Citrus Flavones: An Update on Sources, Biological Functions, and Health Promoting Properties
Abstract
1. Introduction
2. Techniques of Identification and Quantification of Flavones in Citrus Varieties
3. Absorption Uptake and Pharmacokinetics
4. Antioxidant and Anti-Inflammatory Activity of Citrus Flavones
5. Antimicrobial and Antiviral Effect of Citrus Flavones
6. Anticancer Activity of Citrus Flavones
6.1. Apigenin
6.2. Luteolin
6.3. Diosmetin
6.4. Chrysoeriol
7. Conclusion and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- United States Department of Agriculture. Available online: https://www.fas.usda.gov/psdonline/circulars/citrus.pdf (accessed on 28 January 2020).
- Moore, G.A. Oranges and lemons: Clues to the taxonomy of Citrus from molecular markers. Trends Genet. 2001, 17, 536–540. [Google Scholar] [CrossRef]
- Velasco, R.; Licciardello, C. A genealogy of the citrus family. Nat. Biotechnol. 2014, 32, 640–642. [Google Scholar] [CrossRef] [PubMed]
- Mabberley, D.J. A classification for edible Citrus (Rutaceae). Telopea 1997, 7, 167–172. [Google Scholar] [CrossRef]
- Calabrese, F. Considerazioni sulla classificazione botanica delle Aurantioideae (Rutaceae). Webbia J. Plant Taxon. Geogr. 1973, 28, 161–187. [Google Scholar] [CrossRef]
- Wu, G.A.; Terol, J.; Ibanez, V.; López-García, A.; Pérez-Román, E.; Borredá, C.; Domingo, C.; Tadeo, F.R.; Carbonell-Caballero, J.; Alonso, R.; et al. Genomics of the origin and evolution of Citrus. Nature 2018, 554, 311–316. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietar (Poly) phenolics in Human Health: Structures, Bioavailability, and Evidence of Protective Effects Against Chronic Diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef]
- Strack, D.; Wray, V. Anthocyanins. In The Flavonoids: Advances in Research since 1986; Harborne, J.B., Ed.; Chapman and Hall: London, UK, 1992; pp. 1–22. [Google Scholar]
- Stafford, H. Flavonoid Metabolism; CRC: Boca Raton, FL, USA, 1990; pp. 1–59. [Google Scholar]
- Dudek, B.; Warskulat, A.-C.; Schneider, B. The occurrence of flavonoids and related compounds in flower sections of Papaver nudicaule. Plants 2016, 5, 28. [Google Scholar] [CrossRef]
- Tung, Y.-C.; Chou, Y.-C.; Hung, W.-L.; Cheng, A.-C.; Yu, R.-C.; Ho, C.-T.; Pan, M.-H. Polymethoxyflavones: Chemistry and Molecular Mechanisms for Cancer Prevention and Treatment. Curr. Pharmacol. Rep. 2019, 5, 98–113. [Google Scholar] [CrossRef]
- Barreca, D.; Gattuso, G.; Bellocco, E.; Calderaro, A.; Trombetta, D.; Smeriglio, A.; Laganà, G.; Daglia, M.; Meneghini, S.; Nabavi, S.M. Flavanones: Citrus phytochemical with health-promoting properties. BioFactors 2017, 43, 495–506. [Google Scholar] [CrossRef]
- Barreca, D.; Bellocco, E.; Leuzzi, U.; Gattuso, G. First evidence of C- and O-glycosyl flavone in blood orange (Citrus sinensis (L.) Osbeck) juice and their influence on antioxidant properties. Food Chem. 2014, 149C, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Barreca, D.; Gattuso, G.; Laganà, G.; Leuzzi, U.; Bellocco, E. C- and O-glycosyl flavonoids in Sanguinello and Tarocco blood orange (Citrus sinensis (L.) Osbeck) juice: Identification and influence on antioxidant properties and acetylcholinesterase activity. Food Chem. 2016, 196, 619–627. [Google Scholar] [CrossRef]
- Barreca, D.; Bellocco, E.; Caristi, C.; Leuzzi, U.; Gattuso, G. Kumquat (Fortunella japonica Swingle) juice: Flavonoid distribution and antioxidant properties. Food Res. Int. 2011, 44, 2190–2197. [Google Scholar] [CrossRef]
- Gattuso, G.; Barreca, D. Juice analysis in Citrus: Latest developments. In Advances in Citrus Nutrition; Springer Science+Business Media: Berlin/Heidelberg, Germany, 2012; pp. 89–99. [Google Scholar]
- Wang, S.; Yang, C.; Tu, H.; Zhou, J.; Liu, X.; Cheng, Y.; Luo, J.; Deng, X.; Zhang, H.; Xu, J. Characterization and Metabolic Diversity of Flavonoids in Citrus Species. Sci. Rep. 2017, 7, 10549. [Google Scholar] [CrossRef] [PubMed]
- Nogata, Y.; Sakamoto, K.; Shiratsuchi, H.; Ishii, T.; Yano, M.; Ohta, H. Flavonoid composition of fruit tissues of citrus species. Biosci. Biotechnol. Biochem. 2006, 70, 178–192. [Google Scholar] [CrossRef] [PubMed]
- Barreca, D.; Bellocco, E.; Leuzzi, U.; Gattuso, G. Flavonoid C-glycosides in Citrus Juices from Southern Italy: Distribution and Influence on the Antioxidant Activity. In Instrumental Methods for the Analysis and Identification of Bioactive Molecules; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2014; pp. 189–200. [Google Scholar]
- Barreca, D.; Bellocco, E.; Ficarra, S.; Laganà, G.; Galtieri, A.; Tellone, E.; Gattuso, G. Analysis of C-Glycosyl Flavones and 3-Hydroxy-3-methylglutaryl-glycosyl Derivatives in Blood Oranges (Citrus sinensis (L.) Osbeck) Juices and Their Influence on Biological Activity. In Advances in Plant Phenolics: From Chemistry to Human Health; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2018; pp. 67–80. [Google Scholar]
- Gattuso, G.; Barreca, D.; Gargiulli, C.; Leuzzi, U.; Caristi, C. Flavonoid Composition of Citrus Juices. Molecules 2007, 12, 1641–1673. [Google Scholar] [CrossRef]
- Ledesma-Escobar, C.A.; Priego-Capote, F.; Luque de Castro, M.D. Comparative Study of the Effect of Sample Pretreatment and Extraction on the Determination of Flavonoids from Lemon (Citrus limon). PLoS ONE 2016, 11, e0148056. [Google Scholar] [CrossRef]
- Gattuso, G.; Barreca, D.; Caristi, C.; Gargiulli, C.; Leuzzi, U. Distribution of Flavonoids and Furocoumarins in Juices from Cultivars of Citrus bergamia Risso. J. Agric. Food Chem. 2007, 55, 9921–9927. [Google Scholar] [CrossRef]
- Barreca, D.; Bellocco, E.; Caristi, C.; Leuzzi, U.; Gattuso, G. Flavonoid Composition and Antioxidant Activity of Juices from Chinotto (Citrus × myrtifolia Raf.) Fruits at Different Ripening Stages. J. Agric. Food Chem. 2010, 58, 3031–3036. [Google Scholar] [CrossRef]
