Activation of General Control Nonderepressible-2 Kinase Ameliorates Glucotoxicity in Human Peritoneal Mesothelial Cells, Preserves Their Integrity, and Prevents Mesothelial to Mesenchymal Transition
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture Conditions
2.2. Assessment of Halofuginone and Tryptophanol Cytotoxicity
2.3. Assessment of the Proteins of Interest
2.4. Assessment of D-Glucose Consumption and ROS Production
2.5. Assessment of GAPDH and PKC Activity
2.6. Assessment of D-Sorbitol and MGO Cellular Content
2.7. Assessment of TGF-β1 and IL-8 Production
2.8. Statistical Analysis
3. Results
3.1. Both Halofuginone and Tryptophanol, at Nontoxic Concentrations, Activate GCN2 Kinase
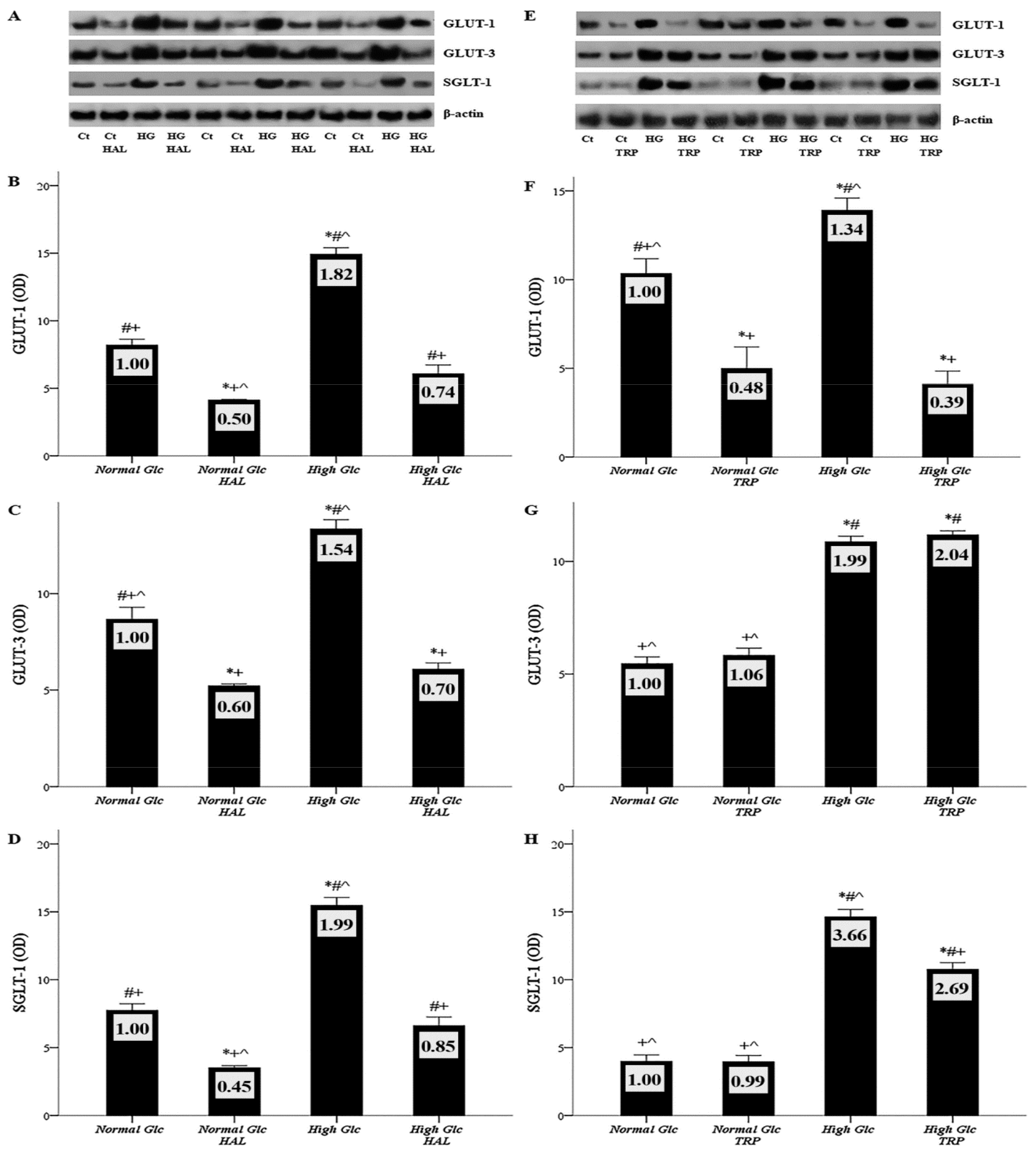
3.2. In Mesothelial Cells Cultured under High-Glucose Conditions, Halofuginone Reduces the Degree of GLUT-1, GLUT-3 and SGLT-1 Increment, and Tryptophanol Exerts a Similar Effect with the Exception of GLUT-3
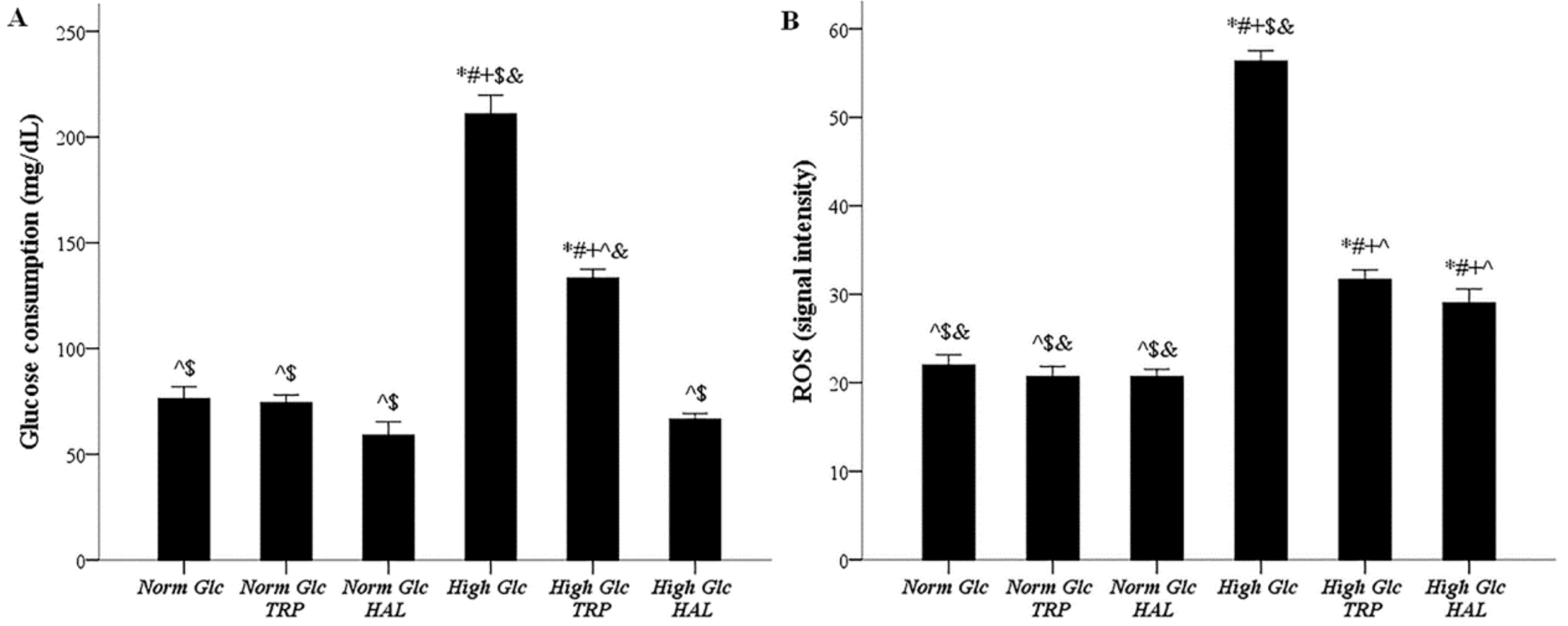
3.3. In Mesothelial Cells Cultured under High-Glucose Conditions, Both Tryptophanol and Halofuginone Decrease the Degree of Enhanced Glucose Consumption and ROS Production
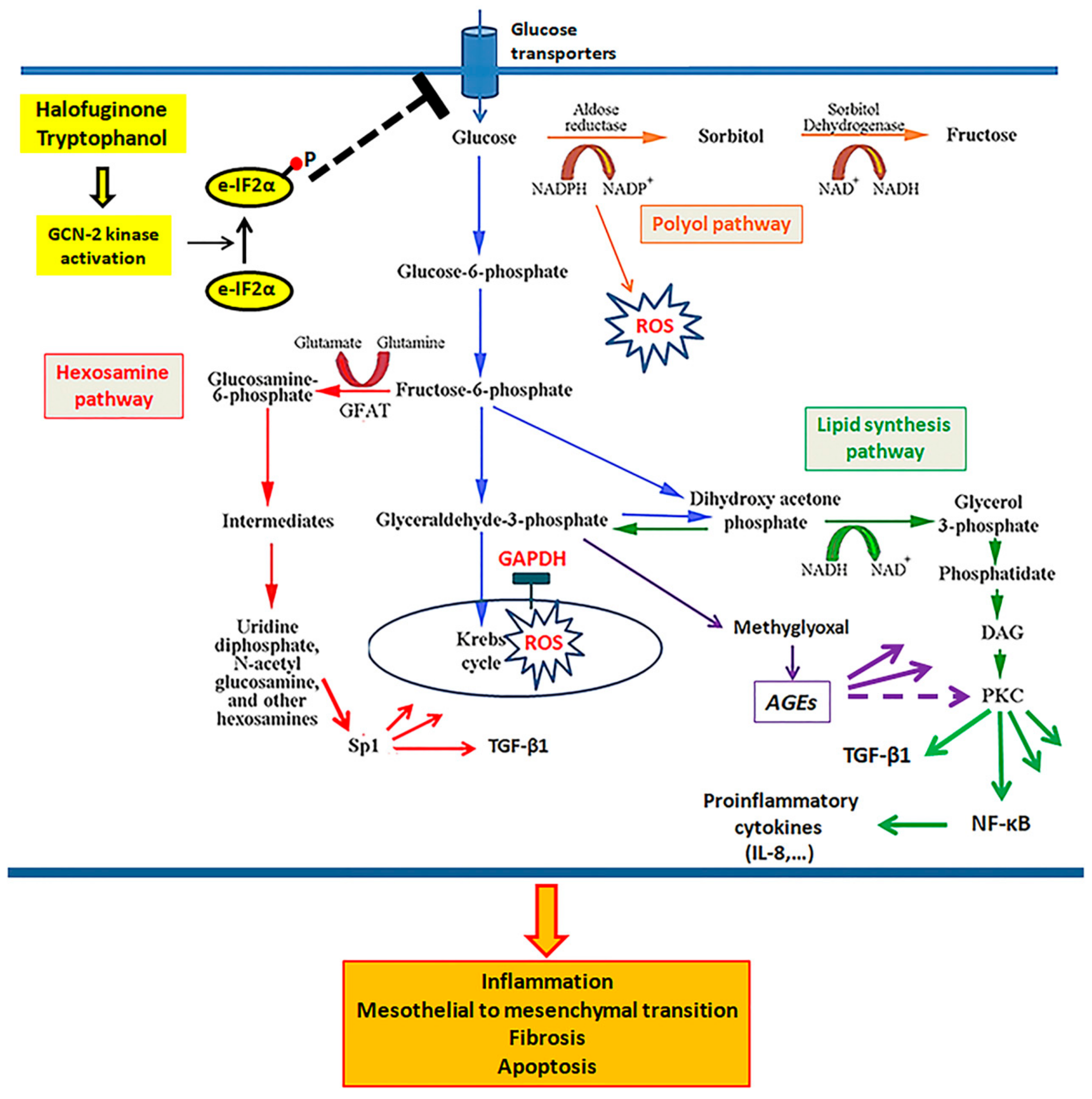
3.4. In Mesothelial Cells Cultured under High-Glucose Conditions, Both Tryptophanol and Halofuginone Prevent GAPDH Inhibition and Ameliorate the High-Glucose-Induced Noxious Pathways
3.5. In Mesothelial Cells Cultured under High-Glucose Conditions, Both Tryptophanol and Halofuginone Reduce the Degree of the TGF-β1 and IL-8 Increment
3.6. Both Tryptophanol and Halofuginone Prevent High-Glucose-Induced Cellular Apoptosis and the Mesothelial to Mesenchymal Transition
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Wong, B.; Ravani, P.; Oliver, M.J.; Holroyd-Leduc, J.; Venturato, L.; Garg, A.X.; Quinn, R.R. Comparison of Patient Survival Between Hemodialysis and Peritoneal Dialysis Among Patients Eligible for Both Modalities. Am. J. Kidney Dis. 2018, 71, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, Y.; Hasegawa, T.; Nakayama, M.; Kubo, H.; Shigematu, T. Issues affecting the longevity of the continuous peritoneal dialysis therapy. Kidney Int. Suppl. 1997, 62, S105–S107. [Google Scholar] [PubMed]
