Study on Thermal Decomposition Behavior, Gaseous Products, and Kinetic Analysis of Bis-(Dimethylglyoximato) Nickel(II) Complex Using TG-DSC-FTIR-MS Technique
Abstract
:1. Introduction
2. Results and Discussion
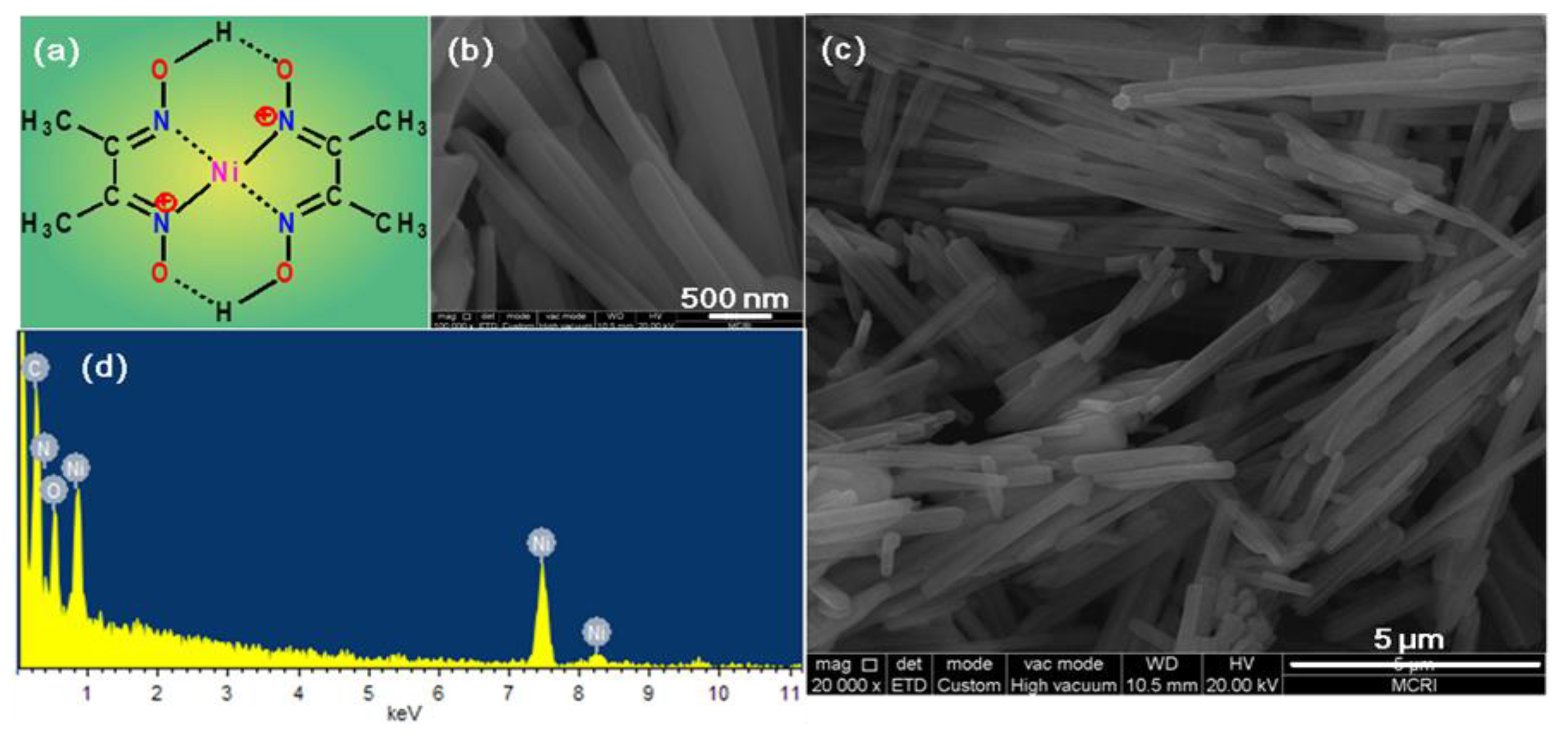
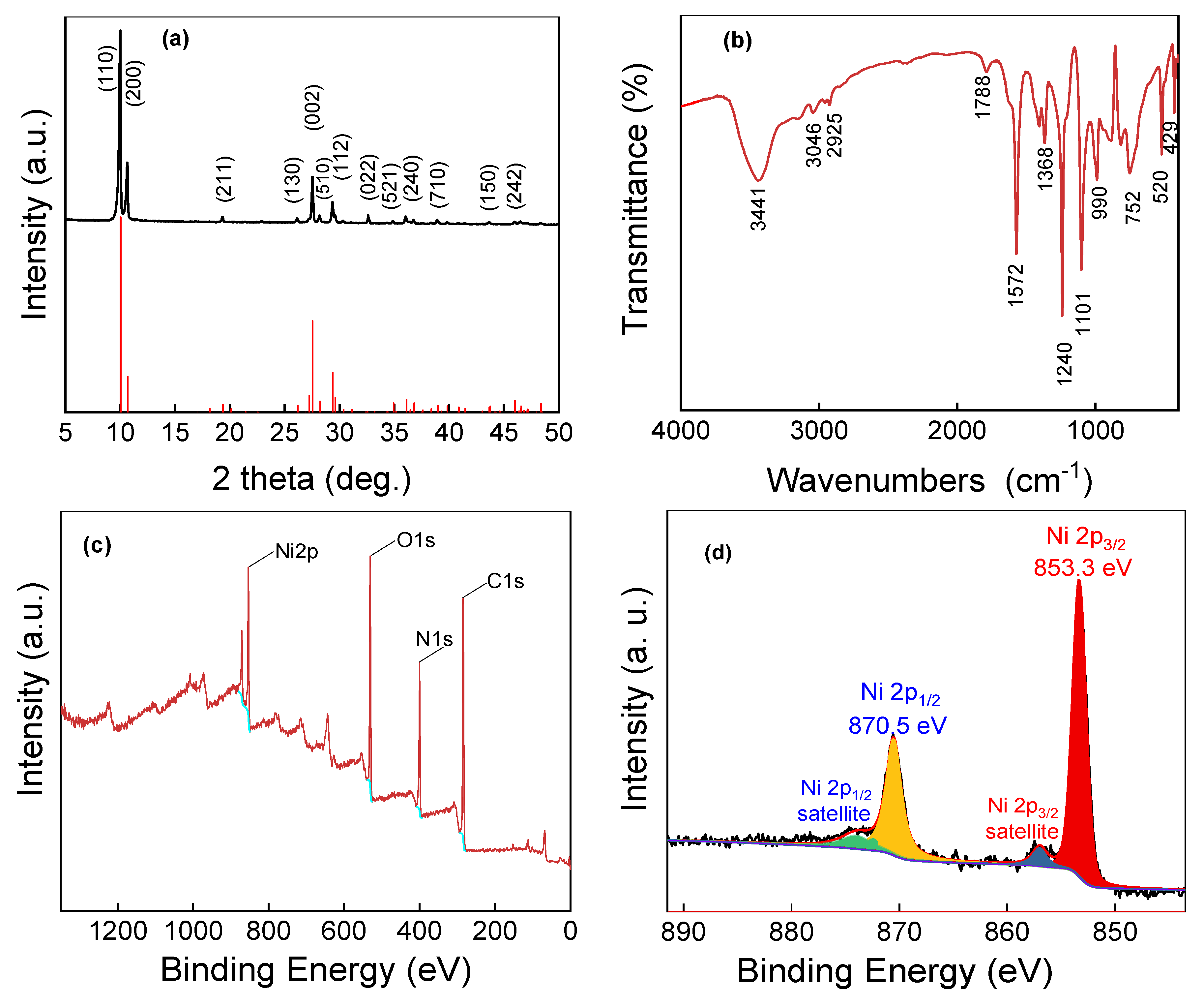
2.1. Morphology and Structure Characterization
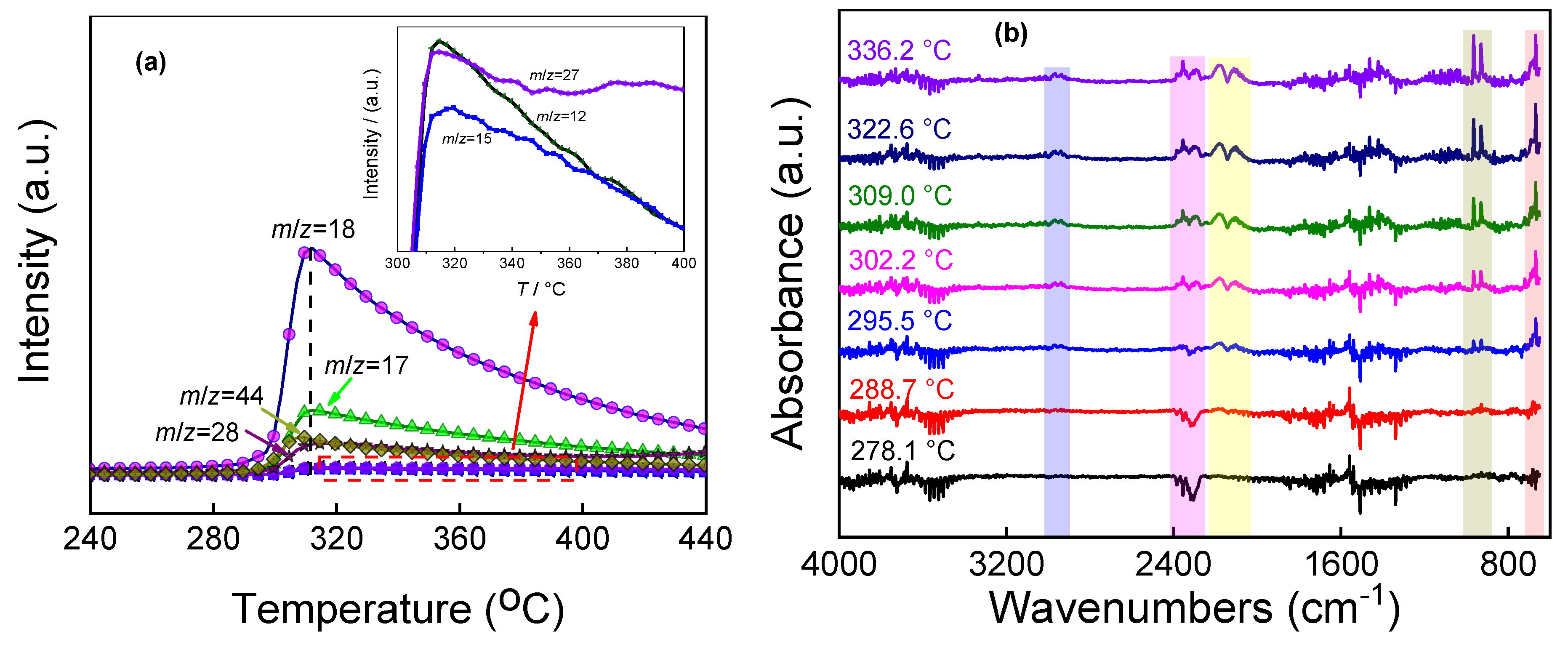
2.2. Thermal Decomposition Behavior
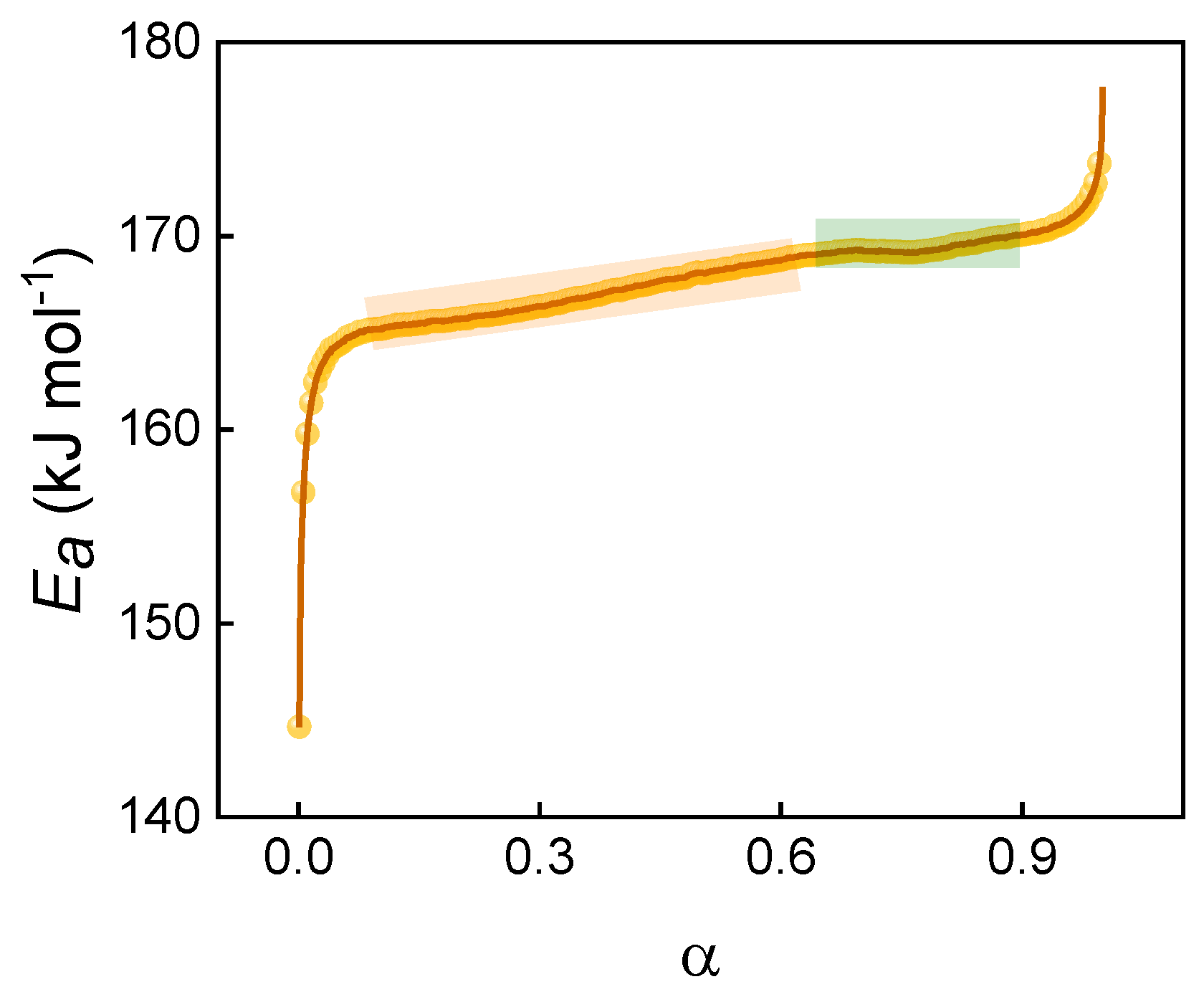
2.3. Non-Isothermal Decomposition Reaction Kinetics
3. Experimental
3.1. Materials
3.2. Synthesis of Ni(DMG)2
3.3. Apparatus and Measurements
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yi, J.; Zhao, F.; Wang, B.; Liu, Q.; Zhou, C.; Hu, R.; Ren, Y.; Xu, S.; Xu, K.; Ren, X. Thermal behaviors, nonisothermal decomposition reaction kinetics, thermal safety and burning rates of BTATz-CMDB propellant. J. Hazard. Mater. 2010, 181, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, F.; Ji, Y.; Yi, J.; An, T.; Liu, W. Synthesis, crystal structure and thermal behavior of 4-amino-3,5-dinitropyrazole copper salt. Chin. Chem. Lett. 2014, 25, 902–906. [Google Scholar] [CrossRef]
- Gao, J.; Wang, L.; Yu, H.; Xiao, A.; Ding, W. Recent research progress in burning rate catalysts. Propellants Explos. Pyrotech. 2011, 36, 404–409. [Google Scholar] [CrossRef]
- Zhao, N.; Li, J.; Gong, H.; An, T.; Zhao, F.; Yang, A.; Hu, R.; Ma, H. Effects of α-Fe2O3 nanoparticles on the thermal behavior and non-isothermal decomposition kinetics of nitrocellulose. J. Anal. Appl. Pyrolysis 2016, 120, 165–173. [Google Scholar] [CrossRef]
