High Relative Abundance of Lactobacillus reuteri and Fructose Intake are Associated with Adiposity and Cardiometabolic Risk Factors in Children from Mexico City
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Outcome Variables
2.2.1. Adiposity
2.2.2. Cardiometabolic Risk Markers
2.3. Independent Variables
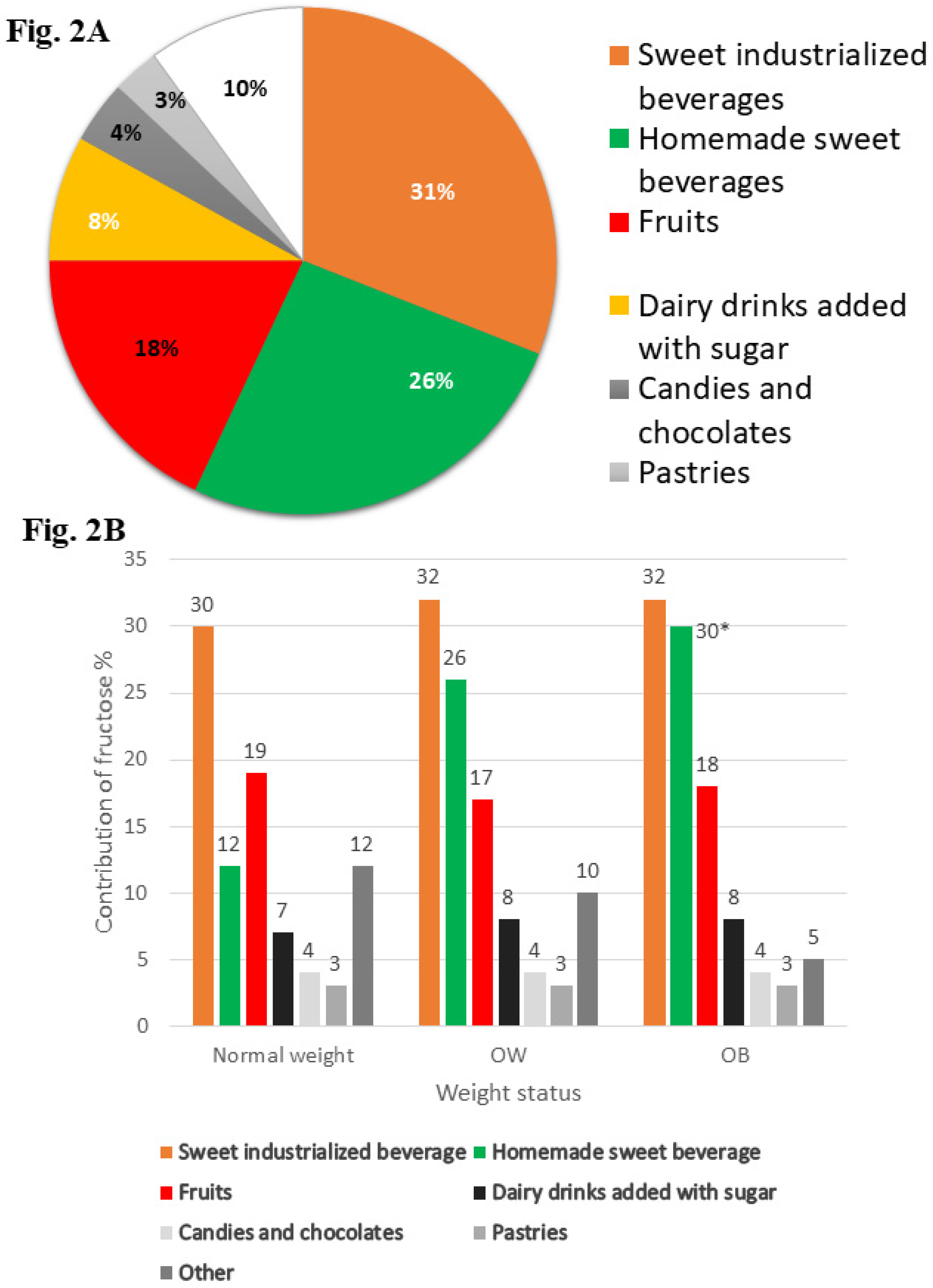
2.3.1. Fructose Intake from Dietary Information
2.3.2. RA of L. reuteri
2.4. Covariates
2.4.1. Leisure Time Physical Activity (LTPA)
2.4.2. Sociodemographic Information
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BMI | Body Mass Index |
| HDL-C | High Density Lipoprotein |
| FHO | Family History of Obesity |
| HOMA-IR | Homeostasis Model Assessment of insulin resistance |
| LDL-C | Low Density Lipoprotein |
| LTPA | Leisure Time Physical Activity |
| METs | Metabolic Equivalent of Task |
| OB | Obesity |
| OW | Overweight |
| RA | Relative Abundance |
| SFFQ | Semi-Quantitative Food Frequency Questionnaire |
| WC | Waist Circumference |
References
- World Health Organization. Obesity and overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 2 May 2018).