- Jager, K.J.; Merkus, M.P.; Dekker, F.W.; Boeschoten, E.W.; Tijssen, J.G.; Stevens, P.; Bos, W.J.; Krediet, R.T. Mortality and technique failure in patients starting chronic peritoneal dialysis: Results of The Netherlands Cooperative Study on the Adequacy of Dialysis. NECOSAD Study Group. Kidney Int. 1999, 55, 1476–1485. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.J.; Bryan, J.; Phillips, L.; Russell, G.I. Longitudinal changes in peritoneal kinetics: The effects of peritoneal dialysis and peritonitis. Nephrol. Dial. Transplant. 1996, 11, 498–506. [Google Scholar] [CrossRef]
- López-Cabrera, M. Mesenchymal Conversion of Mesothelial Cells Is a Key Event in the Pathophysiology of the Peritoneum during Peritoneal Dialysis. Adv. Med. 2014, 2014, 1–17. [Google Scholar] [CrossRef]
- Strippoli, R.; Moreno-Vicente, R.; Battistelli, C.; Cicchini, C.; Noce, V.; Amicone, L.; Marchetti, A.; del Pozo, M.A.; Tripodi, M. Molecular Mechanisms Underlying Peritoneal EMT and Fibrosis. Stem Cells Int. 2016, 2016, 1–11. [Google Scholar] [CrossRef]
- Zhou, Q.; Bajo, M.A.; del Peso, G.; Yu, X.; Selgas, R. Preventing peritoneal membrane fibrosis in peritoneal dialysis patients. Kidney Int. 2016, 90, 515–524. [Google Scholar] [CrossRef]
- Radunz, V.; Pecoits-Filho, R.; Figueiredo, A.E.; Barretti, P.; de Moraes, T.P. Impact of Glucose Exposure on Outcomes of a Nation-Wide Peritoneal Dialysis Cohort–Results of the BRAZPD II Cohort. Front. Physiol. 2019, 10. [Google Scholar] [CrossRef]
- Williams, J.D.; Craig, K.J.; Topley, N.; Von Ruhland, C.; Fallon, M.; Newman, G.R.; Mackenzie, R.K.; Williams, G.T.; Peritoneal Biopsy Study, G. Morphologic changes in the peritoneal membrane of patients with renal disease. J. Am. Soc. Nephrol. 2002, 13, 470–479. [Google Scholar]
- Williams, J.D.; Craig, K.J.; Topley, N.; Williams, G.T. Peritoneal dialysis: Changes to the structure of the peritoneal membrane and potential for biocompatible solutions. Kidney Int. Suppl. 2003, S158–S161. [Google Scholar] [CrossRef]
- Aroeira, L.S.; Aguilera, A.; Selgas, R.; Ramirez-Huesca, M.; Perez-Lozano, M.L.; Cirugeda, A.; Bajo, M.A.; del Peso, G.; Sanchez-Tomero, J.A.; Jimenez-Heffernan, J.A.; et al. Mesenchymal conversion of mesothelial cells as a mechanism responsible for high solute transport rate in peritoneal dialysis: Role of vascular endothelial growth factor. Am. J. Kidney Dis. 2005, 46, 938–948. [Google Scholar] [CrossRef]