- Barreca, D.; Bellocco, E.; Caristi, C.; Leuzzi, U.; Gattuso, G. Distribution of C- and O-glycosyl flavonoids, (3-hydroxy-3-methylglutaryl)glycosyl flavanones and furocoumarins in Citrus aurantium L. juice. Food Chem. 2011, 124, 576–582. [Google Scholar] [CrossRef]
- Barreca, D.; Bellocco, E.; Caristi, C.; Leuzzi, U.; Gattuso, G. Flavonoid profile and radical-scavenging activity of Mediterranean sweet lemon (Citrus limetta Risso) juice. Food Chem. 2011, 129, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Barreca, D.; Bellocco, E.; Caristi, C.; Leuzzi, U.; Gattuso, G. Elucidation of the flavonoid and furocoumarin composition and radical-scavenging activity of green and ripe chinotto (Citrus myrtifolia Raf.) fruit tissues, leaves and seeds. Food Chem. 2011, 129, 1504–1512. [Google Scholar] [CrossRef]
- Barreca, D.; Bisignano, C.; Ginestra, G.; Bisignano, G.; Bellocco, E.; Leuzzi, U.; Gattuso, G. Polymethoxylated, C- and O-glycosyl flavonoids in Tangelo (C. reticulata × C. paradisi) juice and their influence on antioxidant properties. Food Chem. 2013, 141, 1481–1488. [Google Scholar] [CrossRef] [PubMed]
- Theile, D.; Hohmann, N.; Kiemel, D.; Gattuso, G.; Barreca, D.; Mikus, G.; Haefeli, W.E.; Schwenger, V.; Weiss, J. Clementine juice has the potential for drug interactions–In vitro comparison with grapefruit and mandarin juice. Eur. J. Pharm. Sci. 2017, 97, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Smeriglio, A.; Cornara, L.; Denaro, M.; Barreca, D.; Burlando, B.; Xiao, J.; Trombetta, D. Antioxidant and cytoprotective activities of an ancient Mediterranean citrus (Citrus lumia Risso) albedo extract: Microscopic observations and polyphenol characterization. Food Chem. 2019, 279, 347–355. [Google Scholar] [CrossRef]
- Hohmann, N.; Mikus, G.; Haefeli, W.E.; Schwenger, V.; Gattuso, G.; Barreca, D.; Weiss, J. A follow-up report on potential drug interactions with clementines: Two single case experiments show no effect on CYP3A-dependent midazolam clearance. Eur. J. Pharm. Sci. 2019, 133, 54–58. [Google Scholar] [CrossRef]
- Barreca, D.; Bellocco, E.; Caristi, C.; Leuzzi, U.; Gattuso, G. Flavonoid and Antioxidant Properties of Fruits Belonging to the Annona and Citrus Genera. In Tropical and Subtropical Fruit: Flavors, Color, and Health Benefits; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2013; pp. 103–119. [Google Scholar]
- Barreca, D.; Bellocco, E.; Caristi, C.; Leuzzi, U.; Gattuso, G. Flavonoids and Furocoumarins in Bergamot, Myrtle-leaved Orange and Sour Orange Juices: Distribution and Properties. In Emerging Trends in Dietary Components for Preventing and Combating Disease; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2012; pp. 17–35. [Google Scholar]
- Barreca, D.; Bellocco, E.; Caristi, C.; Leuzzi, U.; Gattuso, G. Flavonoid distribution in neglected Citrus species grown in the Mediterranean basin. In Flavonoids: Dietary Sources, Properties and Health Benefits; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2012; pp. 491–509. [Google Scholar]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Remesy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef]
- Hostetler, G.L.; Ralston, R.A.; Schwartz, S.J. Flavones: Food Sources, Bioavailability, Metabolism, and Bioactivity. Adv. Nutr. 2017, 8, 423–435. [Google Scholar] [CrossRef]
- Wang, M.; Firrman, J.; Liu, L.; Yam, K. A Review on Flavonoid Apigenin: Dietary Intake, ADME, Antimicrobial Effects, and Interactions with Human Gut Microbiota. BioMed Res. Int. 2019, 9, 7010467. [Google Scholar] [CrossRef]
- Singh, G.; Verma, A.K.; Kumar, V. Catalytic properties, functional attributes and industrial applications of β-glucosidases. Biotech 2016, 6, 3. [Google Scholar] [CrossRef]
- Cione, E.; La Torre, C.; Cannataro, R.; Caroleo, M.C.; Plastina, P.; Gallelli, L. Quercetin, Epigallocatechin Gallate, Curcumin, and Resveratrol: From Dietary Sources to Human MicroRNA Modulation. Molecules 2019, 25, 63. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zheng, H.; Yu, J.; Zhu, L.; Yan, T.; Wu, P.; Lu, L.; Wang, Y.; Hu, M.; Liu, Z. SGLT-1 Transport and Deglycosylation inside Intestinal Cells Are Key Steps in the Absorption and Disposition of Calycosin-7-O-β-d-Glucoside in Rats. Drug Metab. Dispos. 2016, 44, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, G.B. In vitro bioavailability and cellular bioactivity studies of flavonoids and flavonoid-rich plant extracts: Questions, considerations and future perspectives. Proc. Nutr. Soc. 2017, 76, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Lam, P.Y.; Liu, H.; Lo, C. Completion of Tricin Biosynthesis Pathway in Rice: Cytochrome P450 75B4 Is a Unique Chrysoeriol 5’-Hydroxylase. Plant Physiol. 2015, 168, 1527–1536. [Google Scholar] [CrossRef] [PubMed]
- Hung, W.L.; Chang, W.S.; Lu, W.C.; Wei, G.J.; Wang, Y.; Ho, C.T.; Hwang, L.S. Pharmacokinetics, bioavailability, tissue distribution and excretion of tangeretin in rat. J. Food Drug Anal. 2018, 26, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Burapan, S.; Kim, M.; Han, J. Demethylation of polymethoxyflavones by human gut bacterium, Blautia sp. MRG-PMF1. J. Agric. Food Chem. 2017, 65, 1620–1629. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, N.; Han, J. Metabolism of Kaempferia parviflora polymethoxyflavones by human intestinal bacterium Bautia sp. MRG-PMF1. J. Agric. Food Chem. 2014, 62, 12377–12383. [Google Scholar] [CrossRef]
- Patel, K.; Gadewar, M.; Tahilyani, V.; Patel, D.K. A review on pharmacological and analytical aspects of diosmetin: A concise report. Chin. J. Integr. Med. 2013, 19, 792–800. [Google Scholar] [CrossRef]