- Organization for Economic Cooperation and Development O. Obesity update. Available online: https://www.oecd.org/els/health-systems/Obesity-Update-2017 (accessed on 2 May 2018).
- Tappy, L.; Le, K.-A. Metabolic Effects of Fructose and the Worldwide Increase in Obesity. Physiol. Rev. 2010, 90, 23–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dornas, W.C.; de Lima, W.G.; Pedrosa, M.L.; Silva, M.E. Health implications of high-fructose intake. Adv. Nutr. 2015, 1, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Sievenpiper, J.L.; de Souza, R.J.; Mirrahimi, A.; Yu, M.E.; Carleton, A.J.; Beyene, J.; Chiavaroli, L.; di Buono, M.; Jenkins, A.L.; Leiter, L.A.; et al. Effect of fructose on body weight in controlled feeding trials: A systematic review and meta-analysis. Ann. Intern. Med. 2012, 156, 291–304. [Google Scholar] [CrossRef]
- Wang, D.D.; Sievenpiper, J.L.; de Souza, R.J.; Cozma, A.I.; Chiavaroli, L.; Ha, V.; Mirrahimi, A.; Carleton, A.J.; Di, M.; Jenkins, A.L.; et al. Effect of fructose on postprandial triglycerides: A systematic review and meta-analysis of controlled feeding trials. Atherosclerosis 2014, 232, 125–133. [Google Scholar] [CrossRef] [Green Version]
- Chiavaroli, L.; de Souza, R.J.; Ha, V.; Cozma, A.I.; Mirrahimi, A.; Wang, D.D.; Yu, M.; Carleton, A.J.; Di Buono, M.; Jenkins, A.L.; et al. Effect of fructose on established lipid targets: A systematic review and meta-analysis of controlled feeding trials. J. Am. Heart Assoc. 2015, 4, 1–23. [Google Scholar] [CrossRef]
- Vartanian, L.R.; Schwartz, M.B.; Brownell, K.D. Effects of soft drink consumption on nutrition and health: A systematic review and meta-analysis. Am. J. Public Health 2007, 97, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, D.S.; Peterson, K.E.; Gortmaker, S.L. Relation between consumption of sugar-sweetened drinks and childhood obesity: A prospective, observational analysis. Lancet 2001, 357, 505–508. [Google Scholar] [CrossRef]
- Papandreou, D.; Andreou, E.; Heraclides, A.; Rousso, I. Is beverage intake related to overweight and obesity in school children? Hippokratia 2013, 17, 42–46. [Google Scholar]
- Macdonald, I.A. A review of recent evidence relating to sugars, insulin resistance and diabetes. Eur. J. Nutr. 2016, 55, 17–23. [Google Scholar] [CrossRef] [Green Version]
- Nieuwdorp, M.; Gilijamse, P.W.; Pai, N.; Kaplan, L.M. Role of the microbiome in energy regulation and metabolism. Gastroenterology 2014, 146, 1525–1533. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Kitai, T.; Hazen, S.L. Gut microbiota in cardiovascular health and disease. Circ. Res. 2017, 120, 1183–1196. [Google Scholar] [CrossRef]
- Million, M.; Maraninchi, M.; Henry, M.; Armougom, F.; Richet, H.; Carrieri, P.; Valero, R.; Raccah, D. Obesity-associated gut microbiota is enriched in Lactobacillus reuteri and depleted in Bifidobacterium animalis and Methanobrevibacter smithii. Int. J. Obes. 2011, 36, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Million, M.; Angelakis, E.; Maraninchi, M.; Henry, M.; Giorgi, R.; Valero, R.; Vialettes, B.; Raoult, D. Correlation between body mass index and gut concentrations of Lactobacillus reuteri, Bifidobacterium animalis, Methanobrevibacter smithii and Escherichia coli. Int J Obes. 2013, 37, 1460–1466. [Google Scholar] [CrossRef]