- Perez-Lozano, M.L.; Sandoval, P.; Rynne-Vidal, A.; Aguilera, A.; Jimenez-Heffernan, J.A.; Albar-Vizcaino, P.; Majano, P.L.; Sanchez-Tomero, J.A.; Selgas, R.; Lopez-Cabrera, M. Functional relevance of the switch of VEGF receptors/co-receptors during peritoneal dialysis-induced mesothelial to mesenchymal transition. PLoS ONE 2013, 8, e60776. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, B.; Bartosova, M.; Macher-Goeppinger, S.; Sallay, P.; Vörös, P.; Ranchin, B.; Vondrak, K.; Ariceta, G.; Zaloszyc, A.; Bayazit, A.K.; et al. Neutral pH and low–glucose degradation product dialysis fluids induce major early alterations of the peritoneal membrane in children on peritoneal dialysis. Kidney Int. 2018, 94, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Bartosova, M.; Schaefer, B.; Vondrak, K.; Sallay, P.; Taylan, C.; Cerkauskiene, R.; Dzierzega, M.; Milosevski-Lomic, G.; Büscher, R.; Zaloszyc, A.; et al. Peritoneal Dialysis Vintage and Glucose Exposure but Not Peritonitis Episodes Drive Peritoneal Membrane Transformation During the First Years of PD. Front. Physiol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Szeto, C.C.; Johnson, D.W. Low GDP Solution and Glucose-Sparing Strategies for Peritoneal Dialysis. Semin. Nephrol. 2017, 37, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.J.; Brown, E.A.; Frandsen, N.E.; Rodrigues, A.S.; Rodriguez-Carmona, A.; Vychytil, A.; Macnamara, E.; Ekstrand, A.; Tranaeus, A.; Filho, J.C.; et al. Longitudinal membrane function in functionally anuric patients treated with APD: Data from EAPOS on the effects of glucose and icodextrin prescription. Kidney Int. 2005, 67, 1609–1615. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Johnson, D.W.; Badve, S.; Craig, J.C.; Strippoli, G.F.K.; Wiggins, K.J. Impact of icodextrin on clinical outcomes in peritoneal dialysis: A systematic review of randomized controlled trials. Nephrol. Dial. Transplant. 2013, 28, 1899–1907. [Google Scholar] [CrossRef]
- Moriishi, M.; Kawanishi, H. Icodextrin and intraperitoneal inflammation. Perit. Dial. Int. 2008, 28 (Suppl. S3), S96–S100. [Google Scholar]