- Silvestro, L.; Tarcomnicu, I.; Dulea, C.; Attili, N.R.; Ciuca, V.; Peru, D.; Rizea Savu, S. Confirmation of diosmetin 3-O-glucuronide as major metabolite of diosmin in humans, using micro-liquid-chromatography-mass spectrometry and ion mobility mass spectrometry. Anal. Bioanal. Chem. 2013, 405, 8295–8310. [Google Scholar] [CrossRef]
- Chen, X.; Xu, L.; Guo, S.; Wang, Z.; Jiang, L.; Wang, F.; Zhang, J.; Liu, B. Profiling and comparison of the metabolites of diosmetin and diosmin in rat urine, plasma and feces using UHPLC-LTQ-Orbitrap MS(n). J. Chromatogr. B Anal. Technol. Biomed Life Sci. 2019, 1124, 58–71. [Google Scholar] [CrossRef]
- Szeleszczuk, L.; Pisklak, D.M.; Zielinska-Pisklak, M.; Wawer, I. Spectroscopic and structural studies of the diosmin monohydrate and anhydrous diosmin. Int. J. Pharm. 2017, 529, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Russo, R.; Chandradhara, D.; De Tommasi, N. Comparative Bioavailability of Two Diosmin Formulations after Oral Administration to Healthy Volunteers. Molecules 2018, 23, 2174. [Google Scholar] [CrossRef] [PubMed]
- Bush, R.; Comerota, A.; Meissner, M.; Raffetto, J.D.; Hahn, S.R.; Freeman, K. Recommendations for the medical management of chronic venous disease: The role of Micronized Purified Flavanoid Fraction (MPFF). Phlebology 2017, 32, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Breiter, T.; Laue, C.; Kressel, G.; Gröll, S.; Engelhardt, U.H.; Hahn, A. Bioavailability and antioxidant potential of rooibos flavonoids in humans following the consumption of different rooibos formulations. Food Chem. 2011, 128, 338–347. [Google Scholar] [CrossRef]
- Tripoli, E.; La Guardia, M.; Giammanco, S.; Di Majo, D.; Giammanco, M. Citrus flavonoids: Molecular structure, biological activity and nutritional properties: A review. Food Chem. 2007, 104, 466–479. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef]
- López-Lázaro, M. Distribution and biological activities of the flavonoid luteolin. Mini Rev. Med. Chem. 2009, 10, 31–59. [Google Scholar] [CrossRef]
- Kritas, S.K.; Saggini, A.; Varvara, G.; Murmura, G.; Caraffa, A.; Antinolfi, P.; Toniato, E.; Pantalone, A.; Neri, G.; Frydas, S.; et al. Luteolin inhibits mast cell-mediated allergic inflammation. J. Biol. Regul. Homeost. Agents 2013, 27, 955–959. [Google Scholar]
- Castro-Vazquez, L.; Alañón, M.E.; Rodríguez-Robledo, V.; Pérez-Coello, M.S.; Hermosín-Gutierrez, I.; Díaz-Maroto, M.C.; Jordán, J.; Galindo, M.F.; Arroyo-Jiménez, M. Bioactive Flavonoids, Antioxidant Behaviour, and Cytoprotective Effects of Dried Grapefruit Peels (Citrus paradisi Macf.). Oxidative Med. Cell Longev. 2016, 2016, 8915729. [Google Scholar] [CrossRef]
- Silva, M.M.; Santos, M.R.; Caroço, G.; Rocha, R.; Justino, G.; Mira, L. Structure-antioxidant activity relationships of flavonoids: A re-examination. Free Radic. Res. 2002, 36, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- van Acker, S.A.; van den Berg, D.J.; Tromp, M.N.; Griffioen, D.H.; van Bennekom, W.P.; van der Vijgh, W.J.; Bast, A. Structural aspects of antioxidant activity of flavonoids. Free Radic. Biol. Med. 1996, 20, 331–342. [Google Scholar] [CrossRef]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Galleano, M.; Verstraeten, S.V.; Oteiza, P.I.; Fraga, C.G. Antioxidant actions of flavonoids: Thermodynamic and kinetic analysis. Arch. Biochem. Biophys. 2010, 501, 23–30. [Google Scholar] [CrossRef]
- Bellocco, E.; Barreca, D.; Laganà, G.; Leuzzi, U.; Tellone, E.; Kotyk, A.; Galtieri, A. Influence of L-rhamnosyl-D-glucosyl derivatives on properties and biological interaction of flavonoids. Mol. Cell. Biochem. 2009, 321, 165–171. [Google Scholar] [CrossRef]
- Barreca, D.; Laganà, G.; Tellone, E.; Ficarra, S.; Leuzzi, U.; Galtieri, A.; Bellocco, E. Influences of flavonoids on erythrocyte membrane and metabolic implication through anionic exchange modulation. J. Membr. Biol. 2009, 230, 163–171. [Google Scholar] [CrossRef]
- Barreca, D.; Laganà, G.; Bruno, G.; Magazù, S.; Bellocco, E. Diosmin binding to human serum albumin and its preventive action against degradation due to oxidative injuries. Biochimie 2013, 95, 2042–2049. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–966. [Google Scholar] [CrossRef]
- Zielińska, D.; Zielińskib, H. Antioxidant activity of flavone C-glucosides determined by updated analytical strategies. Food Chem. 2011, 124, 672–678. [Google Scholar] [CrossRef]
- Wolfe, K.L.; Liu, R.H. Structure-activity relationships of flavonoids in the cellular antioxidant activity assay. J. Agric. Food Chem. 2008, 56, 8404–8411. [Google Scholar] [CrossRef]
- Moalin, M.; van Strijdonck, G.P.F.; Beckers, M.; Hagemen, G.J.; Borm, P.J.; Bast, A.; Haenen, G.R. A planar conformation and the hydroxyl groups in the B and C rings play a pivotal role in the antioxidant capacity of quercetin and quercetin derivatives. Molecules 2011, 16, 9636–9950. [Google Scholar] [CrossRef]
- Furusawa, M.; Tanaka, T.; Ito, T.; Nishikawa, A.; Yamazaki, N.; Nakaya, K.-I.; Matsuura, N.; Tsuchiya, H.; Nagayama, M.; Iinuma, M. Antioxidant activity of hydroflavonoids. J. Health Sci. 2005, 51, 376–378. [Google Scholar] [CrossRef]
- Bors, W.; Heller, W.; Michel, C.; Saran, M. Flavonoids as antioxidants: Determination of radical scavenging efficiencies. Methods Enzymol. 1990, 186, 343–355. [Google Scholar] [PubMed]
- Arora, A.; Nair, M.G.; Strasburg, G.M. Structure-activity relationships for antioxidant activities of a series of flavonoids in a liposomal system. Free Radic. Biol Med. 1998, 24, 1355–1363. [Google Scholar] [CrossRef]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant and prooxidant behavior of flavonoids: Structure-activity relationships. Free Radic. Biol. Med. 1997, 22, 749–760. [Google Scholar] [CrossRef]