- Pei, J.; Zhao, F.; Song, X.; Ren, X.; Gao, H.; An, T.; An, J.; Hu, R. Effects of nano-CuO particles on thermal decomposition behavior and decomposition mechanism of BAMO–GAP copolymer. J. Anal. Appl. Pyrolysis 2015, 112, 88–93. [Google Scholar] [CrossRef]
- Patil, P.R.; Krishnamurthy, V.N.; Joshi, S.S. Effect of nano-copper oxide and copper chromite on the thermal decomposition of ammonium perchlorate. Propellants Explos. Pyrotech. 2008, 33, 266–270. [Google Scholar] [CrossRef]
- Mahinroosta, M. Catalytic effect of commercial nano-CuO and nano-Fe2O3 on thermal decomposition of ammonium perchlorate. J. Nanostruct. Chem. 2013, 3, 47. [Google Scholar] [CrossRef] [Green Version]
- Budhwar, A.S.; Gautam, A.; More, P.V.; Pant, C.S.; Banerjee, S.; Khanna, P.K. Modified iron oxide nanoparticles as burn rate enhancer in composite solid propellants. Vacuum 2018, 156, 483–491. [Google Scholar] [CrossRef]
- Hao, G.; Liu, J.; Xiao, L.; Gao, H.; Qiao, Y.; Jiang, W.; Zhao, F.; Gao, H. Effect of drying methods on catalytic performance of nano-sized copper β-resorcylate. J. Therm. Anal. Calorim. 2016, 124, 1367–1374. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, T.; Song, N.; Zhang, L.; Chen, Z.; Yang, L.; Zhou, Z. Assembly of composites into a core–shell structure using ultrasonic spray drying and catalytic application in the thermal decomposition of ammonium perchlorate. RSC Adv. 2016, 6, 71223–71231. [Google Scholar] [CrossRef]
- Liu, J.; Qiu, H.; Han, J.; Yang, L. Synthesis of energetic complexes [Co(en)(H2BTI)2]2 ⋅ en, [Cu2(en)2(HBTI)2]2 and catalytic study on thermal decomposition of ammonium perchlorate. Propellants Explos. Pyrotech. 2019, 4, 816–820. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhao, F.; Yang, Y.; Song, X.; Gao, H.; Xu, S. Comparative investigation of different nano-metal materials on combustion properties of DB and CMDB propellants. Int. J. Energetic Mater. Chem. Propuls. 2017, 16, 219–229. [Google Scholar] [CrossRef]