- Tjiong, H.L.; Rietveld, T.; Wattimena, J.L.; van den Berg, J.W.; Kahriman, D.; van der Steen, J.; Hop, W.C.; Swart, R.; Fieren, M.W. Peritoneal Dialysis with Solutions Containing Amino Acids Plus Glucose Promotes Protein Synthesis during Oral Feeding. Clin. J. Am. Soc. Nephrol. 2007, 2, 74–80. [Google Scholar] [CrossRef]
- Bartosova, M.; Schmitt, C.P. Biocompatible Peritoneal Dialysis: The Target Is Still Way Off. Front. Physiol. 2019, 9. [Google Scholar] [CrossRef]
- Eleftheriadis, T.; Pissas, G.; Yiannaki, E.; Markala, D.; Arampatzis, S.; Antoniadi, G.; Liakopoulos, V.; Stefanidis, I. Inhibition of indoleamine 2,3-dioxygenase in mixed lymphocyte reaction affects glucose influx and enzymes involved in aerobic glycolysis and glutaminolysis in alloreactive T-cells. Hum. Immunol. 2013, 74, 1501–1509. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriadis, T.; Tsogka, K.; Pissas, G.; Antoniadi, G.; Liakopoulos, V.; Stefanidis, I. Activation of general control nonderepressible 2 kinase protects human glomerular endothelial cells from harmful high-glucose-induced molecular pathways. Int. Urol. Nephrol. 2016, 48, 1731–1739. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Matsumura, T.; Edelstein, D.; Rossetti, L.; Zsengellér, Z.; Szabó, C.; Brownlee, M. Inhibition of GAPDH activity by poly(ADP-ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cells. J. Clin. Investig. 2003, 112, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, T.; Edelstein, D.; Du, X.L.; Yamagishi, S.-I.; Matsumura, T.; Kaneda, Y.; Yorek, M.A.; Beebe, D.; Oates, P.J.; Hammes, H.-P.; et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 2000, 404, 787–790. [Google Scholar] [CrossRef] [PubMed]
- Giacco, F.; Du, X.; D’Agati, V.D.; Milne, R.; Sui, G.; Geoffrion, M.; Brownlee, M. Knockdown of Glyoxalase 1 Mimics Diabetic Nephropathy in Nondiabetic Mice. Diabetes 2013, 63, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Wells, L.; Hart, G.W. O-GlcNAc turns twenty: Functional implications for post-translational modification of nuclear and cytosolic proteins with a sugar. FEBS Lett. 2003, 546, 154–158. [Google Scholar] [CrossRef]
- Brownlee, M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef]
- Giacco, F.; Brownlee, M.; Schmidt, A.M. Oxidative Stress and Diabetic Complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef]