- Gerez, C.L.; Cuezzo, S.; Rollán, G.; Font de Valdez, G. Lactobacillus reuteri CRL 1100 as starter culture for wheat dough fermentation. Food Microbiol. 2008, 25, 253–259. [Google Scholar] [CrossRef]
- Walter, J.; Britton, R.A.; Roos, S. Host-microbial symbiosis in the vertebrate gastrointestinal tract and the Lactobacillus reuteri paradigm. Proc. Natl. Acad. Sci. USA 2011, 108, 4645–4652. [Google Scholar] [CrossRef] [PubMed]
- Årsköld, E.; Lohmeier-Vogel, E.; Cao, R.; Roos, S.; Rådström, P.; Van Niel, E.W.J. Phosphoketolase pathway dominates in Lactobacillus reuteri ATCC 55730 containing dual pathways for glycolysis. J. Bacteriol. 2008, 190, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Sanmiguel, C.; Gupta, A.; Mayer, E.A. Gut Microbiome and Obesity: A Plausible Explanation for Obesity. Curr. Obes. Rep. 2015, 4, 250–261. [Google Scholar] [CrossRef] [Green Version]
- Drissi, F.; Merhej, V.; Angelakis, E.; El Kaoutari, A.; Carrière, F.; Henrissat, B.; Raoult, D. Comparative genomics analysis of Lactobacillus species associated with weight gain or weight protection. Nutr. Diabetes 2014, 4, e109. [Google Scholar] [CrossRef]
- Hou, C.; Zeng, X.; Yang, F.; Liu, H.; Qiao, S. Study and use of the probiotic Lactobacillus reuteri in pigs: A review. J. Anim. Sci. Biotechnol. 2015, 6, 14. [Google Scholar] [CrossRef]
- Estrada-Velasco, B.I.; Cruz, M.; García-Mena, J.; Salgado, A.V.; Romero, J.P.; Guna Serrano, M.D.L.R.; Madrid-Marina, V.; Orihuela, C.O.; López Islas, C.; Burguete-García, A.I. Childhood obesity is associated to the interaction between firmicutes and high energy food consumption. Nutr. Hosp. 2015, 31, 1074–1081. [Google Scholar]
- Habich, J. Standardization of quantitative epidemiological methods in the field. Oficina Sanit. Panam. 1974, 75, 375–384. [Google Scholar]
- Lohman, T.G.; Roche, A.F. Anthropometric Standardization Reference Manual; Human Kinetics Books: Champaign, IL, USA, 1988; pp. 39–70. [Google Scholar]
- Rivera, J.Á.; de Cossío, T.G.; Pedraza, L.S.; Aburto, T.C.; Sánchez, T.G.; Martorell, R. Childhood and adolescent overweight and obesity in Latin America: A systematic review. Lancet Diabetes Endocrinol. 2014, 2, 321–332. [Google Scholar] [CrossRef]
- Hosker, J.P.; Matthews, D.R.; Rudenski, A.S.; Burnett, M.A.; Darling, P.; Bown, E.G.; Turner, R.C. Continuous infusion of glucose with model assessment: measurement of insulin resistance and beta-cell function in man. Diabetologia 1985, 28, 401–411. [Google Scholar] [CrossRef]
- Willet, W. Nutritional Epidemiology; Oxford University Press: Oxford, UK, 1990. [Google Scholar]
- INSP. Databases of the nutritional value of foods. Compilation of the National Institute of Public Health. 2012. Available online: http://kin.insp.mx/aplicaciones/Redpidieta (accessed on 2 May 2018).