- Pang, W.; De Luca, L.T.; Fan, X.; Maggi, F.; Xu, H.; Xie, W.; Shi, X. Effects of different nano-sized metal oxide catalysts on the properties of composite solid propellants. Combust. Sci. Technol. 2016, 188, 315–328. [Google Scholar] [CrossRef]
- Pang, W.; Fan, X.; Zhao, F.; Zhang, W.; Xu, H.; Yu, H.; Xie, W.; Yan, N.; Liu, F. Effects of different nano-metric particles on the properties of composite solid propellants. Propellants Explos. Pyrotech. 2014, 39, 329–336. [Google Scholar] [CrossRef]
- Sharma, J.K.; Srivastava, P.; Singh, G.; Akhtar, M.S.; Ameen, S. Biosynthesized NiO nanoparticles: Potential catalyst for ammonium perchlorate and composite solid propellants. Ceram. Int. 2015, 41, 1573–1578. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, D.; Bi, F.; Fan, X.; Zhao, F.; Zhang, G.; Zhang, W.; Gao, Z. Synthesis, characterization, migration studies and combustion catalytic performances of energetic ionic binuclear ferrocene compounds. J. Organomet. Chem. 2014, 762, 1–8. [Google Scholar] [CrossRef]
- Patra, S.G.; Mandal, N.; Datta, A.; Datta, D. On bonding in bis(dimethylglyoximato)nickel(II). Comput. Theor. Chem. 2017, 1114, 118–124. [Google Scholar] [CrossRef]
- Rath, M.; Behera, L.P.; Dash, B.; Sheik, A.R.; Sanjay, K. Recovery of dimethylglyoxime (DMG) from Ni-DMG complexes. Hydrometallurgy 2018, 176, 229–234. [Google Scholar] [CrossRef]
- Farhadi, S.; Kazem, M.; Siadatnasab, F. NiO nanoparticles prepared via thermal decomposition of the bis(dimethylglyoximato)nickel(II) complex: A novel reusable heterogeneous catalyst for fast and efficient microwave-assisted reduction of nitroarenes with ethanol. Polyhedron 2011, 30, 606–613. [Google Scholar] [CrossRef]
