An Introduction to 3D Bioprinting: Possibilities, Challenges and Future Aspects
Abstract
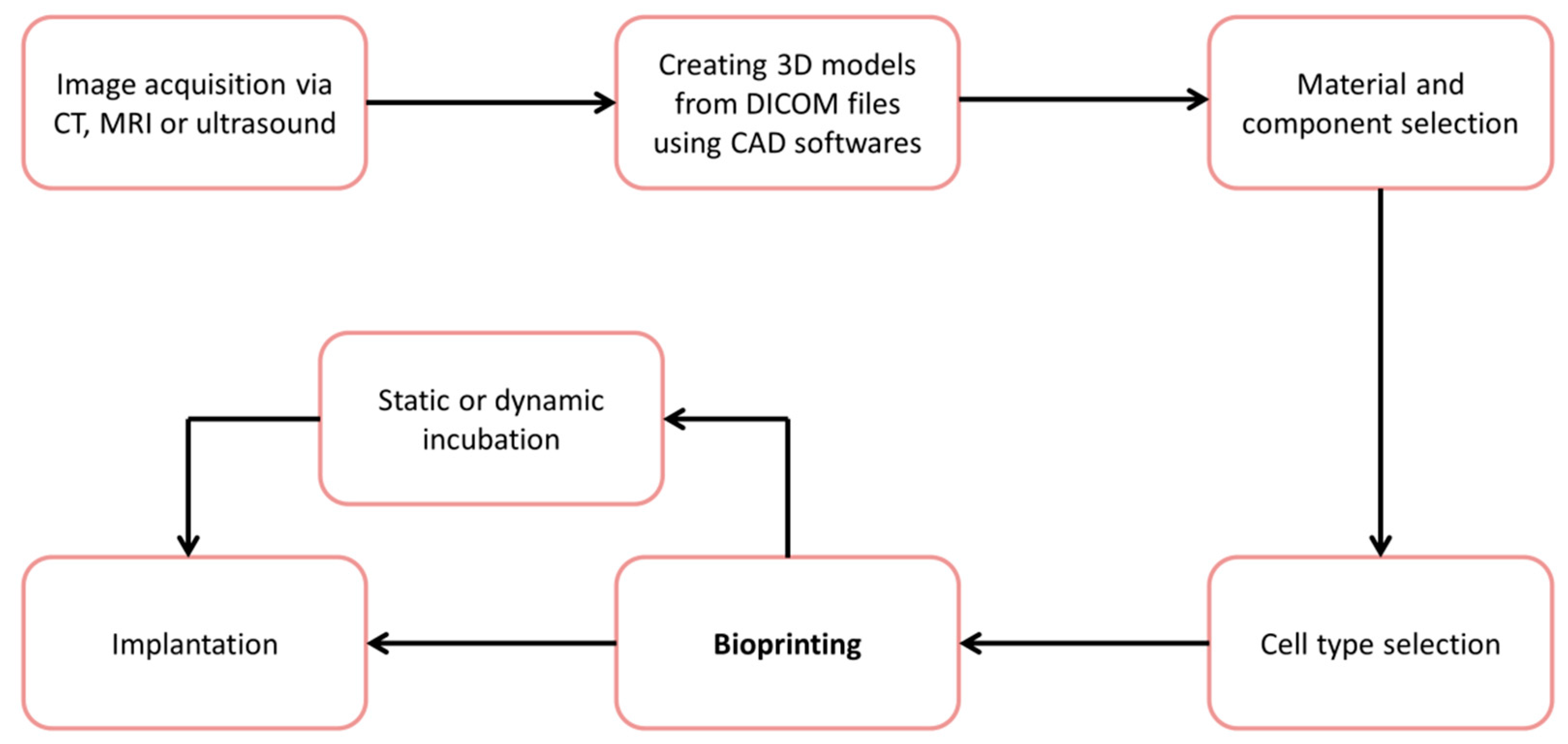
:1. Introduction
2. Inkjet-Based Bioprinting
3. Laser-Based Bioprinting
4. Laser-Assisted Bioprinting
5. Extrusion-Based Bioprinting
6. Discussion
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AFS | Amniotic fluid-derived stem cells |
| AG | Agarose |
| ALP | Alkaline phosphatase |
| AM | Additive manufacturing |
| ATCC | Mouse neural stem cell lines |
| BMSCs | Bone marrow stromal cells |
| BrCa | Breast cancer cells |
| CAD | Computer aided design |
| CT | Computer Tomography |
| dECM | Decellularized extracellular matrix |
| DN | Double network |
| DNA | Deoxyribonucleic acid |
| ECM | Extracellular matrix |
| GelMA | Gelatin methacryloyl |
| HA | Hydroxyapatite |
| hADSCs | Human adipose-derived stem cells |
| HAMa | Hyaluronic acid–methacrylate |
| HMECs | Human microvascular endothelial cells |
| HMVECs | Human dermal microvascular endothelial cells |
| Hs68 | Human dermal fibroblasts |
| hTMSCs | Human inferior turbinate-tissue derived mesenchymal stromal cells |
| HUVECs | Human umbilical vein endothelial cells |
| IPFP | Human infrapatellar fat pad derived adipose stem cells |
| IPNs | Interpenetrating polymer networks |
| LAB | Laser-assisted bioprinting |
| LAP | Lithium phenyl-2,4,6-trimethylbenzoylphosphinate |
| MRI | Magnetic Resonance Imaging |
| MSCs | Human bone marrow mesenchymal stem cells |
| nHA | Nanocrystalline hydroxyapatite |
| NHDFs | Human dermal fibroblasts |
| NHEKs | Neonatal human epidermal keratinocytes |
| PCL | Polycaprolactone |
| PEG | Poly(ethylene-glycol) |
| PEGDA | poly(ethylene glycol) diacrylate |
| PEGDMA | Poly(ethylene glycol) dimethacrylate |
| PLA | Polylactide fibers |
| PVA | polyvinyl alcohol |
| SA | Sodium alginate |
| SLA | Stereolithography |
| UV | Ultraviolet |
| VEGF | Vascular endothelial growth factor |
| β-TCP | Beta-tricalcium phosphate |
References
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Moroni, L.; Boland, T.; Burdick, J.A.; De Maria, C.; Derby, B.; Forgacs, G.; Groll, J.; Li, Q.; Malda, J.; Mironov, V.A.; et al. Biofabrication: A Guide to Technology and Terminology. Trends Biotechnol. 2018, 36, 384–402. [Google Scholar] [CrossRef] [PubMed]
- Freed, L.E.; Marquis, J.C.; Langer, R.; Vunjak-Novakovic, G.; Emmanual, J. Composition of cell-polymer cartilage implants. Biotechnol. Bioeng. 1994, 43, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Galban, C.J.; Locke, B.R. Analysis of cell growth kinetics and substrate diffusion in a polymer scaffold. Biotechnol. Bioeng. 1999, 65, 121–132. [Google Scholar] [CrossRef]
- Wang, X.; Yan, Y.; Pan, Y.; Xiong, Z.; Liu, H.; Cheng, J.; Liu, F.; Lin, F.; Wu, R.; Zhang, R.; et al. Generation of Three-Dimensional Hepatocyte/Gelatin Structures with Rapid Prototyping System. Tissue Eng. 2006, 12, 060127071904002. [Google Scholar] [CrossRef]
- Martin, I.; Wendt, D.; Heberer, M. The role of bioreactors in tissue engineering. Trends Biotechnol. 2004, 22, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Villalona, G.A.; Udelsman, B.; Duncan, D.R.; McGillicuddy, E.; Sawh-Martinez, R.F.; Hibino, N.; Painter, C.; Mirensky, T.; Erickson, B.; Shinoka, T.; et al. Cell-seeding techniques in vascular tissue engineering. Tissue Eng. Part B Rev. 2010, 16, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Malkoc, V. Challenges and the Future of 3D Bioprinting. Available online: http://www.alliedacademies.org/articles/challenges-and-the-future-of-3d-bioprinting.pdf (accessed on 28 March 2018).
- Tappa, K.; Jammalamadaka, U. Novel Biomaterials Used in Medical 3D Printing Techniques. J. Funct. Biomater. 2018, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Auger, F.A.; Gibot, L.; Lacroix, D. The Pivotal Role of Vascularization in Tissue Engineering. Annu. Rev. Biomed. Eng. 2013, 15, 177–200. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, M.; Fan, X.; Zhou, H. Recent advances in bioprinting techniques: Approaches, applications and future prospects. J. Transl. Med. 2016, 14, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Rider, P.; Peri, Ž.; Alkildani, S.; Retnasingh, S.; Barbeck, M. Bioprinting of tissue engineering scaffolds. J. Tissue Eng. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Rider, P.; Alkildani, S.; Retnasingh, S.; Peri, Ž.; Schnettler, R.; Barbeck, M. Additive Manufacturing for Guided Bone Regeneration: A Perspective for Alveolar Ridge Augmentation. Int. J. Mol. Sci. 2018, 19, 3308. [Google Scholar] [CrossRef] [PubMed]