- de Souza, V.T.; de Franco, É.P.; de Araújo, M.E.; Messias, M.C.; Priviero, F.B.; Frankland Sawaya, A.C.; de Oliveira Carvalho, P. Characterization of the antioxidant activity of aglycone and glycosylated derivatives of hesperetin: An in vitro and in vivo study. J. Mol. Recognit. 2016, 29, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Kimata, M.; Shichijo, M.; Miura, T.; Serizawa, I.; Inagaki, N.; Nagai, H. Effects of luteolin, quercetin and baicalein on immunoglobulin E-mediated mediator release from human cultured mast cells. Clin. Exp. Allergy 2000, 30, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Weng, Z.; Patel, A.B.; Panagiotidou, S.; Theoharides, T.C. The novel flavone tetramethoxyluteolin is a potent inhibitor of human mast cells. J. Allergy Clin. Immunol. 2015, 135, 1044–1052. [Google Scholar] [CrossRef]
- Zhu, S.; Xu, T.; Luo, Y.; Zhang, Y.; Xuan, H.; Ma, Y.; Zhu, H. Luteolin enhances sarcoplasmic reticulum Ca2+-ATPase activity through p38 MAPK signaling thus improving rat cardiac function after ischemia/reperfusion. Cell. Physiol. Biochem. 2017, 41, 999–1010. [Google Scholar] [CrossRef]
- Funakoshi-Tago, M.; Nakamura, K.; Tago, K.; Mashino, T.; Kasahara, T. Anti-inflammatory activity of structurally related flavonoids, Apigenin, Luteolin and Fisetin. Int. Immunopharmacol. 2011, 11, 1150–1159. [Google Scholar] [CrossRef]
- Mandalari, G.; Bisignano, C.; D’Arrigo, M.; Ginestra, G.; Arena, A.; Tomaino, A.; Wickham, M.S. Antimicrobial potential of polyphenols extracted from almond skins. Lett. Appl. Microbiol. 2010, 51, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Bisignano, C.; Filocamo, A.; La Camera, E.; Zummo, S.; Fera, M.T.; Mandalari, G. Antibacterial activities of almond skins on cagA-positive and-negative clinical isolates of Helicobacter pylori. BMC Microbiol. 2013, 13, 103. [Google Scholar] [CrossRef] [PubMed]
- Arena, A.; Bisignano, C.; Stassi, G.; Filocamo, A.; Mandalari, G. Almond Skin Inhibits HSV-2 Replication in Peripheral Blood Mononuclear Cells by Modulating the Cytokine Network. Molecules 2015, 20, 8816–8822. [Google Scholar] [CrossRef]
- Bisignano, C.; Mandalari, G.; Smeriglio, A.; Trombetta, D.; Pizzo, M.M.; Pennisi, R.; Sciortino, M.T. Almond Skin Extracts Abrogate HSV-1 Replication by Blocking Virus Binding to the Cell. Virus 2017, 9, 178. [Google Scholar] [CrossRef] [PubMed]
- Mandalari, G.; Bennett, R.N.; Bisignano, G.; Trombetta, D.; Saija, A.; Faulds, C.B.; Gasson, M.J.; Narbad, A. Antimicrobial activity of flavonoids extracted from bergamot (Citrus bergamia Risso) peel, a byproduct of the essential oil industry. J. Appl. Microbiol. 2007, 103, 2056–2064. [Google Scholar] [CrossRef] [PubMed]
- Filocamo, A.; Bisignano, C.; Ferlazzo, N.; Cirmi, S.; Mandalari, G.; Navarra, M. In vitro effect of bergamot (Citrus bergamia) juice against cagA-positive and-negative clinical isolates of Helicobacter pylori. BMC Complementary Altern. Med. 2015, 15, 256. [Google Scholar] [CrossRef] [PubMed]
- Uckoo, R.M.; Jayaprakasha, G.K.; Vikram, A.; Patil, B.S. Polymethoxyflavones Isolated from the Peel of Miaray Mandarin (Citrus miaray) Have Biofilm Inhibitory Activity in Vibrio harveyi. J. Agric. Food Chem. 2015, 63, 7180–7189. [Google Scholar] [CrossRef] [PubMed]
- Johann, S.; Oliveira, V.L.; Pizzolatti, M.G.; Schripsema, J.; Braz-Filho, R.; Branco, A.; Smânia, A., Jr. Antimicrobial activity of wax and hexane extracts from Citrus spp. peels. Memórias Inst. Oswaldo Cruz 2007, 102, 681–685. [Google Scholar] [CrossRef]
- Murakami, A.; Nakamura, Y.; Ohto, Y.; Yano, M.; Koshiba, T.; Koshimizu, K.; Tokuda, H.; Nishino, H.; Ohigashi, H. Suppressive effects of citrus fruits on free radical generation and nobiletin, an anti-inflammatory polymethoxyflavonoid. Biofactors 2000, 12, 187–192. [Google Scholar] [CrossRef]
- Chan, B.C.; Ip, M.; Gong, H.; Lui, S.L.; See, R.H.; Jolivalt, C.; Fung, K.P.; Leung, P.C.; Reiner, N.E.; Lau, C.B. Synergistic effects of diosmetin with erythromycin against ABC transporter over-expressed methicillin-resistant Staphylococcus aureus (MRSA) RN4220/pUL5054 and inhibition of MRSA pyruvate kinase. Phytomedicine 2013, 20, 611–614. [Google Scholar] [CrossRef]
- Ernawita Wahyuono, R.A.; Hesse, J.; Hipler, U.C.; Elsner, P.; Böhm, V. In Vitro Lipophilic Antioxidant Capacity, Antidiabetic and Antibacterial Activity of Citrus Fruits Extracts from Aceh, Indonesia. Antioxidants 2017, 6, 11. [Google Scholar] [CrossRef]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Antibacterial Activity of Some Flavonoids and Organic Acids Widely Distributed in Plants. J. Clin. Med. 2019, 9, 109. [Google Scholar] [CrossRef] [PubMed]
- Echeverría, J.; Opazo, J.; Mendoza, L.; Urzúa, A.; Wilkens, M. Structure-Activity and Lipophilicity Relationships of Selected Antibacterial Natural Flavones and Flavanones of Chilean Flora. Molecules 2017, 22, 608. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.J.; Wu, X.; Li, M.M.; Li, G.Q.; Yang, Y.T.; Luo, H.J.; Huang, W.H.; Chung, H.Y.; Ye, W.C.; Wang, G.C.; et al. Antiviral activity of polymethoxylated flavones from “Guangchenpi”, the edible and medicinal pericarps of citrus reticulata ‘Chachi’. J. Agric. Food Chem. 2014, 62, 2182–2189. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.J.; Liu, Z.; Tang, W.; Wang, G.C.; Chung, H.Y.; Liu, Q.Y.; Zhuang, L.; Li, M.M.; Li, Y.L. Tangeretin from Citrus reticulate Inhibits Respiratory Syncytial Virus Replication and Associated Inflammation in Vivo. J. Agric. Food Chem. 2015, 63, 9520–9527. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; He, S.; Zhang, X.; Guo, J.; Chen, Q.; Yan, F.; Banadyga, L.; Zhu, W.; Qiu, X.; Guo, Y. Tangeretin, an extract from Citrus peels, blocks cellular entry of arenaviruses that cause viral hemorrhagic fever. Antivir. Res. 2018, 160, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Martin-Benlloch, X.; Haid, S.; Novodomska, A.; Rominger, F.; Pietschmann, T.; Davioud-Charvet, E.; Elhabiri, M. Physicochemical Properties Govern the Activity of Potent Antiviral Flavones. ACS Omega 2019, 4, 4871–4887. [Google Scholar] [CrossRef]
