The Effect of Accelerated Aging Conditions on the Properties of Rigid Polyurethane-Polyisocyanurate Foams Modified by Cinnamon Extract
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of the Rigid PUR-PIR Foams
2.3. Methods
2.3.1. Foaming Process
2.3.2. Foam Aging was Carried Out Using Two Methods
Accelerated Aging Tests
Aging in a Climate Chamber
2.3.3. Apparent Density
2.3.4. Flammability Tests
2.3.5. Differential Scanning Calorimetry (DSC)
2.3.6. Softening Point
2.3.7. Compressive Strength and Compressive Strength Ratio
2.3.8. Foam Structure
2.3.9. Absorbability and Water Absorption
2.3.10. Brittleness
2.3.11. Chemical Structure
2.3.12. Content of Closed Cells
2.3.13. Measurement of Foam Color
3. Results and Discussion
3.1. Foaming Process
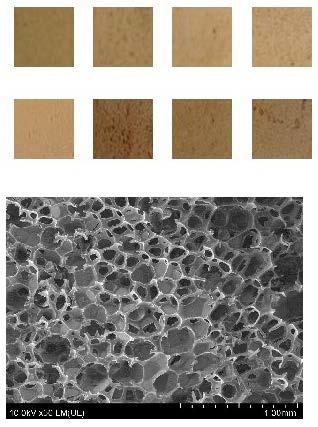
3.2. Organoleptic Assessment of RPU/PIR Foams
3.3. Color of Foam
3.4. Accelerated Aging Tests
3.5. Compressive Strength
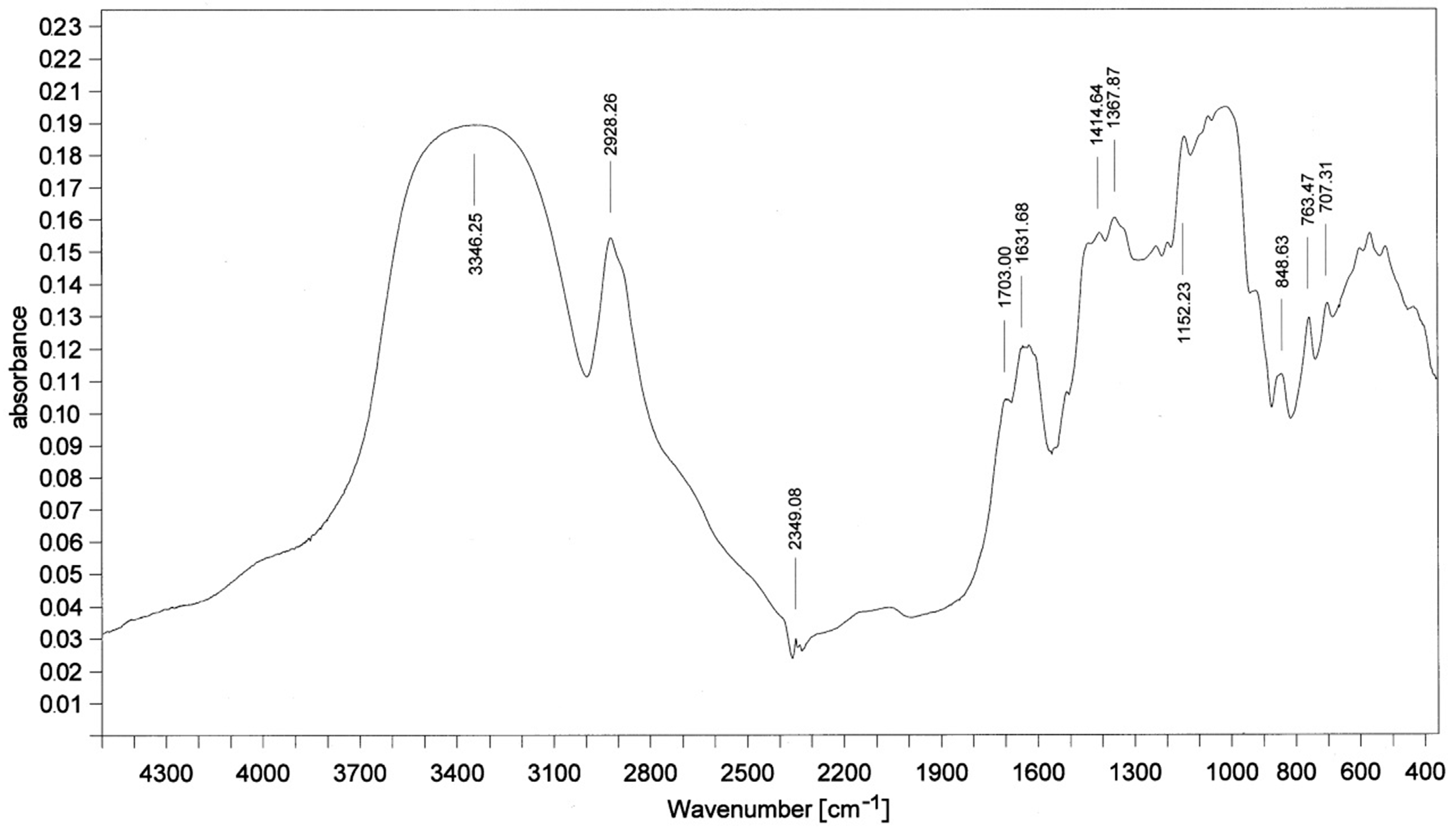
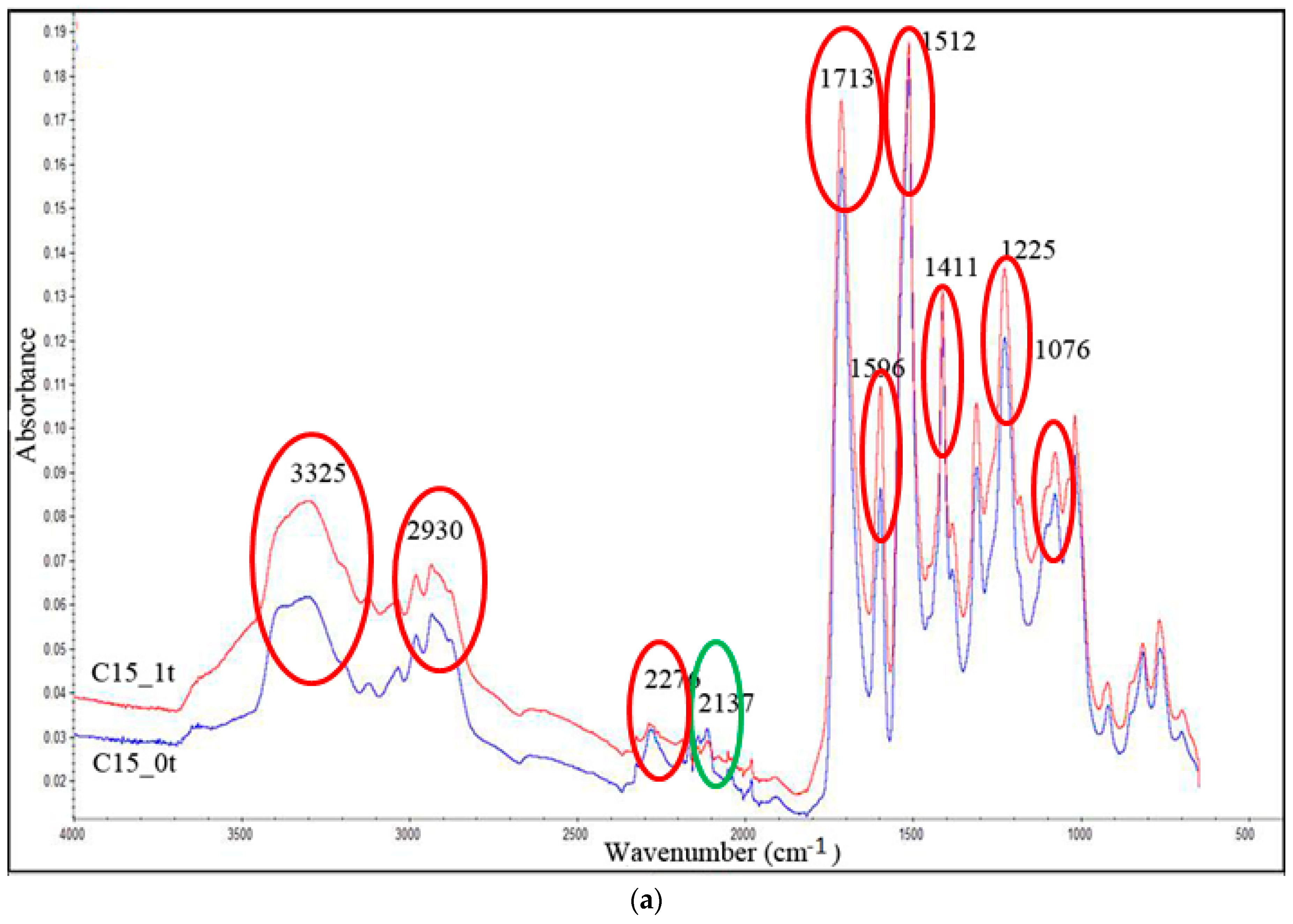
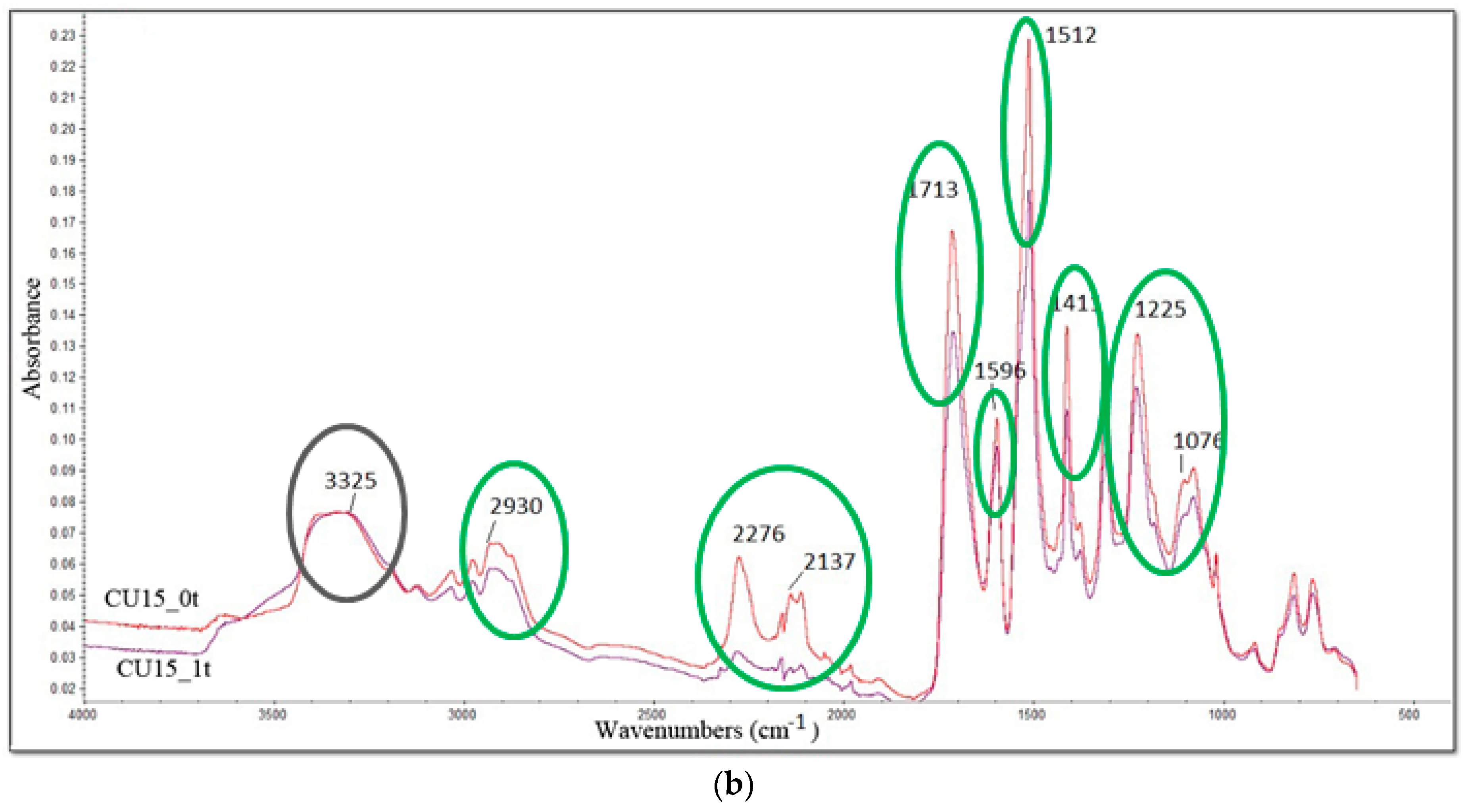
3.6. FTIR Analysis
3.7. Density (d), Water Absorption (WA), Absorbability (A), Softening Point (SP), Brittleness (B), and Content of Closed Cells (CC)
3.8. Flame Properties
3.9. Differential Scanning Calorimetry
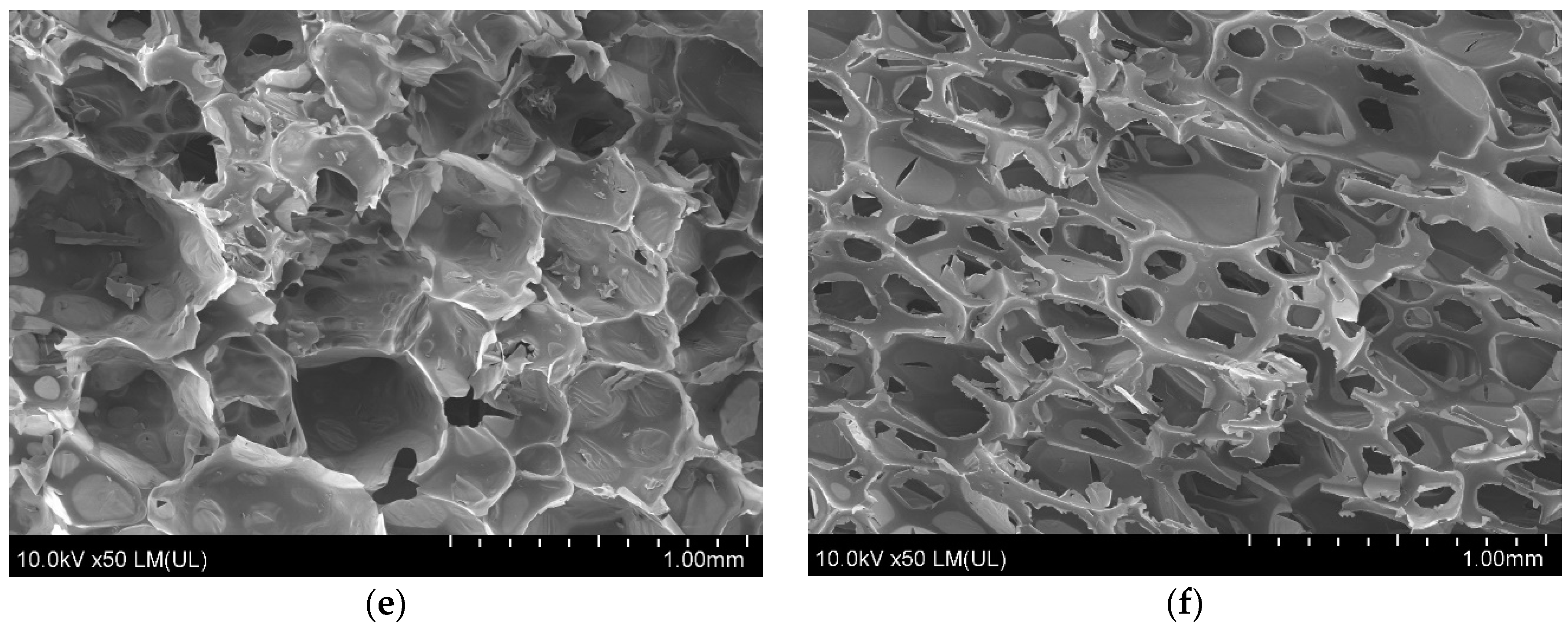
3.10. RPU/PIR Foam Structure
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Czupryński, B. Issues in Chemistry and Technology of Polyurethanes; WN AB: Bydgoszcz, Poland, 2004. [Google Scholar]
- Żabski, L. PIR foams—A new type of insulation: Rigid polyurethane foam. Mater. Bud. 2005, 1, 46–47. [Google Scholar]
- Żuchowska, D. Construction Polymers; WNT: Warsaw, Poland, 2000. [Google Scholar]
- Randall, D.; Lee, S. (Eds.) The Polyurethanes Book; Wiley LTD.: New York, NY, USA, 2002. [Google Scholar]
- Prociak, A.; Pielichowski, J. Characteristics of rigid polyurethane boilings in the aspect of new porophors application. Czas. Tech. 1998, R95, 26–52. [Google Scholar]
- Polyurethane Foam Composition. Available online: https://patents.google.com/patent/US6894083B2/en (accessed on 26 June 2019).