- Haarman, M.; Knol, J. Quantitative Real-Time PCR Analysis of Fecal Lactobacillus Species in Infants Receiving a Prebiotic Infant Formula Quantitative Real-Time PCR Analysis of Fecal Lactobacillus Species in Infants Receiving a Prebiotic Infant Formula. Appl. Environ. Microbiol. 2006, 72, 2359–2365. [Google Scholar] [CrossRef]
- Bacchetti De Gregoris, T.; Aldred, N.; Clare, A.S.; Burgess, J.G. Improvement of phylum- and class-specific primers for real-time PCR quantification of bacterial taxa. J. Microbiol. Methods 2011, 86, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C T method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Hernández, B.; Gortmaker, S.L.; Laird, N.M.; Colditz, G.A.; Parra-Cabrera, S.P.K. Validity and reproducibility of a questionnaire on physical activity and non-activity for school children in Mexico City. Salud Publica Mex. 2000, 42, 315–323. [Google Scholar] [CrossRef]
- Cárdenas-Cárdenas, L.M.; Burguete-Garcia, A.I.; Estrada-Velasco, B.I.; López-Islas, C.; Peralta-Romero, J.; Cruz, M.; Galván-Portillo, M. Leisure-time physical activity and cardiometabolic risk among children and adolescents. J. Pediatr. (Rio J.) 2015, 91, 136–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, R.J.; Andrews, P.; Lanaspa, M.A. Perspective: A Historical and Scienti fi c Perspective of Sugar and Its Relation with Obesity and Diabetes. Adv. Nutr. 2017, 8, 412–422. [Google Scholar]
- Rippe, J.M.; Angelopoulos, T.J. Sucrose, High-Fructose Corn Syrup, and Fructose, Their Metabolism and Potential Health Effects: What Do We Really Know? Adv. Nutr. 2013, 4, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Duthie, S.J.; Duthie, G.G.; Russell, W.R.; Kyle, J.A.M.; Macdiarmid, J.I.; Rungapamestry, V.; Stephen, S.; Megias-Baeza, C.; Kaniewska, J.J.; Shaw, L.; et al. Effect of increasing fruit and vegetable intake by dietary intervention on nutritional biomarkers and attitudes to dietary change: A randomised trial. Eur. J. Nutr. 2018, 57, 1855–1872. [Google Scholar] [CrossRef]
- Hun, S.; Wolfgang, M.; Tokutake, Y.; Chohnan, S.; Lane, M.D. Differential effects of central fructose and glucose on hypothalamic malonyl—CoA and food intake. Proc. Natl. Acad. Sci. USA 2008, 105, 16871–16875. [Google Scholar]
- Basaranoglu, M.; Basaranoglu, G.; Sabuncu, T.; Sentürk, H. Fructose as a key player in the development of fatty liver disease. World J. Gastroenterol. 2013, 19, 1166–1172. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Armougom, F.; Henry, M.; Vialettes, B.; Raccah, D.; Raoult, D. Monitoring bacterial community of human gut microbiota reveals an increase in Lactobacillus in obese patients and Methanogens in anorexic patients. PLoS ONE 2009, 4, 1–8. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J.; Zhang, S.; Yang, F.; Thacker, P.A.; Zhang, G.; Qiao, S.; Ma, X. Oral administration of lactobacillus fermentum I5007 favors intestinal development and alters the intestinal microbiota in formula-fed piglets. J. Agric. Food Chem. 2014, 62, 860–866. [Google Scholar] [CrossRef]