- Sadati, S.M.; Gheibi, N.; Ranjbar, S.; Hashemzadeh, M.S. Docking study of flavonoid derivatives as potent inhibitors of influenza H1N1 virus neuramidases. Biomed. Rep. 2019, 10, 33–38. [Google Scholar]
- Chahar, M.K.; Sharma, N.; Dobhal, M.P.; Joshi, Y.C. Flavonoids: A versatile source of anticancer drugs. Pharmacogn. Rev. 2011, 5, 1–12. [Google Scholar]
- Kanadaswami, C.; Lee, L.-T.; Lee, P.-P.H.; Hwang, J.-J.; Ke, F.-C.; Huang, Y.-T.; Lee, M.-T. The Antitumor Activities of Flavonoids. In Vivo 2005, 19, 895–910. [Google Scholar]
- Takagaki, N.; Sowa, Y.; Oki, T.; Nakanishi, R.; Yogosawa, S.; Sakai, T. Apigenin induces cell cycle arrest and p21/WAF1 expression in a p53-independent pathway. Int. J. Oncol. 2005, 26, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Maggioni, D.; Garavello, W.; Rigolio, R.; Pignataro, L.; Gaini, R.; Nicolini, G. Apigenin impairs oral squamous cell carcinoma growth in vitro inducing cell cycle arrest and apoptosis. Int. J. Oncol. 2013, 43, 1675–1682. [Google Scholar] [CrossRef] [PubMed]
- Iizumi, Y.; Oishi, M.; Taniguchi, T.; Goi, W.; Sowa, Y.; Sakai, T. The flavonoid apigenin downregulates CDK1 by directly targeting ribosomal protein S9. PLoS ONE 2013, 8, e73219. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.S.; Ku, J.M.; Choi, H.S.; Woo, J.K.; Jang, B.H.; Shin, Y.C.; Ko, S.G. Induction of caspase-dependent apoptosis by apigenin by inhibiting STAT3 signaling in HER2-overexpressing MDA-MB-453 breast cancer cells. Anticancer Res. 2014, 34, 2869–2882. [Google Scholar]
- Seo, H.S.; Choi, H.S.; Kim, S.R.; Choi, Y.K.; Woo, S.M.; Shin, I.; Woo, J.K.; Park, S.Y.; Shin, Y.C.; Ko, S.K. Apigenin induces apoptosis via extrinsic pathway, inducing p53 and inhibiting STAT3 and NF-kB signalling in HER2-overexpressing breast cancer cells. Mol. Cell. Biochem. 2012, 366, 319–334. [Google Scholar] [CrossRef]
- Karmakar, S.; Davis, K.A.; Choudhury, S.R.; Deeconda, A.; Banik, N.L.; Ray, S.K. Bcl-2 inhibitor and apigenin worked synergistically in human malignant neuroblastoma cell lines and increased apoptosis with activation of extrinsic and intrinsic pathways. Biochem. Biophys. Res. Commun. 2009, 388, 705–710. [Google Scholar] [CrossRef]
- Peng, Q.; Deng, Z.; Pan, H.; Gu, L.; Liu, O.; Tang, Z. Mitogen-activated protein kinase signaling pathway in oral cancer. Oncol. Lett. 2017, 15, 1379–1388. [Google Scholar] [CrossRef]
- Xie, Y.; Liang, D.; Wu, Q.; Chen, X.; Buabeid, M.A.; Wang, Y. A System-Level Investigation into the Mechanisms of Apigenin against Inflammation. Nat. Prod. Commun. 2019, 14, 1–11. [Google Scholar] [CrossRef]
- Bauer, D.; Mazzio, E.; Soliman, K.F.A. Whole Transcriptomic Analysis of Apigenin on TNFα Immuno-activated MDA-MB-231 Breast Cancer Cells. Cancer Genom. Proteom. 2019, 16, 421–432. [Google Scholar] [CrossRef]
- Lee, H.H.; Jung, J.; Moon, A.; Kang, H.; Cho, H. Antitumor and Anti-Invasive Effect of Apigenin on Human Breast Carcinoma through Suppression of IL-6 Expression. Int. J. Mol. Sci. 2019, 20, 3143. [Google Scholar] [CrossRef]
- Sinha, S.; Patel, S.; Athar, M.; Vora, J.; Chhabria, M.T.; Jha, P.C.; Shrivastava, N. Structure-based identification of novel sirtuin inhibitors against triple negative breast cancer: An in silico and in vitro study. Int. J. Biol. Macromol. 2019, 140, 454–468. [Google Scholar] [CrossRef] [PubMed]
- Pace, E.; Di Vincenzo, S.; Di Salvo, E.; Genovese, S.; Dino, P.; Sangiorgi, C.; Ferraro, M.; Gangemi, S. MiR-21 upregulation increases IL-8 expression and tumorigenesis program in airway epithelial cells exposed to cigarette smoke. J. Cell. Physiol. 2019, 234, 22183–22194. [Google Scholar] [CrossRef]
- Fan, X.; Bai, J.; Zhao, S.; Hu, M.; Sun, Y.; Wang, B.; Ji, M.; Jin, J.; Wang, X.; Hu, J.; et al. Evaluation of inhibitory effects of flavonoids on breast cancer resistance protein (BCRP): From library screening to biological evaluation to structure activity relationship. Toxicol. In Vitro 2019, 61, 104642. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, H.; Yu, X.; Wang, X.; Zhu, X.; Xu, X. Apigenin inhibits in vitro and in vivo tumorigenesis in cisplatin-resistant colon cancer cells by inducing autophagy, programmed cell death and targeting m-TOR/PI3K/Akt signalling pathway. JBUON 2019, 24, 488–493. [Google Scholar] [PubMed]
- Zhan, Y.; Wang, Y.; Qi, M.; Liang, P.; Ma, Y.; Li, T.; Li, H.; Dai, C.; An, Z.; Qi, Y.; et al. BH3 mimetic ABT-263 enhances the anticancer effects of apigenin in tumor cells with activating EGFR mutation. Cell. Biosci. 2019, 9, 60–78. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.G.; Wang, L.; Liu, W.J.; Wang, J.F.; Zhao, E.J.; Zhou, F.M.; Ji, X.B.; Wang, L.H.; Xia, Z.K.; Wang, W.; et al. Apigenin Inhibits IL-6 Transcription and Suppresses Esophageal Carcinogenesis. Front. Pharmacol. 2019, 10, 1002. [Google Scholar] [CrossRef]
- Ahmed, S.; Khan, H.; Fratantonio, D.; Hasan, M.M.; Sharifi, S.; Fathi, N.; Ullah, H.; Rastrelli, L. Apoptosis induced by luteolin in breast cancer: Mechanistic and therapeutic Perspectives. Phytomedicine 2019, 59, 152883. [Google Scholar] [CrossRef]
- Imran, M.; Rauf, A.; Abu-Izneid, T.; Nadeem, M.; Shariati, M.A.; Khan, I.A.; Imran, A.; Orhan, I.E.; Rizwan, M.; Atif, M.; et al. Luteolin, a flavonoid, as an anticancer agent: A review. Biomed. Pharmacother. 2019, 112, 108612. [Google Scholar] [CrossRef]
- Avila-Carrasco, L.; Majano, P.; Sánchez-Toméro, J.A.; Selgas, R.; López-Cabrera, M.; Aguilera, A.; González Mateo, G. Natural Plants Compounds as Modulators of Epithelial-to-Mesenchymal Transition. Front. Pharmacol. 2019, 10, 715. [Google Scholar] [CrossRef]