- Paciorek-Sadowska, J.; Borowicz, M.; Czupryński, B.; Tomaszewska, E.; Liszkowska, J. Oenothera biennis seed oil as an alternative raw material for production of bio-polyol for rigid polyurethane-polyisocyanurate foams. Ind. Crop. Prod. 2018, 126, 208–217. [Google Scholar] [CrossRef]
- Paciorek-Sadowska, J.; Borowicz, M.; Czupryński, B.; Isbrandt, M. Effect of Evening Primrose Oil-Based Polyol on the Properties of Rigid Polyurethane–Polyisocyanurate Foams for Thermal Insulation. Polymers 2018, 10, 1334. [Google Scholar] [CrossRef] [PubMed]
- Liszkowska, J.; Czupryński, B.; Paciorek-Sadowska, J.; Moraczewski, K. The effect of UV radiation on the properties of PUR-PIR foam. Adv. Polym. Technol. 2015, 36, 21631–21639. [Google Scholar] [CrossRef]
- Agrawal, A.; Kaura, R.; Walia, R.S. PU foam derived from renewable sources: Perspective on properties enhancement: An overview. Eur. Polym. J. 2017, 95, 255–274. [Google Scholar] [CrossRef]
- Soares, B.; Gama, N.; Freire, C.S.R.; Timmons, A.B.; Brandão, I.S.; Neto, C.P.; Ferreira, A. Spent coffee grounds as a renewable source for ecopolyols production. J. Chem. Technol. Biotechnol. 2015, 90, 1480–1488. [Google Scholar] [CrossRef]
- Levchik, S.V.; Weil, E.D. Thermal decomposition, combustion and fire-retardancy of polyurethanes-a review of the recent literature. Polym. Int. 2004, 53, 1585–1610. [Google Scholar] [CrossRef]
- Gębura, A.; Kowalska, D.; Tokarski, T. Research on Accelerated Aging of Electric Wires. Available online: http://www.academia.edu/17704721/Badania_przyśpieszonego_starzenia_przewodów_elektrycznych (accessed on 1 December 2018).
- Kolbiński, K.; Słowikowski, J. Materials Electricial; WNT: Warszawa, Poland, 1988. [Google Scholar]
- Broniewski, T.; Kapko, J.; Płaczek, W.; Thomalla, J. Test Methods and Evaluation of Plastic Properties; WNT: Warsaw, Poland, 2000. [Google Scholar]
- Szumniak, J.; Smoczyński, Z.; Szcześniak, K. Armament and Military Equipment Polymer Composite Ageing; Zeszyty Naukowe WSOWL: Wrocław, Poland, 2011; Volume 1, pp. 270–285. [Google Scholar]
- Prociak, A.; Rokicki, G.; Ryszkowska, J. Polyurethane Materials; WN PWN: Warsaw, Poland, 2014. [Google Scholar]
- Bajda, M.; Pogoda, M. Study of the effect of conveyor belt accelerated heat aging on its strength. Transport 2016, 1, 16–22. [Google Scholar]
- Liszkowska, J. Citric acid used in the production of polyols for rigid PUR-PIR foams. Polym. Bull. 2017, 74, 283–305. [Google Scholar] [CrossRef][Green Version]
- Rytlewski, P.; Malinowski, R.; Moraczewski, K.; Żenkiewicz, M. Influence of some crosslinking agents on thermal and mechanical properties of electron beam irradiated polylactide. Radiat. Phys. Chem. 2010, 79, 1052–1057. [Google Scholar] [CrossRef]
- Gramza, A.; Korczak, J.; Amarowicz, R. Tea polyphenols—Their antioxidant properties and biological activity. Pol. J. Food Nutr. Sci. A Rev. 2005, 55, 219–235. [Google Scholar]
- Lee, L.W.; Cheong, M.W.; Curran, F.; Bin, Y.; Shao, Q.L. Coffee fermentation and flavor—An intricate and delicate relationship. Food Chem. 2015, 185, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Kudła, S. Technological aspects of phenolic antioxidants interactions with dicumyl peroxide in the processes of polyolefins’ crosslinking. Polimery 2005, 50, 43–47. [Google Scholar] [CrossRef]
- Półka, M. Analysis of the toxicity of thermal decomposition and combustion products obtained from selected epoxy materials. Res. Dev. 2010, 19, 73–78. [Google Scholar]
- Richard, C.; Thompson, R.C.; Moore, C.J.; Frederick, S.; vom Saal, F.S.; Swan, S.H. Plastics, the environment and human health: current consensus and future trends. Philos. Trans. R Soc. Lond. B Biol. Sci. 2009, 364, 2153–2166. [Google Scholar]
- Liszkowska, J.; Czupryński, B.; Paciorek-Sadowska, J.; Michałowski, S. Thermal and flammable properties of rigid PUR–PIR foams obtained by using new compounds based on 2-hydroxypropane-1.2.3-tricarboxylic acid. J. Cell. Plast. 2016, 52, 321–341. [Google Scholar] [CrossRef]
- Liszkowska, J. Properties of Rigid PUR-PIR Foams Obtained with the Condensation of Citric Acid with Diols and Selected Glycolysates; WU UKW: Bydgoszcz, Poland, 2016. [Google Scholar]
- Technical Card of Cinnamon; Agrema Sp. z o.o: Wrocław, Poland.