- Wang, A.; Yu, H.; Gao, X.; Li, X.; Qiao, S. Influence of Lactobacillus fermentum I5007 on the intestinal and systemic immune responses of healthy and E. coli challenged piglets. Antonie van Leeuwenhoek. Int. J. Gen. Mol. Microbiol. 2009, 96, 89–98. [Google Scholar]
- Fåk, F.; Bäckhed, F. Lactobacillus reuteri Prevents Diet-Induced Obesity, but not Atherosclerosis, in a Strain Dependent Fashion in Apoe-/- Mice. PLoS ONE 2012, 7, 1–8. [Google Scholar] [CrossRef]
- Mu, Q.; Tavella, V.J.; Luo, X.M. Role of Lactobacillus reuteri in human health and diseases. Front. Microbiol. 2018, 9, 757. [Google Scholar] [CrossRef]
- Chen, L.; Chen, Y.; Cheng, K.; Chien, T.; Chan, C. Antiobesity effect of Lactobacillus reuteri 263 associated with energy metabolism remodeling of white adipose tissue in high-energy-diet-fed rats. J. Nutr. Biochem. 2018, 54, 87–94. [Google Scholar] [CrossRef]
- Blaut, M. Gut microbiota and energy balance: Role in obesity. Proc. Nutr. Soc. 2015, 74, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Robinson, L.N.; Towle, H.C. ChREBP·Mlx is the principal mediator of glucose-induced gene expression in the liver. J. Biol. Chem. 2006, 281, 28721–28730. [Google Scholar] [CrossRef]
- Canfora, E.E.; Blaak, E.E. Acetate: A diet-derived key metabolite in energy metabolism: Good or bad in context of obesity and glucose homeostasis? Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 477–483. [Google Scholar]
- Orita, H.M.; Oh, H.T.; Ukuda, S.F.; Orikawa, H.H.; Shima, K.O.; Uzuki, T.S.; Urakami, M.M.; Isamatsu, S.H.; Ato, Y.K.; Akizawa, T.T.; et al. Comparative Genome Analysis of Lactobacillus reuteri and Lactobacillus fermentum Reveal a Genomic Island for Reuterin and Cobalamin Production. DNA Res. 2008, 15, 151–161. [Google Scholar]
- Mobini, R.; Tremaroli, V.; Ståhlman, M.; Karlsson, F.; Levin, M.; Ljungberg, M.; Sohlin, M.; Bertéus Forslund, H.; Perkins, R.; Bäckhed, F.; et al. Metabolic effects of Lactobacillus reuteri DSM 17938 in people with type 2 diabetes: A randomized controlled trial. Diabetes Obes. Metab. 2017, 19, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.L.; Martoni, C.J.; Parent, M.; Prakash, S. Cholesterol-lowering efficacy of a microencapsulated bile salt hydrolase-active Lactobacillus reuteri NCIMB 30242 yoghurt formulation in hypercholesterolaemic adults. Br. J. Nutr. 2012, 107, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.L.; Martoni, C.J.; Prakash, S. Cholesterol lowering and inhibition of sterol absorption by Lactobacillus reuteri NCIMB 30242: A randomized controlled trial. Eur. J. Clin. Nutr. 2012, 66, 1234–1241. [Google Scholar] [CrossRef]
- Simon, M.C.; Strassburger, K.; Nowotny, B.; Kolb, H.; Nowotny, P.; Burkart, V.; Zivehe, F.; Hwang, J.H.; Stehle, P.; Pacini, G.; et al. Intake of lactobacillus reuteri improves incretin and insulin secretion in Glucose-Tolerant humans: A proof of concept. Diabetes Care 2015, 38, 1827–1834. [Google Scholar] [CrossRef]