- Pang, H.; Lu, Q.; Li, Y.; Gao, F. Facile synthesis of nickel oxide nanotubes and their antibacterial, electrochemical and magnetic properties. Chem. Commun. 2009, 7542–7544. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Li, Z.; Qian, Y. Synthesis and characteristics of NiO nanoparticles by thermal decomposition of nickel dimethylglyoximate rods. Solid State Commun. 2006, 137, 581–584. [Google Scholar] [CrossRef]
- Sarkar, B.; Kwek, W.; Verma, D.; Kim, J. Effective vacuum residue upgrading using sacrificial nickel(II) dimethylglyoxime complex in supercritical methanol. Appl. Catal. A 2017, 545, 148–158. [Google Scholar] [CrossRef]
- Dakhel, A.A.; Ali-Mohamed Ahmed, Y. Electrical properties of thermally evaporated nickel-dimethylglyoxime thin films. J. Phys. Chem. Solids 2005, 66, 1080–1084. [Google Scholar] [CrossRef]
- Dakhel, A.A.; Ali-Mohamed Ahmed, Y.; Henari, F.Z. Structural and optical studies of evaporated bis-(dimethylglyoximato)nickel(II) thin films. Opt. Mater. 2006, 28, 925–929. [Google Scholar] [CrossRef]
- Takeda, K.; Hayashi, J.; Shirotani, I.; Fukuda, H.; Yakushi, K. Structural, optical, and electrical properties of one-dimensional bis(dimethylglyoximato)nickel(ii), Ni(dmg)2 at high pressure. Mol. Cryst. Liq. Cryst. 2006, 460, 131–144. [Google Scholar] [CrossRef]
- Godycki, L.E.; Rundle, R.E. The structure of nickel dimethylglyoxime. Acta Crystallogr. 1953, 6, 487–495. [Google Scholar] [CrossRef] [Green Version]
- Williams, D.E.; Wohlauer, G.; Rundle, R.E. Crystal structures of nickel and palladium dimethylglyoximes. J. Am. Chem. Soc. 1959, 81, 755–756. [Google Scholar] [CrossRef]