- Fischereder, M.; Schroppel, B.; Wiese, P.; Fink, M.; Banas, B.; Schmidbauer, S.; Schlondorff, D. Regulation of glucose transporters in human peritoneal mesothelial cells. J. Nephrol. 2003, 16, 103–109. [Google Scholar]
- Schroppel, B.; Fischereder, M.; Wiese, P.; Segerer, S.; Huber, S.; Kretzler, M.; Heiss, P.; Sitter, T.; Schlondorff, D. Expression of glucose transporters in human peritoneal mesothelial cells. Kidney Int. 1998, 53, 1278–1287. [Google Scholar] [CrossRef]
- Pines, M.; Spector, I. Halofuginone-the multifaceted molecule. Molecules 2015, 20, 573–594. [Google Scholar] [CrossRef] [PubMed]
- Castilho, B.A.; Shanmugam, R.; Silva, R.C.; Ramesh, R.; Himme, B.M.; Sattlegger, E. Keeping the eIF2 alpha kinase Gcn2 in check. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2014, 1843, 1948–1968. [Google Scholar] [CrossRef] [PubMed]
- Lowe, G.; Tansley, G. An investigation of the mechanism of activation of tryptophan by tryptophanyl-tRNA synthetase from beef pancreas. Eur. J. Biochem. 1984, 138, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Kolm-Litty, V.; Sauer, U.; Nerlich, A.; Lehmann, R.; Schleicher, E.D. High glucose-induced transforming growth factor beta1 production is mediated by the hexosamine pathway in porcine glomerular mesangial cells. J. Clin. Investig. 1998, 101, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Du, X.L.; Edelstein, D.; Rossetti, L.; Fantus, I.G.; Goldberg, H.; Ziyadeh, F.; Wu, J.; Brownlee, M. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc. Natl. Acad. Sci. USA 2000, 97, 12222–12226. [Google Scholar] [CrossRef]
- Moynagh, P.N. The NF-kappaB pathway. J. Cell Sci. 2005, 118, 4589–4592. [Google Scholar] [CrossRef]
- Koya, D.; Jirousek, M.R.; Lin, Y.W.; Ishii, H.; Kuboki, K.; King, G.L. Characterization of protein kinase C beta isoform activation on the gene expression of transforming growth factor-beta, extracellular matrix components, and prostanoids in the glomeruli of diabetic rats. J. Clin. Investig. 1997, 100, 115–126. [Google Scholar] [CrossRef]
- Suryavanshi, S.V.; Kulkarni, Y.A. NF-κβ: A Potential Target in the Management of Vascular Complications of Diabetes. Front. Pharmacol. 2017, 8. [Google Scholar] [CrossRef]