- Holvoet, P. Stress in obesity and associated metabolic and cardiovascular disorders. Scientifica (Cairo) 2012, 2012, 1–19. [Google Scholar] [CrossRef]
- Bastarrachea, R.A.; López-Alvarenga, J.C.; Bolado-García, V.E.; Téllez-Mendoza, J.; Laviada-Molina, H.C.A. Macrophages, inflammation, adipose tissue, obesity and insulin resistance. Gac. Med. Mex. 2007, 143, 505–512. [Google Scholar]
- Faeh, D.; Minehira, K.; Schwarz, J.M.; Periasami, R.; Seongsu, P.; Tappy, L. Effect of fructose overfeeding and fish oil administration on hepatic de novo lipogenesis and insulin sensitivity in healthy men. Diabetes 2005, 54, 1907–1913. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, J.M.; Noworolski, S.M.; Wen, M.J.; Dyachenko, A.; Prior, J.L.; Weinberg, M.E.; Herraiz, L.A.; Tai, V.W.; Bergeron, N.; Bersot, T.P.; et al. Effect of a high-fructose weight-maintaining diet on lipogenesis and liver fat. J. Clin. Endocrinol. Metab. 2015, 100, 2434–2442. [Google Scholar] [CrossRef]
- Lustig, R.H.; Mulligan, K.; Noworolski, S.M.; Tai, V.W.; Wen, M.J.; Erkin-cakmak, A.; Gugliucci, A.; Schwarz, J.; Francisco, S.; Francisco, S.; et al. Isocaloric fructose restriction and metabolic improvement in children with obesity and metabolic syndrome. Obesity 2017, 24, 453–460. [Google Scholar] [CrossRef]
- Macdiarmid, J.; Blundell, J. Assessing dietary intake. Who, what and why of under-reporting. Nutr. Res. Rev. 1998, 11, 231–253. [Google Scholar] [CrossRef] [PubMed]
- Eck, L.H.; Klesges, R.C.; Hanson, C.L. Recall of a child’s intake from one meal: Are parents accurate? J. Am. Diet. Assoc. 1989, 89, 784–789. [Google Scholar] [PubMed]

| Characteristics 2 | BMI Status | ||
|---|---|---|---|
| Normal Weight (n = 510) | OW (n = 287) | OB (n = 290) | |
| Age (year) | 9.19 ± 1.76 a | 9.70 ± 1.72 b | 9.60 ± 1.80 b |
| Girls (%) | 48.43 a | 46.70 a | 36.21 b |
| LTFA (MET) | 441.81 ± 376.77 a | 444 ± 411.80 a | 448.34 ± 409.40 a |
| FHO (%) | 47.74 a | 57.50 b | 65.20 c |
| BMI for age Z-score | −0.11 ± 0.76 a | 1.60 ± 0.60 b | 2.70 ± 0.50 c |
| WC | 57.45 ± 5.55 a | 68.75 ± 7.60 b | 78.80 ± 9.15 c |
| Glucose (mg/dL) | 81.45 ± 9.80 a | 81.92 ± 8.60 a | 83.70 ± 8.90 b |
| Triglycerides (mg/dL) | 73.30 ± 29.07 a | 101.70 ± 48.60 b | 118.70 ± 53.40 c |
| Total cholesterol (mg/dL) | 155.56 ± 32.18 a | 162.03 ± 33.20 b | 162 ± 33.30 b |
| HDL-C (mg/dL) | 54.80 ± 11.99 a | 50.80 ± 12.77 b | 45.5 ± 11.5 c |
| LDL-C (mg/dL) | 98.06 ± 24.25 a | 107.50 ± 26.40 b | 109.80 ± 27.40 b |
| Insulin (μU/mL) | 4.83 ± 3.63 a | 8.60 ± 7.10 b | 11.30 ± 10.40 c |
| HOMA-IR | 0.97 ± 0.76 a | 1.77 ± 1.50 b | 2.40 ± 2.35 c |
| Exposure variables | |||
| Energy intake (kcal) | 2158.48 ± 722.13 a | 2205.40 ± 766.56 a | 2226.80 ± 824.90 a |
| Fructose intake (g) | 24.31 ± 11.98 a | 25.80 ± 13.50 a | 27.50 ± 17.50 b |
| Fructose contribution (%) | 4.05 ± 1.41 a | 4.23 ± 1.60 a | 4.40 ± 1.70 b |
| L. reuteri (RA) | 0.007 ± 0.025 a | 0.17 ± 1.4 b | 0.40 ± 1.74 c |