- Li, D.; Xu, D.; Xu, Y. Redetermination of bis(dimethylglyoximato-k2N,N′)nickel(II). Acta Crystallogr. Sect. E Struct. Rep. Online 2003, 59, m1094–m1095. [Google Scholar] [CrossRef] [Green Version]
- Bruce-Smith, I.F.; Zakharov, B.A.; Stare, J.; Boldyreva, E.V.; Pulham, C.R. Structural properties of nickel dimethylglyoxime at high pressure: Single-crystal X-ray diffraction and DFT studies. J. Phys. Chem. C 2014, 118, 24705–24713. [Google Scholar] [CrossRef]
- Xavier, K.O.; Chacko, J.; Mohammed Yusuff, K.K. Zeolite-encapsulated Co(II), Ni(II) and Cu(II) complexes as catalysts for partial oxidation of benzyl alcohol and ethylbenzene. Appl. Catal. A 2004, 258, 251–259. [Google Scholar] [CrossRef]
- Coropceanu, E.; Rudic, V.; Cepoi, L.; Rudi, L.; Lozan, V.; Chiriac, T.; Miscu, V.; Bulhac, I.; Kravtsov, V.; Bourosh, P. Synthesis and crystal structure of [Co(DmgH)2(Thio)2]2F[PF6]. The effect of fluorine-containing Co(III) dioximates on the physiological processes of the microalga porphyridium cruentum. Russ. J. Coord. Chem. 2019, 45, 200–207. [Google Scholar] [CrossRef]
- Yang, B.; Li, J.; Zhang, L.; Xu, G. A molecularly imprinted electrochemiluminescence sensor based on the mimetic enzyme catalytic effect for ultra-trace Ni2+ determination. Analyst 2016, 141, 5822–5828. [Google Scholar] [CrossRef] [PubMed]
- Szabó, A.; Kovács, A. Vibrational analysis of the bis(dimethylglyoximato)nickel(II) complex. J. Mol. Struct. 2003, 651–653, 547–553. [Google Scholar] [CrossRef]
- Adkhis, A.; Djebbar, S.; Benali-Baïtich, O.; Kadri, A.; Khan, M.A.; Bouet, G. Synthesis, characterization, and electrochemical behavior of mixed-ligand complexes of Cobalt(III) with dimethylglyoxime and some amino acids. Synth. React. Inorg. Met. Org. Chem. 2003, 33, 35–50. [Google Scholar] [CrossRef]