- Sachlos, E.; Czernuszka, J. Making Tissue Engineering Scaffolds Work. Review: The application of solid freeform fabrication technology to the production of tissue engineering scaffolds. Eur. Cells Mater. 2003, 5, 29–40. [Google Scholar] [CrossRef]
- Townsend, A.; Racasan, R.; Leach, R.; Senin, N.; Thompson, A.; Ramsey, A.; Bate, D.; Woolliams, P.; Brown, S.; Blunt, L. An interlaboratory comparison of X-ray computed tomography measurement for texture and dimensional characterisation of additively manufactured parts. Addit. Manuf. 2018, 23, 422–432. [Google Scholar] [CrossRef]
- Krolczyk, G.; Raos, P.; Legutko, S. Experimental Analysis of Surface Roughness and Surface Texture of Machined and Fused Deposition Modelled Parts. Tehnicki Vjesnik 2014, 21, 217–221. [Google Scholar]
- Mandić, M.; Galeta, T. Dimensional accuracy of camera casing models 3D printed on Mcor IRIS: A case study. Adv. Prod. Eng. Manag. 2016, 11, 324–332. [Google Scholar] [CrossRef] [Green Version]
- Obregon, F.; Vaquette, C.; Ivanovski, S.; Hutmacher, D.W.; Bertassoni, L.E. The application of three-dimensional printing techniques in the field of oral and maxillofacial surgery. J. Korean Assoc. Oral Maxillofac. Surg. 2015, 41, 169–170. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Arneri, A.; Bersini, S.; Shin, S.R.; Zhu, K.; Goli-Malekabadi, Z.; Aleman, J.; Colosi, C.; Busignani, F.; Dell’Erba, V.; et al. Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip. Biomaterials 2016, 110, 45–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pourchet, L.J.; Thepot, A.; Albouy, M.; Courtial, E.J.; Boher, A.; Blum, L.J.; Marquette, C.A. Human Skin 3D Bioprinting Using Scaffold-Free Approach. Adv. Healthc. Mater. 2017, 6, 1601101. [Google Scholar] [CrossRef] [PubMed]
- Michael, S.; Sorg, H.; Peck, C.T.; Koch, L.; Deiwick, A.; Chichkov, B.; Vogt, P.M.; Reimers, K. Tissue Engineered Skin Substitutes Created by Laser-Assisted Bioprinting Form Skin-Like Structures in the Dorsal Skin Fold Chamber in Mice. PLoS ONE 2013, 8, e57741. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Tomaskovic-Crook, E.; Lozano, R.; Chen, Y.; Kapsa, R.M.; Zhou, Q.; Wallace, G.G.; Crook, J.M. Functional 3D Neural Mini-Tissues from Printed Gel-Based Bioink and Human Neural Stem Cells. Adv. Healthc. Mater. 2016, 5, 1429–1438. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Choi, J.-C.; Shim, J.-H.; Lee, J.-S.; Park, H.; Kim, S.W.; Doh, J.; Cho, D.-W. A comparative study on collagen type I and hyaluronic acid dependent cell behavior for osteochondral tissue bioprinting. Biofabrication 2014, 6, 035004. [Google Scholar] [CrossRef] [PubMed]
- Gruene, M.; Deiwick, A.; Koch, L.; Schlie, S.; Unger, C.; Hofmann, N.; Bernemann, I.; Glasmacher, B.; Chichkov, B. Laser Printing of Stem Cells for Biofabrication of Scaffold-Free Autologous Grafts. Tissue Eng. Part C Methods 2011, 17, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Malda, J.; Visser, J.; Melchels, F.P.; Jüngst, T.; Hennink, W.E.; Dhert, W.J.A.; Groll, J.; Hutmacher, D.W. 25th anniversary article: Engineering hydrogels for biofabrication. Adv. Mater. 2013, 25, 5011–5028. [Google Scholar] [CrossRef] [PubMed]
- Miri, A.K.; Nieto, D.; Iglesias, L.; Goodarzi Hosseinabadi, H.; Maharjan, S.; Ruiz-Esparza, G.U.; Khoshakhlagh, P.; Manbachi, A.; Dokmeci, M.R.; Chen, S.; et al. Microfluidics-Enabled Multimaterial Maskless Stereolithographic Bioprinting. Adv. Mater. 2018, 30, 1800242. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lu, Z.; Wu, H.; Li, W.; Zheng, L.; Zhao, J. Collagen-alginate as bioink for three-dimensional (3D) cell printing based cartilage tissue engineering. Mater. Sci. Eng. C 2018, 83, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Kosik-Kozioł, A.; Costantini, M.; Bolek, T.; Szöke, K.; Barbetta, A.; Brinchmann, J.; Święszkowski, W. PLA short sub-micron fiber reinforcement of 3D bioprinted alginate constructs for cartilage regeneration. Biofabrication 2017, 9, 044105. [Google Scholar] [CrossRef] [PubMed]