- Lee, J.; Park, S.H.; Lee, J.; Chun, H.; Choi, M.K.; Yoon, J.H.; Pham, T.H.; Kim, K.H.; Kwon, T.; Ryu, H.W.; et al. Differential effects of luteolin and its glycosides on invasion and apoptosis in MDA-MB-231 triple-negative breast cancer cells. EXCLI J. 2019, 18, 750–763. [Google Scholar]
- Yao, X.; Jiang, W.; Yu, D.; Yan, Z. Luteolin inhibits proliferation and induces apoptosis of human melanoma cells in vivo and in vitro by suppressing MMP-2 and MMP-9 through the PI3K/AKT pathway. Food Funct. 2019, 10, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Jin, K.; Lan, H. Luteolin inhibits cell cycle progression and induces apoptosis of breast cancer cells through downregulation of human telomerase reverse transcriptase. Oncol. Lett. 2019, 17, 3842–3850. [Google Scholar] [CrossRef] [PubMed]
- Aryappalli, P.; Shabbiri, K.; Masad, R.J.; Al-Marri, R.H.; Haneefa, S.M.; Mohamed, Y.A.; Arafat, K.; Attoub, S.; Cabral-Marques, O.; Ramadi, K.B.; et al. Inhibition of Tyrosine-Phosphorylated STAT3 in Human Breast and Lung Cancer Cells by Manuka Honey is Mediated by Selective Antagonism of the IL-6 Receptor. Int. J. Mol. Sci. 2019, 20, 4340. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Zhang, M.; Ying, Q.; Xie, X.; Yue, S.; Tong, B.; Wei, Q.; Bai, Z.; Ma, L. Decrease of AIM2 mediated by luteolin contributes to non-small cell lung cancer treatment. Cell Death Dis. 2019, 10, 218. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.J.; Wen-Hsien Hsu, W.H.; Lee, K.H.; Chen, K.C.; Lin, C.W.; Lee, Y.L.A.; Ko, T.P.; Lee, L.A.; Lee, M.T.; Chang, M.S.; et al. Dietary Flavonoids Luteolin and Quercetin Inhibit Migration and Invasion of Squamous Carcinoma through Reduction of Src/Stat3/S100A7. Signal. Antioxid. 2019, 8, 557. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Ge, R.; Li, Y.; Liu, S. Luteolin exhibits anti-breast cancer property through up-regulating miR-203. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3265–3271. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Rao, C.; Zheng, G.; Wang, S. Luteolin suppresses colorectal cancer cell metastasis via regulation of the miR-384/pleiotrophin axis. Oncol. Rep. 2019, 42, 131–141. [Google Scholar] [CrossRef]
- Ferino, A.; Rapozzi, V.; Xodo, L.E. The ROS-KRAS-Nrf2 axis in the control of the redox homeostasis and the intersection with survival-apoptosis pathways: Implications for photodynamic therapy. J. Photochem. Photobiol. B 2020, 202, 111672. [Google Scholar] [CrossRef]
- Kang, K.A.; Piao, M.J.; Hyun, Y.J.; Zhen, A.X.; Cho, S.J.; Ahn, M.J.; Yi, J.M.; Hyun, J.W. Luteolin promotes apoptotic cell death via upregulation of Nrf2 expression by DNA demethylase and the interaction of Nrf2 with p53 in human colon cancer cells. Exp. Mol. Med. 2019, 51, 40. [Google Scholar] [CrossRef]
- Soliman, N.A.; Abd-Ellatif, R.N.; ELSaadany, A.A.; Shalaby, S.M.; Bedeer, A.E. Luteolin and 5-flurouracil act synergistically to induce cellular weapons in experimentally induced Solid Ehrlich Carcinoma: Realistic role of P53; a guardian fights in a cellular battle. Chem. Biol. Interact. 2019, 310, 108740. [Google Scholar] [CrossRef]
- Wu, C.; Xu, Q.; Chen, X.; Liu, J. Delivery luteolin with folacin-modified nanoparticle for glioma therapy. Int. J. Nanomed. 2019, 14, 7515–7531. [Google Scholar] [CrossRef] [PubMed]
- Androutsopoulos, V.P.; Mahale, S.; Arroo, R.R.; Potter, G. Anticancer effects of the flavonoid diosmetin on cell cycle progression and proliferation of MDA-MB 468 breast cancer cells due to CYP1 activation. Oncol. Rep. 2009, 21, 1525–1528. [Google Scholar] [PubMed]
- Androutsopoulos, V.; Wilsher, N.; Arroo, R.R.; Potter, G.A. Bioactivation of the phytoestrogen diosmetin by CYP1 cytochromes P450. Cancer Lett. 2009, 274, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Androutsopoulos, V.P.; Spandidos, D.A. The flavonoids diosmetin and luteolin exert synergistic cytostatic effects in human hepatoma HepG2 cells via CYP1A-catalyzed metabolism, activation of JNK and ERK and P53/P21 up-regulation. J. Nutr. Biochem. 2013, 24, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, S.; Ren, H.; Sheng, Y.; Wang, T.; Li, M.; Zhou, Q.; He, H.; Liu, C. Anti-Proliferation and Pro-Apoptotic Effects of Diosmetin via Modulating Cell Cycle Arrest and Mitochondria-Mediated Intrinsic Apoptotic Pathway in MDA-MB-231 Cells. Med. Sci. Monit. 2019, 25, 4639–4647. [Google Scholar] [CrossRef]
- Koosha, S.; Mohamed, Z.; Sinniah, A.; Alshawsh, M.A. Investigation into the Molecular mechanisms underlying the Anti-proliferative and Anti-tumorigenesis activities of Diosmetin against HCT-116 Human Colorectal Cancer. Sci. Rep. 2019, 9, 5148–5164. [Google Scholar] [CrossRef]
- Oak, C.; Khalifa, A.O.; Isali, I.; Bhaskaran, N.; Walker, E.; Shukla, S. Diosmetin suppresses human prostate cancer cell proliferation through the induction of apoptosis and cell cycle arrest. Int. J. Oncol. 2018, 53, 835–843. [Google Scholar] [CrossRef]
- Xu, Z.; Yan, Y.; Xiao, L.; Dai, S.; Zeng, S.; Qian, L.; Wang, L.; Yang, X.; Xiao, Y.; Gong, Z. Radiosensitizing effect of diosmetin on radioresistant lung cancer cells via Akt signaling pathway. PLoS ONE 2017, 12, e0175977. [Google Scholar] [CrossRef]
- Liu, B.; Shi, Y.; Peng, W.; Zhang, Q.; Liu, J.; Chen, N.; Zhu, R. Diosmetin induces apoptosis by upregulating p53 via the TGF-β signal pathway in HepG2 hepatoma cells. Mol. Med. Rep. 2016, 14, 159–164. [Google Scholar] [CrossRef]
- Qiao, J.; Liu, J.; Jia, K.; Li, N.; Liu, B.; Zhang, Q.; Zhu, R. Diosmetin triggers cell apoptosis by activation of the p53/Bcl-2 pathway and inactivation of the Notch3/NF-κB pathway in HepG2 cells. Oncol. Lett. 2016, 12, 5122–5128. [Google Scholar] [CrossRef]