| Diet Fructose Contribution (%) | Relative Abundance of L. reuteri | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Medium-Tertile 4 (3.96 ± 0.33) | High-Tertile 4 (5.85 ± 1.40) | Medium-Tertile 5 (0.0007 ± 0.0004) | High-Tertile 5 (0.50 ± 1.86) | |||||||||
| Path Coefficient | 95% CI | P Value | Path Coefficient | 95% CI | P Value | Path Coefficient | 95% CI | P Value | Path Coefficient | 95% CI | P Value | |
| Adiposity Indicators | ||||||||||||
| Direct Effect | ||||||||||||
| BMI for age Z-score | −0.07 2 | −0.30, 0.12 | 0.50 | 0.24 2 | 0.04, 0.44 | 0.02 | 0.27 2,6 | 0.07, 0.47 | 0.009 | 0.52 2,6 | 0.32, 0.72 | <0.001 |
| WC, cm | 0.30 2 | −1.2, 1.75 | 0.70 | 2.40 2 | 0.95, 3.84 | 0.001 | 1.60 2,6 | 0.12, 3.04 | 0.03 | 3.40 2,6 | 1.95, 4.90 | <0.001 |
| Indirect Effects (Via RA L reuteri) | ||||||||||||
| BMI for age Z-score | 0.02 2 | −0.12, 0.05 | 0.25 | 0.01 2 | −0.02, 0.04 | 0.51 | - | - | - | - | - | |
| WC, cm | 0.11 2 | −0.08, 0.31 | 0.25 | 0.06 2 | −0.12, 0.25 | 0.60 | - | - | - | - | - | |
| Total Effects | ||||||||||||
| BMI for age Z-score | −0.05 2 | −0.25, 0.15 | 0.60 | 0.24 2 | 0.05, 0.45 | 0.02 | 0.27 2,6 | 0.07, 0.47 | 0.009 | 0.52 2,6 | 0.32, 0.72 | <0.001 |
| WC, cm | 0.40 2 | −1.06, 1.88 | 0.54 | 2.45 2 | 1.00, 3.90 | 0.001 | 1.60 2,6 | 0.12, 3.04 | 0.03 | 3.40 2,6 | 1.95, 4.90 | <0.001 |
| Diet Fructose Contribution (%) 2 | Relative Abundance of L. Reuteri 2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Medium-Tertile 3 | High-Tertile 3 | Medium-Tertile 4 | High-Tertile 4 | |||||||||
| Path Coefficient | 95% CI | P Value | Path Coefficient | 95% CI | P Value | Path Coefficient | 95% CI | P Value | Path Coefficient | 95% CI | P Value | |
| Cardiometabolic Markers | ||||||||||||
| Direct Effect | ||||||||||||
| Glucose, mg/dL | 1.24 | −0.17, 2.65 | 0.08 | 0.34 | −1.06, 1.75 | 0.63 | −0.27 | −1.70, 1.14 | 0.70 | 0.23 | −1.18, 1.65 | 0.75 |
| Insulin, μU/mL | 0.49 | −0.51, 1.50 | 0.96 | 0.87 | −0.13, 1.88 | 0.09 | −1.00 | −2.01, 0.11 | 0.06 | 0.007 | −1.01, 1.02 | 0.98 |
| HOMA-IR | 0.13 | −0.09, 0.36 | 0.25 | 0.20 | −0.03, 0.43 | 0.09 | −0.22 | −0.45, 0.01 | 0.06 | 0.03 | −0.20, 0.26 | 0.80 |
| LDL-C, mg/dL | 2.70 | −1.33, 6.73 | 0.18 | −1.27 | −5.30, 2.76 | 0.53 | −0.32 | −4.37. 3.71 | 0.87 | −0.70 | −4.75, 3.35 | 0.73 |
| HDL-C, mg/dL | 0.03 | −1.76, 1.81 | 0.97 | 0.50 | −1.28, 2.30 | 0.60 | −0.45 | −2.24, 1.34 | 0.62 | 0.19 | −1.60, 2.00 | 0.83 |
| Triglycerides, mg/dL | 1.97 | −4.31, 8.27 | 0.53 | −2.60 | −8.88, 3.69 | 0.41 | −2.47 | −8.79, 3.83 | 0.44 | −6.03 | −12.36, 0.30 | 0.06 |
| Indirect Effect (Via Waist Circumference) | ||||||||||||
| Glucose, mg/dL | −0.03 | −0.20, 0.12 | 0.70 | 0.14 | −0.03, 0.33 | 0.11 | 0.12 | −0.03, 0.27 | 0.10 | 0.25 | 0.02, 0.50 | 0.03 |
| Insulin, μU/mL | 0.06 | −0.42, 0.55 | 0.80 | 0.75 | 0.25, 1.24 | <0.01 | 0.44 | −0.04, 0.93 | 0.07 | 0.91 | 0.41, 1.41 | <0.001 |
| HOMA-IR | 0.01 | −0.09, 0.11 | 0.78 | 0.15 | 0.05, 0.26 | <0.01 | 0.09 | −0.01, 0.20 | 0.08 | 0.19 | 0.08, 0.30 | <0.001 |