- Kawecki, F.; Clafshenkel, W.P.; Auger, F.A.; Bourget, J.-M.; Fradette, J.; Devillard, R. Self-assembled Human Osseous Cell Sheets as Living Biopapers for the Laser-assisted Bioprinting of Human Endothelial Cells. Biofabrication 2018, 10, 035006. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Harris, B.T.; Zhang, L.G. Gelatin methacrylamide hydrogel with graphene nanoplatelets for neural cell-laden 3D bioprinting. Proc. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. EMBS 2016, 4185–4188. [Google Scholar] [CrossRef]
- Zhou, X.; Zhu, W.; Nowicki, M.; Miao, S.; Cui, H.; Holmes, B.; Glazer, R.I.; Zhang, L.G. 3D Bioprinting a Cell-Laden Bone Matrix for Breast Cancer Metastasis Study. ACS Appl. Mater. Interfaces 2016, 8, 30017–30026. [Google Scholar] [CrossRef] [PubMed]
- Tse, C.; Whiteley, R.; Yu, T.; Stringer, J.; MacNeil, S.; Haycock, J.W.; Smith, P.J. Inkjet printing Schwann cells and neuronal analogue NG108-15 cells. Biofabrication 2016, 8, 015017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, T.; Baicu, C.; Aho, M.; Zile, M.; Boland, T. Fabrication and characterization of bio-engineered cardiac pseudo tissues. Biofabrication 2009, 1, 035001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seol, Y.J.; Lee, H.; Copus, J.S.; Kang, H.W.; Cho, D.W.; Atala, A.; Lee, S.J.; Yoo, J.J. 3D bioprinted biomask for facial skin reconstruction. Bioprinting 2018. [Google Scholar] [CrossRef]
- Pati, F.; Ha, D.H.; Jang, J.; Han, H.H.; Rhie, J.W.; Cho, D.W. Biomimetic 3D tissue printing for soft tissue regeneration. Biomaterials 2015, 62, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, F.Y.; Lin, H.H.; Hsu, S. hui 3D bioprinting of neural stem cell-laden thermoresponsive biodegradable polyurethane hydrogel and potential in central nervous system repair. Biomaterials 2015, 71, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Yanez, M.; Rincon, J.; Dones, A.; De Maria, C.; Gonzales, R.; Boland, T. In vivo assessment of printed microvasculature in a bilayer skin graft to treat full-thickness wounds. Tissue Eng. Part A 2015, 21, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Maiullari, F.; Costantini, M.; Milan, M.; Pace, V.; Chirivì, M.; Maiullari, S.; Rainer, A.; Baci, D.; Marei, H.E.S.; Seliktar, D.; et al. A multi-cellular 3D bioprinting approach for vascularized heart tissue engineering based on HUVECs and iPSC-derived cardiomyocytes. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.W.; Lee, K.W.; Park, J.H.; Lee, J.H.; Jung, C.R.; Yu, J.J.; Kim, H.Y.; Kim, D.H. 3D bioprinted artificial trachea with epithelial cells and chondrogenic-differentiated bone marrow-derived mesenchymal stem cells. Int. J. Mol. Sci. 2018, 19, 1624. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Ruan, C.; Ma, Y.; Cheng, D.; Wu, M.; Liu, W.; Zhao, X.; Pan, H.; Lu, W.W. Nanocomposite Hydrogels: 3D-Bioprinted Osteoblast-Laden Nanocomposite Hydrogel Constructs with Induced Microenvironments Promote Cell Viability, Differentiation, and Osteogenesis both In Vitro and In Vivo (Adv. Sci. 3/2018). Adv. Sci. 2018, 5, 1870013. [Google Scholar] [CrossRef]
- Kang, H.W.; Lee, S.J.; Ko, I.K.; Kengla, C.; Yoo, J.J.; Atala, A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 2016, 34, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Boland, T. Human microvasculature fabrication using thermal inkjet printing technology. Biomaterials 2009, 30, 6221–6227. [Google Scholar] [CrossRef] [PubMed]
- Marchioli, G.; Van Gurp, L.; Van Krieken, P.P.; Stamatialis, D.; Engelse, M.; Van Blitterswijk, C.A.; Karperien, M.B.J.; De Koning, E.; Alblas, J.; Moroni, L.; et al. Fabrication of three-dimensional bioplotted hydrogel scaffolds for islets of Langerhans transplantation. Biofabrication 2015, 7, 25009. [Google Scholar] [CrossRef] [PubMed]