- Ha, H.; Yu, M.R.; Lee, H.B. High glucose–induced PKC activation mediates TGF-β1 and fibronectin synthesis by peritoneal mesothelial cells. Kidney Int. 2001, 59, 463–470. [Google Scholar] [CrossRef]
- Wang, L.; Balzer, M.S.; Rong, S.; Menne, J.; von Vietinghoff, S.; Dong, L.; Gueler, F.; Jang, M.-S.; Xu, G.; Timrott, K.; et al. Protein kinase C α inhibition prevents peritoneal damage in a mouse model of chronic peritoneal exposure to high-glucose dialysate. Kidney Int. 2016, 89, 1253–1267. [Google Scholar] [CrossRef]
- Sanajou, D.; Ghorbani Haghjo, A.; Argani, H.; Aslani, S. AGE-RAGE axis blockade in diabetic nephropathy: Current status and future directions. Eur. J. Pharmacol. 2018, 833, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Thallas-Bonke, V.; Lindschau, C.; Rizkalla, B.; Bach, L.A.; Boner, G.; Meier, M.; Haller, H.; Cooper, M.E.; Forbes, J.M. Attenuation of extracellular matrix accumulation in diabetic nephropathy by the advanced glycation end product cross-link breaker ALT-711 via a protein kinase C-alpha-dependent pathway. Diabetes 2004, 53, 2921–2930. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Xiong, X.; Chen, Y.-G. Feedback regulation of TGF-β signaling. Acta Biochim. Biophys. Sin. 2018, 50, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.N.; Tang, S.C.; Leung, J.C. Mediators of inflammation and fibrosis. Perit. Dial. Int. 2007, 27 (Suppl. S2), S65–S71. [Google Scholar]
- Riesenhuber, A.; Kratochwill, K.; Bender, T.O.; Vargha, R.; Kasper, D.C.; Herzog, R.; Salzer, E.; Aufricht, C. Peritoneal dialysis fluid induces p38-dependent inflammation in human mesothelial cells. Perit. Dial. Int. 2010, 31, 332–339. [Google Scholar] [CrossRef]
- Fernandez-Perpen, A.; Perez-Lozano, M.L.; Bajo, M.A.; Albar-Vizcaino, P.; Correa, P.S.; del Peso, G.; Castro, M.J.; Aguilera, A.; Ossorio, M.; Peter, M.E.; et al. Influence of Bicarbonate/Low-GDP Peritoneal Dialysis Fluid (Bicavera) on In Vitro and Ex Vivo Epithelial-to-Mesenchymal Transition of Mesothelial Cells. Perit. Dial. Int. 2012, 32, 292–304. [Google Scholar] [CrossRef]
- Elliott, C.L. Nuclear factor-kappa B is essential for up-regulation of interleukin-8 expression in human amnion and cervical epithelial cells. Mol. Hum. Reprod. 2001, 7, 787–790. [Google Scholar] [CrossRef]