- Saxena, V.K.; Gupta, M.; Srivastava, M.N. Synthesis and characterization of complexes of Copper(II), Nickel(II), Cobalt(II) and Zinc(II) with histidine and glycine or alanine. Synth. React. Inorg. Met. Org. Chem. 1996, 26, 1661–1676. [Google Scholar] [CrossRef]
- Ponnuswamy, T.; Chyan, O. Detection of Ni2+ by a dimethylglyoxime probe using attenuated total-reflection infrared spectroscopy. Anal. Sci. 2002, 18, 449–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caleb Noble Chandar, S.; Sangeetha, D.; Arumugham, M.N. Synthesis, structure, CMC values, thermodynamics of micellization, steady-state photolysis and biological activities of hexadecylamine cobalt(III) dimethyl glyoximato complexes. Transit. Met. Chem. 2011, 36, 211–216. [Google Scholar] [CrossRef]
- Salih, B.M.M.; Satyanarayana, S. Vitamin B12 models: Synthesis and characterization of cyano bridged dicobaloximes and antimicrobial activity. Afr. J. Pure Appl. Chem. 2009, 3, 170–176. [Google Scholar]
- Cardoso, W.S.; Dias, V.L.N.; Costa, W.M.; de Araujo Rodrigues, I.; Marques, E.P.; Sousa, A.G.; Boaventura, J.; Bezerra, C.W.B.; Song, C.; Liu, H.; et al. Nickel-dimethylglyoxime complex modified graphite and carbon paste electrodes: Preparation and catalytic activity towards methanol/ethanol oxidation. J. Appl. Electrochem. 2009, 39, 55–64. [Google Scholar] [CrossRef]
- Panja, P.K.; Bala, S.; Pal, C.; Ghosh, P.N. Infrared spectroscopic studies of dimethylglyoxime chelates of nickel, cobalt, copper, palladium and platinum. J. Mol. Struct. 1991, 249, 277–283. [Google Scholar] [CrossRef]