- Di Bella, C.; Duchi, S.; O’Connell, C.D.; Blanchard, R.; Augustine, C.; Yue, Z.; Thompson, F.; Richards, C.; Beirne, S.; Onofrillo, C.; et al. In situ handheld three-dimensional bioprinting for cartilage regeneration. J. Tissue Eng. Regen. Med. 2018, 12, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Keriquel, V.; Guillemot, F.; Arnault, I.; Guillotin, B.; Miraux, S.; Amédée, J.; Fricain, J.-C.; Catros, S. In vivo bioprinting for computer- and robotic-assisted medical intervention: Preliminary study in mice. Biofabrication 2010, 2, 014101. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Breitenkamp, K.; Finn, M.G.; Lotz, M.; D’Lima, D.D. Direct human cartilage repair using three-dimensional bioprinting technology. Tissue Eng. Part A 2012, 18, 1304–1312. [Google Scholar] [CrossRef] [PubMed]
- Skardal, A.; Mack, D.; Kapetanovic, E.; Atala, A.; Jackson, J.D.; Yoo, J.S.S. Bioprinted amniotic fluid-derived stem cells accelerate healing of large skin wounds. Stem Cells Transl. Med. 2012, 1, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Iwanaga, S.; Arai, K.; Nakamura, M. Inkjet Bioprinting; Elsevier Inc.: Amsterdam, The Netherlands, 2015; ISBN 9780128010150. [Google Scholar]
- Nakamura, M.; Kobayashi, A.; Takagi, F.; Watanabe, A.; Hiruma, Y.; Ohuchi, K.; Iwasaki, Y.; Horie, M.; Morita, I.; Takatani, S. Biocompatible Inkjet Printing Technique for Designed Seeding of Individual Living Cells. Tissue Eng. 2005, 11, 1658–1666. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Jin, S.; Ye, K. Tissue and Organ 3D Bioprinting. SLAS Technol. Transl. Life Sci. Innov. 2018. [CrossRef]
- Jammalamadaka, U.; Tappa, K. Recent Advances in Biomaterials for 3D Printing and Tissue Engineering. J. Funct. Biomater. 2018, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Boland, T.; D’Lima, D.D.; Lotz, M.K. Thermal inkjet printing in tissue engineering and regenerative medicine. Recent Patents Drug Deliv. Formul. 2012, 6, 149–155. [Google Scholar] [CrossRef]
- Tekin, E.; Smith, P.J.; Schubert, U.S. Inkjet printing as a deposition and patterning tool for polymers and inorganic particles. Soft Matter 2008, 4, 703. [Google Scholar] [CrossRef]
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D.H. 3D bioprinting for engineering complex tissues. Biotechnol. Adv. 2016, 34, 422–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boland, T.; Xu, T.; Damon, B.; Cui, X. Application of inkjet printing to tissue engineering. Biotechnol. J. 2006, 1, 910–917. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Dean, D.; Ruggeri, Z.M.; Boland, T. Cell damage evaluation of thermal inkjet printed Chinese hamster ovary cells. Biotechnol. Bioeng. 2010, 106, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Schilling, A.F.; Yonezawa, T.; Wang, J.; Dai, G.; Cui, X. Bioactive nanoparticles stimulate bone tissue formation in bioprinted three-dimensional scaffold and human mesenchymal stem cells. Biotechnol. J. 2014, 9, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Min, D.; Lee, W.; Bae, I.-H.; Lee, T.R.; Croce, P.; Yoo, S.-S. Bioprinting of biomimetic skin containing melanocytes. Exp. Dermatol. 2018, 27, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Derakhshanfar, S.; Mbeleck, R.; Xu, K.; Zhang, X.; Zhong, W.; Xing, M. 3D bioprinting for biomedical devices and tissue engineering: A review of recent trends and advances. Bioact. Mater. 2018, 3, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Binder, K.W.; Albanna, M.Z.; Dice, D.; Zhao, W.; Yoo, J.J.; Atala, A. Hybrid printing of mechanically and biologically improved constructs for cartilage tissue engineering applications. Biofabrication 2012, 5, 015001. [Google Scholar] [CrossRef] [PubMed]
- Raman, R.; Bhaduri, B.; Mir, M.; Shkumatov, A.; Lee, M.K.; Popescu, G.; Kong, H.; Bashir, R. High-Resolution Projection Microstereolithography for Patterning of Neovasculature. Adv. Healthc. Mater. 2016, 5, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mondschein, R.J.; Kanitkar, A.; Williams, C.B.; Verbridge, S.S.; Long, T.E. Polymer structure-property requirements for stereolithographic 3D printing of soft tissue engineering scaffolds. Biomaterials 2017, 140, 170–188. [Google Scholar] [CrossRef] [PubMed]