- Liu, J.; Ren, H.; Liu, B.; Zhang, Q.; Li, M.; Zhu, R. Diosmetin inhibits cell proliferation and induces apoptosis by regulating autophagy via the mammalian target of rapamycin pathway in hepatocellular carcinoma HepG2 cells. Oncol. Lett. 2016, 12, 4385–4392. [Google Scholar] [CrossRef] [PubMed]
- Roma, A.; Rota, S.G.; Spagnuolo, P.A. Diosmetin Induces Apoptosis of Acute Myeloid Leukemia. Cells Mol. Pharm. 2018, 15, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wu, Q.; Chen, Y.; Zhang, J.; Li, H.; Yang, Z.; Yang, Y.; Deng, Y.; Zhang, L.; Liu, B. Diosmetin induces apoptosis and enhances the chemotherapeutic efficacy of paclitaxel in non-small cell lung cancer cells via Nrf2 inhibition. Br. J. Pharmacol. 2019, 176, 2079–2094. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wen, X.; Liu, B.; Zhang, Q.; Zhang, J.; Miao, H.; Zhu, R. Diosmetin inhibits the metastasis of hepatocellular carcinoma cells by downregulating the expression levels of MMP-2 and MMP-9. Mol. Med. Rep. 2016, 13, 2401–2408. [Google Scholar] [CrossRef] [PubMed]
- Koosha, S.; Mohamed, Z.; Sinniah, A.; Alshawsh, M.A. Evaluation of Anti-Tumorigenic Effects of Diosmetin against Human Colon Cancer Xenografts in Athymic Nude Mice. Molecules 2019, 24, 2522. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Lee, D.H.; Park, S.Y.; Seol, J.W. Diosmetin inhibits tumor development and block tumor angiogenesis in skin cancer. Biomed. Pharmacother. 2019, 117, 109091. [Google Scholar] [CrossRef]
- Takemura, H.; Uchiyama, H.; Ohura, T.; Sakakibara, H.; Kuruto, R.; Amagai, T.; Shimoi, K. A methoxyflavonoid, chrysoeriol, selectively inhibits the formation of a carcinogenic estrogen metabolite in MCF-7 breast cancer cells. J. Steroid Biochem. Mol. Biol. 2010, 118, 70–76. [Google Scholar] [CrossRef]
- Baier, A.; Galicka, A.; Nazaruk, J.; Szyszka, R. Selected flavonoid compounds as promising inhibitors of protein kinase CK2α and CK2α’, the catalytic subunits of CK2. Phytochemistry 2017, 136, 39–45. [Google Scholar] [CrossRef]
- Tan, K.W.; Li, Y.; Paxton, J.W.; Birch, N.P.; Scheepens, A. Identification of novel dietary phytochemicals inhibiting the efflux transporter breast cancer resistance protein (BCRP/ABCG2). Food Chem. 2013, 138, 2267–2274. [Google Scholar] [CrossRef]
- Lai, S.; Chen, J.N.; Huang, H.W.; Zhang, X.Y.; Jiang, H.L.; Li, W.; Wang, P.L.; Wang, J.; Liu, F.N. Structure activity relationships of chrysoeriol and analogs as dual c-Met and VEGFR2 tyrosine kinase inhibitors. Oncol. Rep. 2018, 40, 1650–1656. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Min, Q.; Palida, A.; Zhang, Y.; Tang, R.; Chen, L.; Li, H. 8-Chrysoeriol, as a potential BCL-2 inhibitor triggers apoptosis of SW1990 pancreatic cancer cells. Bioorg. Chem. 2018, 77, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Amrutha, K.; Nanjan, P.; Shaji, S.K.; Sunilkumar, D.; Subhalakshmi, K.; Rajakrishna, L.; Banerji, A. Discovery of lesser known flavones as inhibitors of NF-κB signaling in MDA-MB-231 breast cancer cells--A SAR study. Bioorg. Med. Chem. Lett. 2014, 24, 4735–4742. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhou, X.; Xiao, M.; Hong, Z.; Gong, Q.; Jiang, L.; Zhou, J. Discovery of chrysoeriol, a PI3K-AKT-mTOR pathway inhibitor with potent antitumor activity against human multiple myeloma cells in vitro. J. Huazhong Univ. Sci. Technol. Med. Sci. 2010, 30, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; He, J.; Ruan, H.; Wang, Y. In vitro and in vivo cytotoxic effects of chrysoeriol in human lung carcinoma are facilitated through activation of autophagy, sub-G1/G0 cell cycle arrest, cell migration and invasion inhibition and modulation of MAPK/ERK signaling pathway. J. BUON 2019, 24, 936–942. [Google Scholar]
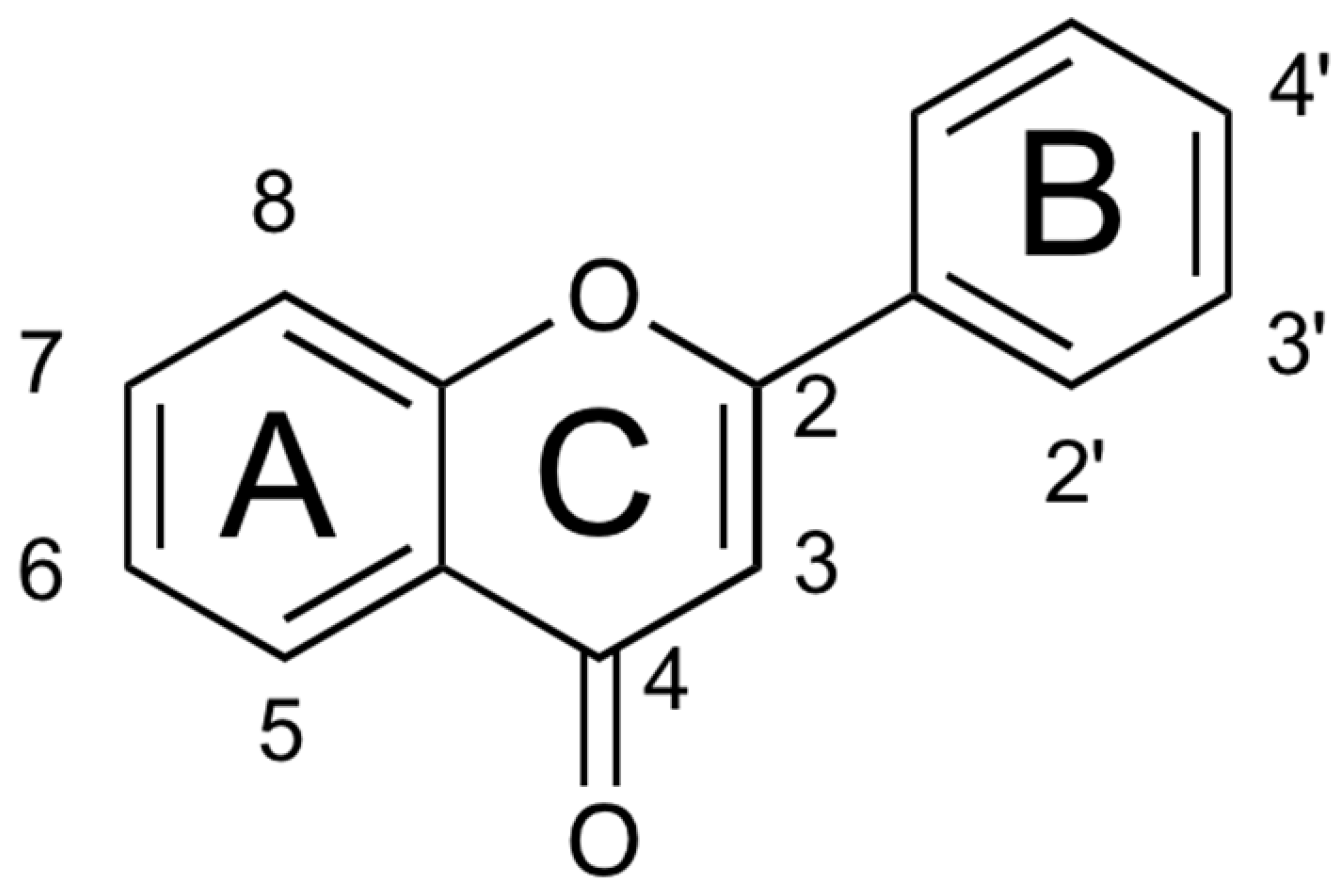

 | |||
|---|---|---|---|
| Compound Name | R1 | R2 | R3 |
| Acacetin | H | H | OMe |
| Isoscutellarein | OH | H | OH |
| Luteolin | H | OH | OH |
| Apigenin | H | H | OH |
| Diosmetin | H | OH | OMe |
| Chrysoeriol | H | OMe | OH |
 | |||||
|---|---|---|---|---|---|
| Compound Name | R1 | R2 | R3 | R4 | R5 |
| Quercetogetin | OMe | H | OMe | OMe | OMe |
| 3,3’,4’,5,6,7,8-Heptamethoxyflavone | OMe | OMe | OMe | OMe | OMe |
| Natsudaidain | OMe | OMe | OMe | OH | OMe |
| Nobiletin | OMe | OMe | OMe | H | OMe |
| Sinensetin | OMe | H | OMe | H | OMe |
| Tangeretin | OMe | OMe | H | H | OMe |
| Tetramethylscutellarein | OMe | H | H | H | OMe |
| 5-Hydroxy-3,7,3’,4’-tetramethoxyflavone | H | H | OMe | OMe | OH |
| 3,3’,4’,5,7,8-Hexamethoxyflavone | H | OMe | OMe | OMe | OMe |
| Pentamethylquercetin | H | H | OMe | OMe | OMe |
| 3’,4’,5,7,8-Pentamethoxyflavone | H | OMe | OMe | H | OMe |
| 4’,5,7,8-Tetramethoxyflavone | H | OMe | H | H | OMe |
| 4’,5,7-Trimethoxyflavone | H | H | H | H | OMe |
 | ||||||
|---|---|---|---|---|---|---|
| Compound Name | R1 | R2 | R3 | R4 | R5 | R6 |
| Luteolin 6,8-di-C-glucoside (Lucenin-2) | H | Glu | OH | Glu | OH | OH |
| Apigenin 6,8-di-C-glucoside (Vicenin-2) | H | Glu | OH | Glu | H | OH |
| Chrysoeriol 6,8-di-C-glucoside (Stellarin-2) | H | Glu | OH | Glu | OMe | OH |
| Diosmetin 6,8-di-C-glucoside (Lucenin-2 4’-methyl ether) | H | Glu | OH | Glu | OH | OMe |
| Apigenin 7-O-neohesperidoside-4’-glucoside (Rhoifolin 4’-glucoside) | H | H | O-Nh | H | OH | O-Glu |
| Chrysoeriol 7-O-neohesperidoside-4’-glucoside | H | H | O-Nh | H | OMe | OH |
| Apigenin 6-C-glucoside (Isovitexin) | H | Glu | OH | H | H | OH |
| Luteolin 7-O-rutinoside | H | H | O-Ru | H | OH | OH |
| Chrysoeriol 8-C-glucoside (Scoparin) | H | H | OH | Glu | OMe | OH |
| Diosmetin 8-C-glucoside (Orientin 4’-methyl ether) | H | H | OH | Glu | OH | OMe |
| Apigenin 7-O-neohesperidoside (Rhoifolin) | H | H | O-Nh | H | OH | OH |
| Apigenin 7-O-rutinoside (Isorhoifolin) | H | H | O-Ru | H | OH | OH |
| Chrysoeriol 7-O-neohesperidoside | H | H | O-Nh | H | OMe | OH |
| Diosmetin 7-O-rutinoside (Diosmin) | H | H | O-Ru | H | OH | OMe |