| LDL-C, mg/dL | −0.24 | −1.09, 0.61 | 0.60 | 1.02 | 0.12, 1.93 | 0.03 | 0.90 | 0.04, 1.75 | 0.04 | 1.80 | 0.80, 2.80 | <0.001 |
| HDL-C, mg/dL | −0.12 | −0.80, 0.54 | 0.71 | −1.03 | −1.71, −0.35 | <0.01 | −0.60 | −1.26, 0.08 | 0.08 | −1.21 | −1.91, −0.50 | 0.001 |
| Triglycerides, mg/dL | −0.17 | −3.37, 3.02 | 0.91 | 4.62 | 1.40, 7.84 | <0.01 | 3.24 | 0.03, 6.45 | 0.05 | 6.58 | 3.30, 9.90 | <0.001 |
| Total Effect | ||||||||||||
| Glucose, mg/dL | 1.21 | −0.20, 2.64 | 0.09 | 0.50 | −0.92, 1.90 | 0.50 | −0.14 | −1.60, 1.30 | 0.83 | 0.48 | −0.92, 1.90 | 0.50 |
| Insulin, μU/mL | 0.56 | −0.55, 1.68 | 0.32 | 1.62 | 0.50, 2.73 | <0.01 | −0.55 | −1.68, 0.56 | 0.33 | 0.92 | −0.19, 2.04 | 0.10 |
| HOMA-IR | 0.14 | −0.10, 0.40 | 0.25 | 0.35 | 0.10, 0.61 | <0.01 | −0.12 | −0.38, 0.12 | 0.31 | 0.22 | −0.02, 0.50 | 0.09 |
| LDL-C, mg/dL | 2.46 | −1.64, 6.56 | 0.24 | −0.25 | −4.33, 3.85 | 0.90 | 0.60 | −3.55, 4.68 | 0.80 | 1.10 | −3.00, 5.20 | 0.60 |
| HDL-C, mg/dL | −0.10 | −2.00, 1.80 | 0.92 | −0.54 | −2.44, 1.35 | 0.57 | −1.04 | −2.95, 0.87 | 0.30 | −1.02 | −2.92, 0.88 | 0.30 |
| Triglycerides, mg/dL | 1.81 | −5.22, 8.84 | 0.60 | 2.02 | −4.98, 9.03 | 0.60 | 0.76 | −6.3, 7.81 | 0.83 | 0.55 | −6.47, 7.57 | 0.94 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huerta-Ávila, E.E.; Ramírez-Silva, I.; Torres-Sánchez, L.E.; Díaz-Benítez, C.E.; Orbe-Orihuela, Y.C.; Lagunas-Martínez, A.; Galván-Portillo, M.; Flores, M.; Cruz, M.; Burguete-García, A.I. High Relative Abundance of Lactobacillus reuteri and Fructose Intake are Associated with Adiposity and Cardiometabolic Risk Factors in Children from Mexico City. Nutrients 2019, 11, 1207. https://doi.org/10.3390/nu11061207
Huerta-Ávila EE, Ramírez-Silva I, Torres-Sánchez LE, Díaz-Benítez CE, Orbe-Orihuela YC, Lagunas-Martínez A, Galván-Portillo M, Flores M, Cruz M, Burguete-García AI. High Relative Abundance of Lactobacillus reuteri and Fructose Intake are Associated with Adiposity and Cardiometabolic Risk Factors in Children from Mexico City. Nutrients. 2019; 11(6):1207. https://doi.org/10.3390/nu11061207
Chicago/Turabian StyleHuerta-Ávila, Eira E., Ivonne Ramírez-Silva, Luisa E. Torres-Sánchez, Cinthya E. Díaz-Benítez, Yaneth C. Orbe-Orihuela, Alfredo Lagunas-Martínez, Marcia Galván-Portillo, Mario Flores, Miguel Cruz, and Ana I. Burguete-García. 2019. "High Relative Abundance of Lactobacillus reuteri and Fructose Intake are Associated with Adiposity and Cardiometabolic Risk Factors in Children from Mexico City" Nutrients 11, no. 6: 1207. https://doi.org/10.3390/nu11061207
APA StyleHuerta-Ávila, E. E., Ramírez-Silva, I., Torres-Sánchez, L. E., Díaz-Benítez, C. E., Orbe-Orihuela, Y. C., Lagunas-Martínez, A., Galván-Portillo, M., Flores, M., Cruz, M., & Burguete-García, A. I. (2019). High Relative Abundance of Lactobacillus reuteri and Fructose Intake are Associated with Adiposity and Cardiometabolic Risk Factors in Children from Mexico City. Nutrients, 11(6), 1207. https://doi.org/10.3390/nu11061207