- Ikehata, H.; Ono, T. The Mechanisms of UV Mutagenesis. J. Radiat. Res. 2011, 52, 115–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smeds, K.A.; Pfister-Serres, A.; Miki, D.; Dastgheib, K.; Inoue, M.; Hatchell, D.L.; Grinstaff, M.W. Photocrosslinkable polysaccharides forin situ hydrogel formation. J. Biomed. Mater. Res. 2001, 55, 254–255. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.; Zhang, D.; Alexander, P.G.; Yang, G.; Tan, J.; Cheng, A.W.M.; Tuan, R.S. Application of visible light-based projection stereolithography for live cell-scaffold fabrication with designed architecture. Biomaterials 2013, 34, 331–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffmann, A.; Leonards, H.; Tobies, N.; Pongratz, L.; Kreuels, K.; Kreimendahl, F.; Apel, C.; Wehner, M.; Nottrodt, N. New stereolithographic resin providing functional surfaces for biocompatible three-dimensional printing. J. Tissue Eng. 2017, 8, 204173141774448. [Google Scholar] [CrossRef] [PubMed]
- Loong, W.; Liew, A.; Zhang, Y. using bessel beams Laser-based fabrication of 3D hydrogel constructs using bessel beams. Bioprinting 2018, 9, 44–51. [Google Scholar] [CrossRef]
- Lin, H.; Tang, Y.; Lozito, T.P.; Oyster, N.; Kang, R.B.; Fritch, M.R.; Wang, B.; Tuan, R.S. Projection Stereolithographic Fabrication of BMP-2 Gene-activated Matrix for Bone Tissue Engineering. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.; Maclean, M.; MacGregor, S.J.; Anderson, J.G.; Grant, M.H. Exposure of 3T3 mouse Fibroblasts and Collagen to High Intensity Blue Light. IFMBE Proc. 2009, 23, 1352–1355. [Google Scholar] [CrossRef]
- Wang, Z.; Abdulla, R.; Parker, B.; Samanipour, R.; Ghosh, S.; Kim, K. A simple and high-resolution stereolithography-based 3D bioprinting system using visible light crosslinkable bioinks. Biofabrication 2015, 7, 45009. [Google Scholar] [CrossRef] [PubMed]
- Morris, V.B.; Nimbalkar, S.; Younesi, M.; McClellan, P.; Akkus, O. Mechanical Properties, Cytocompatibility and Manufacturability of Chitosan:PEGDA Hybrid-Gel Scaffolds by Stereolithography. Ann. Biomed. Eng. 2017, 45, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Merten, O.-W. Advances in cell culture: Anchorage dependence. Philos. Trans. R. Soc. B Biol. Sci. 2014, 370. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Member, S.; Tian, Z.; Jin, X.; Holzman, J.F.; Menard, F.; Kim, K.; Preparation, A.H. Visible Light-based Stereolithography Bioprinting of Cell-adhesive Gelatin Hydrogels. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2017, 2017, 1599–1602. [Google Scholar]
- Hu, J.; Hou, Y.; Park, H.; Choi, B.; Hou, S.; Chung, A.; Lee, M. Visible light crosslinkable chitosan hydrogels for tissue engineering. Acta Biomater. 2012, 8, 1730–1738. [Google Scholar] [CrossRef] [PubMed]
- Atsumi, T.; Murata, J.; Kamiyanagi, I.; Fujisawa, S.; Ueha, T. Cytotoxicity of photosensitizers camphorquinone and 9-fluorenone with visible light irradiation on a human submandibular-duct cell line in vitro. Arch. Oral Biol. 1998, 43, 73–81. [Google Scholar] [CrossRef]
- Okada, N.; Muraoka, E.; Fujisawa, S.; Machino, M. Effects of visible light-irradiated camphorquinone and 9-fluorenone on murine oral mucosa. Dent. Mater. J. 2008, 27, 809–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, K.S.; Schon, B.S.; Mekhileri, N.V.; Brown, G.C.J.; Chia, C.M.; Prabakar, S.; Hooper, G.J.; Woodfield, T.B.F. New Visible-Light Photoinitiating System for Improved Print Fidelity in Gelatin-Based Bioinks. ACS Biomater. Sci. Eng. 2016, 2, 1752–1762. [Google Scholar] [CrossRef]
- Bohandy, J.; Kim, B.F.; Adrian, F.J. Metal deposition from a supported metal film using an excimer laser. J. Appl. Phys. 1986, 60, 1538–1539. [Google Scholar] [CrossRef]
- Kim, B.; Bohandy, J.; Adrian, F. Method and Apparatus for The Thin Film Deposition of Materials With a High Power Pulsed Laser. Available online: https://patents.google.com/patent/US4970196A/en (accessed on 6 November 2018).