- Wirpsza, Z. Poliuretanes. Chemistry, Technology, Application; WNT: Warsaw, Poland, 1991. [Google Scholar]
- Liszkowska, J.; Czupryński, B.; Paciorek-Sadowska, J. Thermal properties of polyurethane-polyisocyanurate (PUR-PIR) foams modified with tris(5-hydroxypentyl)-2-hydroxypropane-1,2,3-tricarboxylate. J. Adv. Chem. Eng. 2016, 6, 148–154. [Google Scholar] [CrossRef]
- Czupryński, B.; Liszkowska, J.; Paciorek-Sadowska, J. Modification of rigid polyurethane-polyisocyanurate foam selected powder fillers. Polimery 2008, 3, 48–52. [Google Scholar]
- Mańczak, K. Experiment Planning Technique; WNT: Warszawa, Poland, 1976. [Google Scholar]
- ASTM Standard D7487–13e1. Standard Practice for Polyurethane Raw Materials: Polyurethane Foam Cup Test; ASTM International: West Conshohocken, PA, USA, 2016. [Google Scholar] [CrossRef]
- Operating Instructions for the Climate Chamber Model CCK-40/300 NG, Es-tor.
- Szczepaniak, W. Instrumental Methods in Chemical Analysis; PWN: Warsaw, Poland, 2011. [Google Scholar]
- Prociak, A. High-Tech Insulation Polyurethane Materials; WU PK: Cracow, Poland, 2008. [Google Scholar]
- Paciorek-Sadowska, J.; Borowicz, M.; Czupryński, B.; Liszkowska, J.; Tomaszewska, E. Application of halloysite as filler in the production of rigid PUR-PIR foams. Polimery 2018, 63, 185–190. [Google Scholar] [CrossRef]
- Gama, N.V.; Silva, R.; Mohseni, F.; Davarpanah, A.; Amaral, V.S.; Ferreira, A.; Barros-Timmons, A. Enhancement of physical and reaction to fire properties of crude glicerol polyurethane foams filled with expanded graphite. Polym. Test. 2018, 69, 199–207. [Google Scholar] [CrossRef]
- Paciorek-Sadowska, J.; Borowicz, M.; Czupryński, B.; Liszkowska, J. Composites of rigid polyurethane-polyisocyanurate foams with oak bark. Polimery 2017, 62, 666–672. [Google Scholar] [CrossRef]
- Rojek, M. Methodology of Diagnostic Tests of Layered Composite Materials with a Polymer Matrix (in Polish). Open Access Libr. 2011, 2, 11–31. [Google Scholar]
- Pączkowski, J. Photochemistry of Polymers. Theory and Applications; WU UMK: Toruń, Poland, 2003. [Google Scholar]
- Czaplińska, M.; Czepas, J.; Gwoździński, K. Structure, antioxidant and anti-cancer properties flavonoid bike. Adv. Biochem. 2012, 58, 235–244. [Google Scholar]
- Yi-Fang, C. (Ed.) Coffee: Emerging Health Effects and Disease Prevention; John Wiley & Sons, Inc.: New York, NY, USA; Blackwell Publishing Ltd.: Oxford, UK, 2012. [Google Scholar]
- Gawlik-Dziki, U. Phenolacids as bioactive food ingredients. Food Sci. Technol. Qual. 2004, 4, 29–40. [Google Scholar]
- Liszkowska, J. The effect of ground coffee on the mechanical and application properties of rigid polyurethane-polyisocyanurate foams. Polimery 2018, 63, 305–310. [Google Scholar] [CrossRef]
- Liszkowska, J.; Paciorek-Sadowska, J.; Czupryński, B. Temperature stability and thermal properties of polyurethane-polyisocyanurate foams obtained using products of citric acid condensation. Polimery 2018, 63, 503–514. [Google Scholar] [CrossRef]
- Czupryński, B.; Paciorek-Sadowska, J.; Liszkowska, J. Heat and thermal reistance of rigid polyurethane-polyisocyanurate foams with addition of polyethylene, polypropylene and foamed polystyrene recyclates. Inżynieria I Apar. Chem. 2008, 4, 15–16. [Google Scholar]