| Diosmetin 7-O-neohesperidoside (Neodiosmin) | H | H | O-Nh | H | OH | OMe |
| Acacetin 3,6-di-C-glucoside | Glu | Glu | OH | H | H | OMe |
| Apigenin 8-C-neohesperidoside | H | H | OH | Nh | H | OH |
| Acacetin 8-C-neohesperidoside (2’’-O-rhamnosyl cytisoside) | H | H | OH | Nh | H | OMe |
| Acacetin 6-C-neohesperidoside (2’’-O-rhamnosyl isocytisoside) | H | Nh | OH | H | H | OMe |
| Acacetin 7-O-neohesperidoside (Fortunellin) | H | H | O-Nh | H | H | OMe |
| Species | Chinotto | Sour Orange | Sweet Lemon | Kumquat | Tangelo | |
|---|---|---|---|---|---|---|
| Ripe | Unripe | |||||
| Flavone | ||||||
| Vicenin-2 | 0.58 ± 0.06 | 1.54 ± 0.15 | 0.37 ± 0.02 | Traces | 0.13 ± 0.01 | 3.89 ± 0.11 |
| Diosmin | 0.20 ± 0.02 | 0.15 | 0.39 ± 0.07 | ------ | ------ | |
| Lucenin-2 | ------ | 0.12 ± 0.012 | ------ | ------ | ------ | 0.10 ± 0.012 |
| Scoparin | ------ | ------ | 0.10 ± 0.03 | ------ | ------ | ------ |
| Lucenin-2 4’- methyl ether | ------ | 0.45 ± 0.06 | 0.75 ± 0.05 | Traces | Traces | ------ |
| Rhoifolin | 0.11 ± 0.02 | 0.79 ± 0.08 | 0.15 ± 0.02 | Traces | ------ | ------ |
| Neodiosmin | 0.11 ± 0.01 | ------ | ------ | ------ | ------ | ------ |
| Rhoifolin 4’-O-glucoside | ------ | 0.36 ± 0.04 | ------ | ------ | ------ | ------ |
| Orientin 4’-methyl ether | ------ | ------ | 0.10 ± 0.04 | ------ | ------ | ------ |
| Acacetin 3,6-di-C-glucoside | ------ | ------ | ------ | Traces | Traces | ------ |
| Apigenin 8-C-neohesperidoside | ------ | ------ | ------ | 0.26 ± 0.03 | 0.81 ± 0.08 | ------ |
| Acacetin 8-C-neohesperidoside | ------ | ------ | ------ | 0.60 ± 0.06 | 1.32 ± 0.13 | ------ |
| Acacetin 6-C-neohesperidoside | ------ | ------ | ------ | 0.70 ± 0.07 | 1.77 ± 0.2 | ------ |
| Acacetin 7-O-neohesperidoside | ------ | ------ | ------ | 0.82 ± 0.08 | 2.72 ± 0.3 | ------ |
| Sinensetin | ------ | ------ | ------ | ------ | ------ | 0.06 ± 0.02 |
| Tangeretin | ------ | 0.08 | ------ | ------ | ------ | 0.08 ± 0.012 |
| Nobiletin | ------ | 0.2 | ------ | ------ | ------ | ------ |
| [25,28] | [26] | [27] | [16] | [16] | [22] | |
| Species | Bergamot | Blood Orange | ||||
|---|---|---|---|---|---|---|
| Cultivar | Castagnaro | Fantastico | Femminello | Moro | Sanguinello | Tarocco |
| Flavone | ||||||
| Vicenin-2 | 47.5 ± 4.7 | 44.1 ± 4.4 | 55.2 ± 5.5 | 37.53 ± 0.40 | 36.2 ± 2.40 | 32.22 ± 0.34 |
| Lucenin-2 | 2.2 ± 0.22 | 2.5 ± 0.25 | 3.3 ± 0.3 | 10.48 ± 0.56 | 4.5 ± 0.42 | 7.23 ± 0.34 |
| Stellarin-2 | 0.6 ± 0.06 | 0.7 ± 0.07 | 1.1 ± 0.1 | 22.13 ± 2.27 | 6.46 ± 1.64 | 0.78 ± 0.02 |
| Isovitexin | 2.5 ± 0.25 | 2.2 ± 0.2 | 3.1 ± 0.3 | ------ | ------ | ------ |
| Scoparin | 5.4 ± 0.54 | 5.9 ± 0.6 | 9.1 ± 0.9 | 7.14 ± 0.88 | 7.43 ± 0.90 | ------ |
| Lucenin-2 4’-methyl ether | 25.4 ± 2.5 | 32.7 ± 3.3 | 62.8 ± 6.3 | 11.73 ± 1.10 | 0.70 ± 0.03 | 0.26 ± 0.01 |
| Rhoifolin | 28.9 ± 2.9 | 26.2 ± 2.6 | 22.8 ± 2.3 | ------ | ------ | ------ |
| Neodiosmin | 15.3 ± 1.5 | 80.0 ± 8.0 | 27.1 ± 2.7 | ------ | ------ | ------ |
| Rhoifolin 4’-O-glucoside | 1.2 ± 0.12 | 1.2 ± 0.12 | 1.3 ± 0.1 | ------ | ------ | ------ |
| Orientin 4’-methyl ether | 1.7 ± 0.2 | 1.9 ± 0.2 | 4.2 ± 0.4 | ------ | ------ | ------ |
| Chrysoeriol 7-O- neoesperidoside | 10.6 ± 1.1 | 9.3 ± 0.9 | 17.2 ± 1.7 | 0.79 ± 0.09 | 0.50 ± 0.07 | 0.23 ± 0.01 |
| Chrysoeriol 7-O- neohesperidoside -4′-O- glucoside | 3.8 ± 0.4 | 4.1 ± 0.4 | 5.3 ± 0.5 | ------ | ------ | ------ |
| [22,24] | [22,24] | [22,24] | [14] | [15] | [15] | |
| Species | Lumia | Sweet Orange | Grapefruit | Clementine | Mandarine |
|---|---|---|---|---|---|
| Flavone | |||||
| Vicenin-2 | ------ | 5.72 ± 2.02 | 1.30 ± 0.16 | 0.70 ± 0.09 | 11.4 ± 0.60 |
| Diosmin | 22.8 ± 0.84 | 0.09 ± 0.009 | ------ | 1.25 ± 0.51 | ------ |
| Lucenin-2 4’-methyl ether | ------ | 0.35 ± 0.14 | ------ | 0.30 ± 0.02 | 4.80 ± 0.70 |
| Rhoifolin | 2.58 ± 0.025 | 0.05 ± 0.005 | ------ | ------ | ------ |
| Neodiosmin | 3.33 ± 0.02 | 0.08 ± 0.008 | ------ | ------ | ------ |
| Isorhoifolin | ------ | 0.07 ± 0.007 | ------ | ------ | ------ |
| Sinensetin | ------ | 0.37 ± 0.04 | ------ | 1.85 ± 0.11 | 1.03 ± 0.07 |
| Tangeretin | 23.37 ± 0.42 | 0.04 ± 0.004 | 1.04 ± 0.07 | 1.32 ± 0.12 | 0.61 ± 0.08 |
| Chrysoeriol 7-O- neohesperidoside -4′-O- glucoside | ------ | ------ | ------ | 0.70 ± 0.03 | ------ |
| Nobiletin | ------ | 0.33 ± 0.19 | 1.04 ± 0.10 | 1.25 ± 0.17 | 0.84 ± 0.05 |
| Apigenin | 9.67 ± 0.12 | ------ | ------ | ------ | ------ |
| Heptamethoxy-flavone | ------ | 0.08 ± 0.06 | ------ | ------ | 0.07 ± 0.007 |
| Quercetogetin | ------ | ------ | ------ | ------ | 0.06 ± 0.006 |
| [31] | [22] | [30] | [30] | [30] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barreca, D.; Mandalari, G.; Calderaro, A.; Smeriglio, A.; Trombetta, D.; Felice, M.R.; Gattuso, G. Citrus Flavones: An Update on Sources, Biological Functions, and Health Promoting Properties. Plants 2020, 9, 288. https://doi.org/10.3390/plants9030288
Barreca D, Mandalari G, Calderaro A, Smeriglio A, Trombetta D, Felice MR, Gattuso G. Citrus Flavones: An Update on Sources, Biological Functions, and Health Promoting Properties. Plants. 2020; 9(3):288. https://doi.org/10.3390/plants9030288
Chicago/Turabian StyleBarreca, Davide, Giuseppina Mandalari, Antonella Calderaro, Antonella Smeriglio, Domenico Trombetta, Maria Rosa Felice, and Giuseppe Gattuso. 2020. "Citrus Flavones: An Update on Sources, Biological Functions, and Health Promoting Properties" Plants 9, no. 3: 288. https://doi.org/10.3390/plants9030288
APA StyleBarreca, D., Mandalari, G., Calderaro, A., Smeriglio, A., Trombetta, D., Felice, M. R., & Gattuso, G. (2020). Citrus Flavones: An Update on Sources, Biological Functions, and Health Promoting Properties. Plants, 9(3), 288. https://doi.org/10.3390/plants9030288