- Odde, D.J.; Renn, M.J. Laser-guided direct writing of living cells. Biotechnol. Bioeng. 2000, 67, 312–318. [Google Scholar] [CrossRef]
- Keriquel, V.; Oliveira, H.; Rémy, M.; Ziane, S.; Delmond, S.; Rousseau, B.; Rey, S.; Catros, S.; Amédée, J.; Guillemot, F.; et al. In situ printing of mesenchymal stromal cells, by laser-assisted bioprinting, for in vivo bone regeneration applications. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Barron, J.A.; Wu, P.; Ladouceur, H.D.; Ringeisen, B.R. Biological Laser Printing: A Novel Technique for Creating Heterogeneous 3-dimensional Cell Patterns. Biomed. Microdevices 2004, 6, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Catros, S.; Guillemot, F.; Nandakumar, A.; Ziane, S.; Moroni, L.; Habibovic, P.; van Blitterswijk, C.; Rousseau, B.; Chassande, O.; Amédée, J.; et al. Layer-by-Layer Tissue Microfabrication Supports Cell Proliferation In Vitro and In vivo. Tissue Eng. Part C Methods 2012, 18, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Bishop, E.S.; Mostafa, S.; Pakvasa, M.; Luu, H.H.; Lee, M.J.; Wolf, J.M.; Ameer, G.A.; He, T.C.; Reid, R.R. 3-D bioprinting technologies in tissue engineering and regenerative medicine: Current and future trends. Genes Dis. 2017, 4, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Falguni, P.; Jinah, J.; Lee, J.W.; Dong-Woo, C. Extrusion Bioprinting. In Essentials of 3D Biofabrication and Translation; Elsevier: Amsterdam, The Netherlands, 2015; pp. 123–152. [Google Scholar]
- Leberfinger, A.N.; Ravnic, D.J.; Dhawan, A.; Ozbolat, I.T. Concise Review: Bioprinting of Stem Cells for Transplantable Tissue Fabrication. Stem Cells Transl. Med. 2017, 6, 1940–1948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, Q.; Tomaskovic-Crook, E.; Wallace, G.G.; Crook, J.M. 3D Bioprinting Human Induced Pluripotent Stem Cell Constructs for In Situ Cell Proliferation and Successive Multilineage Differentiation. Adv. Healthc. Mater. 2017, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Nair, K.; Gandhi, M.; Khalil, S.; Yan, K.C.; Marcolongo, M.; Barbee, K.; Sun, W. Characterization of cell viability during bioprinting processes. Biotechnol. J. 2009, 4, 1168–1177. [Google Scholar] [CrossRef] [PubMed]
- Wüst, S.; Müller, R.; Hofmann, S. Controlled Positioning of Cells in Biomaterials-Approaches Towards 3D Tissue Printing. J. Funct. Biomater. 2011, 2, 119–154. [Google Scholar] [CrossRef] [PubMed]
- Kraut, G.; Yenchesky, L.; Prieto, F.; Tovar, G.E.M.; Southan, A. Influence of shear thinning and material flow on robotic dispensing of poly(ethylene glycol) diacrylate/poloxamer 407 hydrogels. J. Appl. Polym. Sci. 2017, 134, 45083. [Google Scholar] [CrossRef]
- Chung, J.H.Y.; Naficy, S.; Yue, Z.; Kapsa, R.; Quigley, A.; Moulton, S.E.; Wallace, G.G. Bio-ink properties and printability for extrusion printing living cells. Biomater. Sci. 2013, 1, 763. [Google Scholar] [CrossRef] [Green Version]
- Castilho, M.; Hochleitner, G.; Wilson, W.; van Rietbergen, B.; Dalton, P.D.; Groll, J.; Malda, J.; Ito, K. Mechanical behavior of a soft hydrogel reinforced with three-dimensional printed microfibre scaffolds. Sci. Rep. 2018, 8, 1245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melchels, F.P.W.; Domingos, M.A.N.; Klein, T.J.; Malda, J.; Bartolo, P.J.; Hutmacher, D.W. Additive manufacturing of tissues and organs. Prog. Polym. Sci. 2012, 37, 1079–1104. [Google Scholar] [CrossRef] [Green Version]
- Skoog, S.A.; Goering, P.L.; Narayan, R.J. Stereolithography in tissue engineering. J. Mater. Sci. Mater. Med. 2014, 25, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Skardal, A.; Atala, A. Biomaterials for Integration with 3-D Bioprinting. Ann. Biomed. Eng. 2015, 43, 730–746. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.; Sousa, A.; Barrias, C.; Bayat, A.; Ben, R.; Pereira, F.; Aureliana, S.; Barrias, C.C.; Granja, P.L.; Bártolo, P.J. Advances in bioprinted cell-laden hydrogels for skin tissue engineering International Dupuytren Data Bank View project ACHILLES View project Advances in bioprinted cell-laden hydrogels for skin tissue engineering. Biomanuf. Rev. 2017, 2, 1. [Google Scholar] [CrossRef]
- He, Y.; Yang, F.; Zhao, H.; Gao, Q.; Xia, B.; Fu, J. Research on the printability of hydrogels in 3D bioprinting. Sci. Rep. 2016, 6, 29977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Highley, C.B.; Rodell, C.B.; Burdick, J.A. Direct 3D Printing of Shear-Thinning Hydrogels into Self-Healing Hydrogels. Adv. Mater. 2015, 27, 5075–5079. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Adokoh, C.K.; Narain, R. Recent development and biomedical applications of self-healing hydrogels. Expert Opin. Drug Deliv. 2018, 15, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for Biomedical Applications: Their Characteristics and the Mechanisms behind Them. Gels 2017, 3, 6. [Google Scholar] [CrossRef]
- Dragan, E.S. Advances in interpenetrating polymer network hydrogels and their applications. Pure Appl. Chem. 2014, 86, 1707–1721. [Google Scholar] [CrossRef]