- Paciorek-Sadowska, J. Research on the Influence of Boric Acid and N, N–(dihydroxymethyl) urea Derivatives on the Properties of Rigid Polyurethane-Polyisocyanurate Foams; WU UKW: Bydgoszcz, Poland, 2011. (In Polish) [Google Scholar]
- Dick, C.; Dominges-Rosado, E.; Eling, B.; Liggat, J.J.; Lindsay, C.I.; Martin, S.C.; Mohammed, M.H.; Seeley, G.; Snape, C.E. The flammability the flammability of urethane-modified polyisocyanurates and its relationship to thermal degradation chemistry. Polymer 2001, 42, 913–923. [Google Scholar] [CrossRef]
- Janik, H. Progress in the studies of the supermolecular structure of segmented polyurethanes. Polimery 2010, 55, 421–430. [Google Scholar] [CrossRef]
- Thirumal, M.; Khastgir, D.; Singha, N.K.; Manjunath, B.S.; Naik, Y.P. Water blown rigid polyurethane foam. J. Appl. Polym. Sci. 2008, 8, 1810–1817. [Google Scholar] [CrossRef]
- Piszczyk, Ł.; Hejna, A.; Formela, K.; Danowska, M.; Strankowski, M. Morphology, mechanical and thermal properties of flexible polyurethane foams modified. Polimery 2014, 59, 783–791. [Google Scholar] [CrossRef]




| Compound | Content |
|---|---|
| (a) polyphenols | |
| Chlorogenic acid | together |
| 5% | |
| Flavonoids Phenol acids | |
| heavy metals | |
| lead | 0.49 ppm |
| (b) cadmium | 0.15 ppm |
| arsenic | 0.81 ppm |
| mercury | 0.09 ppm |
| chromium | 1.06 ppm |
| chloride | 0.51 ppm |
| sulphate | 3.00 ppm |
| (c) carrier of the active substance (cellulose) | about 94.9% |
| Foam | Ground Cinnamon Extract (g) | Antiblaze TCMP (g) |
|---|---|---|
| W_0t | 0.0 | 54.0 |
| WU_0t | 0.0 | 0.0 |
| C5_0t | 15.9 | 54.0 |
| C10_0t | 31.8 | 54.0 |
| C15_0t | 47.6 | 54.0 |
| CU5_0t | 15.9 | 0.0 |
| CU10_0t | 31.8 | 0.0 |
| CU15_0t | 47.6 | 0.0 |
| Foam | Cream Time (s) | Free Rise Time (s) | String Gel Time (s) | Tack Free Time (s) | Tmax (°C) |
|---|---|---|---|---|---|
| W_0t | 8 | 34 | 23 | 21 | 126 |
| WU_0t | 8 | 34 | 23 | 21 | 125 |
| C5_0t | 10 | 46 | 28 | 24 | 151 |
| C10_0t | 10 | 48 | 30 | 25 | 146 |
| C15_0t | 10 | 54 | 30 | 26 | 148 |
| CU5_0t | 9 | 36 | 25 | 24 | 144 |
| CU10_0t | 9 | 40 | 26 | 25 | 144 |
| CU15_0t | 10 | 44 | 33 | 27 | 142 |
| Foam | L | a | b | ∆E |
|---|---|---|---|---|
| W_0t | 82.8 | −2.6 | 28.0 | 87.5 |
| C5_0t | 85.1 | 0.5 | 14.9 | 86.4 |
| C15_0t | 76.6 | 3.4 | 15.8 | 78.3 |
| WU_0t | 81.8 | −3.6 | 27.0 | 86.2 |
| CU10_0t | 71.0 | 2.7 | 15.9 | 72.8 |
| W_48h | 71.4 | 8.4 | 27.9 | 77.1 |
| C5_48h | 70.7 | 6.8 | 27.3 | 76.1 |
| WU_48h | 58.7 | 15.8 | 35.1 | 70.2 |
| CU5_48h | 62.6 | 5.9 | 23.1 | 67.0 |
| CU15_48h | 65.2 | 5.6 | 19.1 | 68.1 |
| W_1t | 56.4 | 18.2 | 17.3 | 61.8 |
| C5_1t | 57.3 | 17.4 | 16.1 | 60.1 |
| C15_1t | 50.8 | 21.6 | 29.2 | 62.4 |
| WU1_t | 56.3 | 16.0 | 21.3 | 62.3 |
| CU5_1t | 53.4 | 16.7 | 21.7 | 60.0 |
| CU15_1t | 57.1 | 13.7 | 18.1 | 61.4 |
| Foam | ∆l (%) | ∆V (%) | ∆m (%) |
|---|---|---|---|
| W | +0.39 ± 0.00 | −1.07 ± 0.01 | 2.27 ± 0.01 |
| C5 | +0.00 ± 0.00 | −1.37 ± 0.01 | 2.09 ± 0.01 |
| C10 | +0.80 ± 0.00 | −1.94 ± 0.01 | 2.35 ± 0.01 |
| C15 | +0.99 ± 0.00 | −2.07 ± 0.01 | 2.91 ± 0.01 |
| WU | +0.21 ± 0.00 | −1.89 ± 0.01 | 2.40 ± 0.01 |
