. [Google Scholar] [CrossRef]
- Molano-Flores, B. Herbivory and calcium concentrations affect calcium oxalate crystal formation in leaves of Sida (Malvaceae). Ann. Bot. 2001, 88, 387–391. [Google Scholar] [CrossRef]
- Innocenti, A.; del Monaco, S. Patologia dovuta a polveri di legno. In Contributi Scientifico Pratici per una Migliore Conoscenza del Legno CNR Instituto del Legno Firenze; Consiglio Nazionale delle Ricerche: Rome, Italy, 1980; Volume 27, pp. 1–64. [Google Scholar]
- Mcnaughton, S.J.; Tarrants, J.L. Grass leaf silicification: Natural selection for an inducible defense against herbivores. Proc. Natl. Acad. Sci. USA 1983, 80, 790–791. [Google Scholar] [CrossRef]













Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esteban, L.G.; de Palacios, P.; Gasson, P.; García-Iruela, A.; García-Fernández, F.; García-Esteban, L. Hardwoods: Anatomy and Functionality of Their Elements—A Short Review. Forests 2024, 15, 1162. https://doi.org/10.3390/f15071162
Esteban LG, de Palacios P, Gasson P, García-Iruela A, García-Fernández F, García-Esteban L. Hardwoods: Anatomy and Functionality of Their Elements—A Short Review. Forests. 2024; 15(7):1162. https://doi.org/10.3390/f15071162
Chicago/Turabian StyleEsteban, Luis G., Paloma de Palacios, Peter Gasson, Alberto García-Iruela, Francisco García-Fernández, and Lydia García-Esteban. 2024. "Hardwoods: Anatomy and Functionality of Their Elements—A Short Review" Forests 15, no. 7: 1162. https://doi.org/10.3390/f15071162
APA StyleEsteban, L. G., de Palacios, P., Gasson, P., García-Iruela, A., García-Fernández, F., & García-Esteban, L. (2024). Hardwoods: Anatomy and Functionality of Their Elements—A Short Review. Forests, 15(7), 1162. https://doi.org/10.3390/f15071162