- Vega, S.; Kwon, M.; Burdick, J. Recent advances in hydrogels for cartilage tissue engineering. Eur. Cells Mater. 2017, 33, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Olsen, B.D.; Khademhosseini, A. The mechanical properties and cytotoxicity of cell-laden double-network hydrogels based on photocrosslinkable gelatin and gellan gum biomacromolecules. Biomaterials 2012, 33, 3143–3152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozbolat, I.T.; Hospodiuk, M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials 2016, 76, 321–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.S.; Hong, J.M.; Jung, J.W.; Shim, J.H.; Oh, J.H.; Cho, D.W. 3D printing of composite tissue with complex shape applied to ear regeneration. Biofabrication 2014, 6, 024103. [Google Scholar] [CrossRef] [PubMed]
- Kundu, J.; Shim, J.-H.; Jang, J.; Kim, S.-W.; Cho, D.-W. An additive manufacturing-based PCL-alginate-chondrocyte bioprinted scaffold for cartilage tissue engineering. J. Tissue Eng. Regen. Med. 2015, 9, 1286–1297. [Google Scholar] [CrossRef] [PubMed]
- Pati, F.; Jang, J.; Ha, D.H.; Won Kim, S.; Rhie, J.W.; Shim, J.H.; Kim, D.H.; Cho, D.W. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat. Commun. 2014, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G. Biomimicry in biomedical research. Organogenesis 2012, 8, 101–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serena, E.; Cimetta, E.; Zatti, S.; Zaglia, T.; Zagallo, M.; Keller, G.; Elvassore, N. Micro-Arrayed Human Embryonic Stem Cells-Derived Cardiomyocytes for In Vitro Functional Assay. PLoS ONE 2012, 7, e48483. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, C.; Wen, J.; Wu, Y.; Shan, Y.; Liao, J. The design, mechanism and biomedical application of self-healing hydrogels. Chin. Chem. Lett. 2017, 28, 1857–1874. [Google Scholar] [CrossRef]
- Villa, M.M.; Wang, L.; Huang, J.; Rowe, D.W.; Wei, M. Bone tissue engineering with a collagen-hydroxyapatite scaffold and culture expanded bone marrow stromal cells. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Butler, D.L.; Goldstein, S.A.; Guldberg, R.E.; Guo, X.E.; Kamm, R.; Laurencin, C.T.; McIntire, L.V.; Mow, V.C.; Nerem, R.M.; Sah, R.L.; et al. The Impact of Biomechanics in Tissue Engineering and Regenerative Medicine. Tissue Eng. Part B Rev. 2009, 15, 477–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.S.; Lee, J.S.; Gao, G.; Cho, D.W. Direct 3D cell-printing of human skin with functional transwell system. Biofabrication 2017, 9, 025034. [Google Scholar] [CrossRef] [PubMed]




| Biomaterials | Cells | Results | Significance | Reference |
|---|---|---|---|---|
| Extrusion-based techniques | ||||
| SA SA/collagen SA/AG | Chondrocytes | Printed SA/collagen scaffold in cell culture showed enhanced cell proliferation, cartilage specific gene expression and cell adhesion. | SA/collagen is a potential bioink base material for cartilage regeneration | Yang et al., 2017 [27] |
| Alginate PLA fibers | Human chondrocytes | Printed cells showed a high cell viability (80%). | The addition of sub-micron PLA fibers can be used to improve hydrogel mechanical properties | Kosik-Kozioł 2017 [28] |
| GelMA | HUVECs | Printed cells form lumen- like structure of the endothelium and contracted with an approximate rate of 60 bpm for up to 7–10 days when cultured. | Successfully demonstrated the 3D printing of endothelialized-myocardium-on-a chip. | Zhang 2016 [19] |
| Laser-assisted bioprinting | ||||
| Human Osseous Cell Sheets | HUVECs | Printed cell exhibits the formation of tubule-like structures within the biopaper after 21 days of culture. | Demonstration of self-assembled cell sheets for the soft tissue regeneration. | Kawecki 2018 [29] |
| Stereolithography | ||||
| PEGDA and GelMA | MCF-7 breast cancer cell, HUVECs, C2C12 skeletal muscle cells, osteoblasts, fibroblasts, mesenchymal cells. | Fabricated structure exhibited high cell viability, proliferation and metabolic activity. | Demonstrated the flexibility of stereolithography for printing different cell types | Miri 2018 [26] |
| GelMA and graphene nanoplatelets | ATCC | The printed cells had differentiated, produced well-defined architectures and homogenous cell distribution. | Successfully demonstrated the printing neural stem cells | Zhu 2016 [30] |
| GelMA and nHA | BrCa and MSCs | Printed MSCs secreted macromolecules that promoted BrCa growth. | Successful model for the investigation of post-metastatic breast cancer progression in bone. | Zhou 2016 [31] |
| Inkjet-based techniques | ||||
| Cell suspension | Porcine Schwann cells, Neuronal analogue NG108-15 cells | Printed neuronal cells exhibited high cell viabilities as well as earlier and longer neurite growth than unprinted cells. | Can be incorporated into large tissue models to include an established neuronal network before implantation. | Tse 2016 [32] |
| Alginate | Primary feline adult cardiomyocytes, HL1 cardiac muscle cell line | Cells remained viable in a large scaffold. Scaffold pulsated under electrical stimulation. | Successfully printed myogenic tissue | Xu 2009 [33] |
| Biomaterials | Cells | Results | Significance | Reference |
|---|---|---|---|---|
| Extrusion-based techniques | ||||
| Hyaluronic acid, Gelatin, Glycerol, Fibrinogen, PU | Human fibroblasts, Human keratinocytes | Subcutaneous implants in rats reduced wound area to <40% after 14 days. Regenerated skin tissue consisted of epidermis and dermis layers | Novel method to fabricate patient-specific tissue construct to reconstruct facial skin wounds | Seol, 2018 [34] |