| CU5 | +0.50 ± 0.00 | −2.28 ± 0.01 | 2.31 ± 0.01 |
| CU10 | +0.95 ± 0.00 | −2.41 ± 0.01 | 1.70 ± 0.01 |
| CU15 | +0.85 ± 0.00 | −2.85 ± 0.01 | 1.42 ± 0.01 |
| Foam | CS_0t (kPa) | CS_1t (kPa) | CV (%) |
|---|---|---|---|
| W | 251.6 ± 6 | 140.5 ± 4 | 74.7 ± 2 |
| WU | 361.4 ± 9 | 256.2 ± 6 | 70.9 ± 2 |
| C5 | 212.7 ± 6 | 200.1 ± 5 | 94.1 ± 2 |
| C10 | 160.4 ± 4 | 143.9 ± 4 | 89.7 ± 2 |
| C15 | 147.3 ± 3 | 126.6 ± 4 | 86.0 ± 2 |
| CU5 | 121.4 ± 3 | 151.1 ± 4 | 124.4 ± 3 |
| CU10 | 109.4 ± 2 | 132.8 ± 4 | 121.4 ± 3 |
| CU15 | 91.9 ± 2 | 109.6 ± 3 | 119.2 ± 3 |
| Band, cm−1 | Bond |
|---|---|
| 3325 | N–H |
| 2930 | C-H |
| 2276 | –N=C=O |
| 2137 | –N=C=N– |
| 1713 | –C=O in urethane bond |
| 1596 | N–H |
| 1512 | N–H |
| 1411 | Isocyanurate ring |
| 1225 | C=N in trimer |
| 1076 | C–O |
| Foam | A (%) | WA (%) | SP (°C) | B (%) | d (kg/m3) | CC (%) |
|---|---|---|---|---|---|---|
| W | 12.33 ± 0.37 | 5.15 ± 0.05 | 184 ± 5 | 13.27 ± 0.01 | 39.67 ± 0.03 | 83.2 ± 0.2 |
| C5 | 13.41 ± 0.40 | 5.67 ± 0.06 | 189 ± 6 | 14.34 ± 0.01 | 39.60 ± 0.03 | 74.3 ± 0.2 |
| C10 | 22.34 ± 0.67 | 6.14 ± 0.06 | 181 ± 5 | 13.04 ± 0.01 | 35.72 ± 0.02 | 39.1 ± 0.1 |
| C15 | 36.05 ± 1.08 | 9.64 ± 0.10 | 166 ± 5 | 11.93 ± 0.01 | 33.97 ± 0.02 | 5.7 ± 0.0 |
| WU | 8.14 ± 0.04 | 3.12 ± 0.01 | 204 ± 6 | 16.42 ± 0.01 | 49.60 ± 0.03 | 89.5 ± 0.3 |
| CU5 | 23.22 ± 0.69 | 3.78 ± 0.01 | 185 ± 6 | 45.60 ± 0.31 | 30.94 ± 0.02 | 17.4 ± 0.5 |
| CU10 | 18.04 ± 0.54 | 4.02 ± 0.01 | 179 ± 6 | 44.22 ± 0.31 | 29.19 ± 0.02 | 23.4 ± 0.7 |
| CU15 | 17.14 ± 0.51 | 4.99 ± 0.02 | 172 ± 6 | 42.13 ± 0.29 | 29.43 ± 0.02 | 28.1 ± 0.8 |
| Foam | CR (%) | LOI (%vol. of O2) | HT |
|---|---|---|---|
| W | 83.4 ± 1.6 | 24.7 ± 0.2 | self-extinguishing |
| C5 | 91.2 ± 1.8 | 24.4 ± 0.2 | |
| C10 | 91.6 ± 1.8 | 24.6 ± 0.2 | |
| C15 | 91.7 ± 1.8 | 23.7 ± 0.2 | |
| WU | 37.7 ± 0.8 | 18.5 ± 0.1 | |
| CU5 | 650 ± 0.9 | 21.0 ± 0.2 | |
| CU10 | 63.3 ± 0.9 | 20.3 ± 0.1 | |
| CU15 | 63.3 ± 0.9 | 20.2 ± 0.1 |
| Foam | Peak P1 | Peak P2 | Peak P3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tonset, (°C) | Tmax1 (°C) | Tk1 (°C) | H1 (J/g) | Tonset2 (°C) | Tmax2 (°C) | Tk2 (°C) | H2 (J/g) | Tonset3 (°C) | Tmax3 (°C) | Tk3 (°C) | H3 (J/g) | |
| W_0t | 21.7 | 71.8 | 146.6 | 25.8 | 278.6 | 294.8 | 325.3 | 44.9 | 326.3 | 330.4 | 347.3 | 5.1 |
| C5_0t | 44.6 | 82.3 | 131.4 | 26.5 | 266.0 | 285.9 | 316.6 | 28.0 | 330.2 | 335.9 | 351.6 | 4.6 |
| C10_0t | 46.7 | 86.4 | 139.4 | 33.4 | 261.9 | 274.5 | 319.1 | 35.6 | 324.0 | 329.8 | 352.7 | 6.1 |
| C15_0t | 46.7 | 85.6 | 140.8 | 36.5 | 262.6 | 271.0 | 314.4 | 30.8 | 327.1 | 333.2 | 357.7 | 8.9 |
| W_1t | 54.7 | 91.3 | 179.8 | 41.8 | 271.5 | 289.0 | 314.4 | 19.0 | 318.2 | 323.6 | 342.2 | 4.7 |
| C5_1t | 39.7 | 93.9 | 133.9 | 47.5 | 259.7 | 285.3 | 313.0 | 13.8 | 331.2 | 337.5 | 358.8 | 5.2 |
| C10_1t | 40.1 | 94.0 | 140.0 | 50.0 | 249.7 | 271.2 | 310.4 | 15.5 | 329.6 | 334.1 | 342.5 | 7.1 |
| C15_1t | 47.4 | 94.4 | 142.2 | 53.0 | 244.8 | 267.3 | 305.8 | 17.8 | 323.0 | 333.4 | 363.2 | 11.1 |
| Foam | Peak P1 | Peak P2 | Peak P3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tonset1 (°C) | Tmax1 (°C) | Tk1 (°C) | H1 (J/g) | Tonset2 (°C) | Tmax2 (°C) | Tk2 (°C) | H2 (J/g) | Tonset3 (°C) | Tmax3 (°C) | Tk3 (°C) | H3 (J/g) | |