- Palena, C.; Hamilton, D.H.; Fernando, R.I. Influence of IL-8 on the epithelial–mesenchymal transition and the tumor microenvironment. Future Oncol. 2012, 8, 713–722. [Google Scholar] [CrossRef]
- Fadeel, B.; Orrenius, S. Apoptosis: A basic biological phenomenon with wide-ranging implications in human disease. J. Intern. Med. 2005, 258, 479–517. [Google Scholar] [CrossRef]
- Sappino, A.P.; Schurch, W.; Gabbiani, G. Differentiation repertoire of fibroblastic cells: Expression of cytoskeletal proteins as marker of phenotypic modulations. Lab. Investig. 1990, 63, 144–161. [Google Scholar]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eleftheriadis, T.; Pissas, G.; Antoniadi, G.; Nikolaou, E.; Golfinopoulos, S.; Liakopoulos, V.; Stefanidis, I. Activation of General Control Nonderepressible-2 Kinase Ameliorates Glucotoxicity in Human Peritoneal Mesothelial Cells, Preserves Their Integrity, and Prevents Mesothelial to Mesenchymal Transition. Biomolecules 2019, 9, 832. https://doi.org/10.3390/biom9120832
Eleftheriadis T, Pissas G, Antoniadi G, Nikolaou E, Golfinopoulos S, Liakopoulos V, Stefanidis I. Activation of General Control Nonderepressible-2 Kinase Ameliorates Glucotoxicity in Human Peritoneal Mesothelial Cells, Preserves Their Integrity, and Prevents Mesothelial to Mesenchymal Transition. Biomolecules. 2019; 9(12):832. https://doi.org/10.3390/biom9120832
Chicago/Turabian StyleEleftheriadis, Theodoros, Georgios Pissas, Georgia Antoniadi, Evdokia Nikolaou, Spyridon Golfinopoulos, Vassilios Liakopoulos, and Ioannis Stefanidis. 2019. "Activation of General Control Nonderepressible-2 Kinase Ameliorates Glucotoxicity in Human Peritoneal Mesothelial Cells, Preserves Their Integrity, and Prevents Mesothelial to Mesenchymal Transition" Biomolecules 9, no. 12: 832. https://doi.org/10.3390/biom9120832
APA StyleEleftheriadis, T., Pissas, G., Antoniadi, G., Nikolaou, E., Golfinopoulos, S., Liakopoulos, V., & Stefanidis, I. (2019). Activation of General Control Nonderepressible-2 Kinase Ameliorates Glucotoxicity in Human Peritoneal Mesothelial Cells, Preserves Their Integrity, and Prevents Mesothelial to Mesenchymal Transition. Biomolecules, 9(12), 832. https://doi.org/10.3390/biom9120832