| Human decellularized adipose tissue, PCL | hASCs | The scaffolds proved to be adipo-inductive and exhibited adequate tissue infiltration | Demonstration of a clinically viable method of soft tissue regeneration | Pati, 2015 [35] |
| PU nanoparticles | NSCs | Implanted in adult zebrafish repaired traumatic brain injuries and restored function | 3D printing system that does not involve the use of heat, toxic organic solvents, toxic photoinitiators or UV for crosslinking | Hsieh, 2015 [36] |
| Alginate/gelatin, Alginate/hyaluronic acid, Alginate/Matrigel | INS1E-ß cells, Islets, (human and mouse) | Implanted subcutaneously in mice, exhibited metabolic activity after 7 days | Demonstrates possibility of encapsulating and printing human islets for islet transplantation applications | Yanez, 2015 [37] |
| Alginate, Fibrinogen, PEG | HUVECs, iPSCs-derived CMs | Subcutaneous implants in NOD-SCID mice developed a vascular network and CMs exhibited maturation after 2 weeks | Demonstrates an advantageous printing design where extruded filament was composed of 2 different inks | Maiullari, 2018 [38] |
| PCL, Sodium alginate | Rabbit bMSCs, Rabbit chondrogenic bMSCs, Rabbit respiratory endothelial cells | Neocartilage and neovascularization in rabbits after 12 weeks of tracheal implantation | Demonstrates fabrication of an artificial trachea with two cell types via additive manufacturing | Bae, 2018 [39] |
| PEG, Laponite XLG, Hyaluronic acid | ROBs | Implanted into rat tibias, exhibited new bone formation after 12 weeks | Demonstrates benefit of extruding the scaffold support material and bioink separately, however combined into one printing process | Xinyun Zhai, 2018 [40] |
| PCL/TCP/Pluronic® F127, PCL/Pluronic® F127 | Human amniotic-derived stem cells, Rabbit ear chondrocytes, Rabbit myoblasts | Implanted into rats, scaffolds with different cell types produced: newly formed vascularized bone tissue; vasculature with physiologically relevant mechanical properties; nerve integration | Showed significant improvements compared to acellular scaffolds for myogenic and osteogenic tissues | Kang, 2016 [41] |
| Laser-based techniques | ||||
| Collagen | Mouse fibroblasts, Human keratinocytes | Subcutaneous implants in nude mice form multi-layered epidermis and vascularization towards the printed cells, after 11 days | Utilization of a laser-assisted printing process in adding cells to commercially available skin grafts | Michael, 2013 [21] |
| Inkjet-based techniques | ||||
| Fibrin | HMECs | Printed cells form confluent tubular structure after 21 days | Promising approach for human microvascular tissue engineering | Cui, 2009 [42] |
| Collagen, Thrombin, Fibrinogen | Neonatal human dermal fibroblasts and epidermal keratinocytes, Dermal microvascular endothelial cells | Printed scaffolds exhibited 17% better wound contraction after 6 weeks in nude mice | Positioning of microvascular endothelial cells on fibroblast/keratinocyte grafts seemed to be advantageous over commercially available fibroblast/keratinocyte grafts | Marchioli, 2015 [43] |
| Biomaterials | Cells | Results | Significance | Reference |
|---|---|---|---|---|
| Extrusion-based techniques | ||||
| HA-GelMA | MSCs | Demonstrated cultured cells directly into the cartilage defect in sheep. | Directly reconstruction of cartilage using extrusion printing. | Di Bella 2017 [44] |
| Laser-based techniques | ||||
| nHA | MSCs | Printed cells exhibits the presence of pulsating blood vessels after bone defect achievement. | Scaffold was successfully printed in the mouse calvaria defect model in vivo. | Keriquel 2010 [45] |
| Inkjet-based techniques | ||||
| PEGDMA | Human chondrocytes | Printed directly onto the femoral condyles defects showed enhanced tissue integration. | Improved integration by direct in situ printing. | Cui 2012 [46] |
| Fibrinogen-collagen | AFS and MSCs | Used to repair full thickness wounds in the backs of mice, histological test shows the presence of blood vessel in the subcutaneous adipose tissue. | Potential to quickly close full thickness burns and enable revascularization of the tissue. | Skardal 2012 [47] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kačarević, Ž.P.; Rider, P.M.; Alkildani, S.; Retnasingh, S.; Smeets, R.; Jung, O.; Ivanišević, Z.; Barbeck, M. An Introduction to 3D Bioprinting: Possibilities, Challenges and Future Aspects. Materials 2018, 11, 2199. https://doi.org/10.3390/ma11112199
Kačarević ŽP, Rider PM, Alkildani S, Retnasingh S, Smeets R, Jung O, Ivanišević Z, Barbeck M. An Introduction to 3D Bioprinting: Possibilities, Challenges and Future Aspects. Materials. 2018; 11(11):2199. https://doi.org/10.3390/ma11112199
Chicago/Turabian StyleKačarević, Željka P., Patrick M. Rider, Said Alkildani, Sujith Retnasingh, Ralf Smeets, Ole Jung, Zrinka Ivanišević, and Mike Barbeck. 2018. "An Introduction to 3D Bioprinting: Possibilities, Challenges and Future Aspects" Materials 11, no. 11: 2199. https://doi.org/10.3390/ma11112199
APA StyleKačarević, Ž. P., Rider, P. M., Alkildani, S., Retnasingh, S., Smeets, R., Jung, O., Ivanišević, Z., & Barbeck, M. (2018). An Introduction to 3D Bioprinting: Possibilities, Challenges and Future Aspects. Materials, 11(11), 2199. https://doi.org/10.3390/ma11112199