| WU_0t s | 29.6 | 78.1 | 129.6 | 33.1 | 227.8 | 325.6 | 354.0 | 76.6 | 354.0 | 374.7 | 391.6 | 5.71 |
| CU1_0t | 47.6 | 81.2 | 121.8 | 14.2 | 213.5 | 261.3 | 322.0 | 93.7 | 327.9 | 356.7 | 392.3 | 37.1 |
| CU5_0t | 37.1 | 82.5 | 129.6 | 27.4 | 219.3 | 279.6 | 315.3 | 136.7 | 315.3 | 348.7 | 394.2 | 46.1 |
| CU15_0t | 35.6 | 84.1 | 138.0 | 55.0 | 221.3 | 282.3 | 320.0 | 116.0 | 307.1 | 336.2 | 395.1 | 10.1 |
| WU_1t | 54.7 | 91.1 | 179.8 | 41.6 | 271.5 | 289.0 | 314.4 | 19.0 | 318.2 | 323.6 | 342.2 | 4.4 |
| CU1_1t | 37.8 | 82.8 | 136.1 | 49.6 | 221.3 | 308.1 | 326.9 | 147.6 | 326.9 | 352.0 | 391.6 | 36.8 |
| CU5_1t | 35.6 | 84.1 | 141.4 | 54.9 | 218.6 | 287.3 | 320.0 | 146.0 | 320.0 | 346.3 | 386.2 | 20.6 |
| CU15_1t | 38.3 | 91.0 | 150.9 | 82.9 | 279.9 | 325.2 | 355.8 | 49.2 | 354.7 | 368.7 | 390.5 | 4.0 |
| Foam Symbol | Cell/Hole Height (µm) | Cell/Hole Width (µm) | Anisotropy Coefficient | Cell/Hole Surface Area (mm2) | Content of Cell/Hole per Area Unit (cell/mm2) |
|---|---|---|---|---|---|
| W_0t | 606.6 ± 2 | 540.7 ± 2 | 1.12 ± 0.00 | 0.258 ± 0.001 | 3.88 ± 0.01 |
| C5_0t | 399.0 ± 1 | 326.4 ± 1 | 1.22 ± 0.00 | 0.102 ± 0.001 | 9.80 ± 0.01 |
| C15_0t | 325.3 ± 1 | 322.4 ± 1 | 1.01 ± 0.00 | 0.084 ± 0.001 | 11.92 ± 0.03 |
| W_1t | 367.7 ± 1 | 340.9 ± 1 | 1.08 ± 0.00 | 0.098 ± 0.001 | 10.20 ± 0.03 |
| C5_1t | 359.7 ± 2 | 348.1 ± 1 | 1.05 ± 0.00 | 0.085 ± 0.001 | 11.77 ± 0.03 |
| C15_1t | 260.4 ± 2 | 249.0 ± 2 | 1.04 ± 0.00 | 0.051 ± 0.001 | 19.69 ± 0.04 |
| CU5_0t | 598.4 ± 2 | 613.0 ± 2 | 0.98 ± 0.00 | 0.288 ± 0.001 | 3.47 ± 0.01 |
| CU15_0t | 563.8 ± 2 | 558.2 ± 2 | 0.97 ± 0.00 | 0.255 ± 0.001 | 3.52 ± 0.01 |
| CU5_1t | 981.1 ± 2 | 978.2 ± 2 | 1.00 ± 0.00 | 0.754 ± 0.001 | 1.33 ± 0.00 |
| CU15_1t | 980.3 ± 2 | 916.4 ± 2 | 1.06 ± 0.00 | 0.705 ± 0.001 | 1.42 ± 0.00 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liszkowska, J.; Moraczewski, K.; Borowicz, M.; Paciorek-Sadowska, J.; Czupryński, B.; Isbrandt, M. The Effect of Accelerated Aging Conditions on the Properties of Rigid Polyurethane-Polyisocyanurate Foams Modified by Cinnamon Extract. Appl. Sci. 2019, 9, 2663. https://doi.org/10.3390/app9132663
Liszkowska J, Moraczewski K, Borowicz M, Paciorek-Sadowska J, Czupryński B, Isbrandt M. The Effect of Accelerated Aging Conditions on the Properties of Rigid Polyurethane-Polyisocyanurate Foams Modified by Cinnamon Extract. Applied Sciences. 2019; 9(13):2663. https://doi.org/10.3390/app9132663
Chicago/Turabian StyleLiszkowska, Joanna, Krzysztof Moraczewski, Marcin Borowicz, Joanna Paciorek-Sadowska, Bogusław Czupryński, and Marek Isbrandt. 2019. "The Effect of Accelerated Aging Conditions on the Properties of Rigid Polyurethane-Polyisocyanurate Foams Modified by Cinnamon Extract" Applied Sciences 9, no. 13: 2663. https://doi.org/10.3390/app9132663
APA StyleLiszkowska, J., Moraczewski, K., Borowicz, M., Paciorek-Sadowska, J., Czupryński, B., & Isbrandt, M. (2019). The Effect of Accelerated Aging Conditions on the Properties of Rigid Polyurethane-Polyisocyanurate Foams Modified by Cinnamon Extract. Applied Sciences, 9(13), 2663. https://doi.org/10.3390/app9132663