- Toma, H.E.; Morino, L.A. Thermal decomposition of bis(dimethylglyoximate) iron(II) complexes containing axial N-heterocyclic ligands. J. Therm. Anal. 1990, 36, 7–15. [Google Scholar] [CrossRef]
- Ding, L.; Zhao, F.; Pan, Q.; Xu, H. Research on the thermal decomposition behavior of NEPE propellant containing CL-20. J. Anal. Appl. Pyrolysis 2016, 121, 121–127. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Tan, G.; Wang, Q.; Zheng, H.; Zhao, W.; Zhang, S.; Liu, Z. Concept of variable activation energy and its validity in nonisothermal kinetics. J. Phys. Chem. A 2011, 115, 5517–5524. [Google Scholar] [CrossRef]
- Zhou, D.; Grant, D.J.W. Model Dependence of the activation energy derived from nonisothermal kinetic data. J. Phys. Chem. A 2004, 108, 4239–4246. [Google Scholar] [CrossRef]
- Maraii, D.; Farjas, J.; Fontrodona, X.; Dammak, M. Synthesis, structural study, thermal, optical properties and characterization of the new compound [C6H7N2O2]3TeCl5·2Cl. Chin. Chem. Lett. 2017, 28, 1773–1779. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Maqueda, L.A.; Criado, J.M.; Sánchez-Jiménez, P.E. Combined kinetic analysis of solid-state reactions: A powerful tool for the simultaneous determination of kinetic parameters and the kinetic model without previous assumptions on the reaction mechanism. J. Phys. Chem. A 2006, 110, 12456–12462. [Google Scholar] [CrossRef]
- Sánchez-Jiménez, P.E.; Pérez-Maqueda, L.A.; Perejón, A.; Criado, J.M. Combined kinetic analysis of thermal degradation of polymeric materials under any thermal pathway. Polym. Degrad. Stab. 2009, 94, 2079–2085. [Google Scholar] [CrossRef] [Green Version]
- García-Garrido, C.; Pérez-Maqueda, L.A.; Criado, J.M.; Sánchez-Jiménez, P.E. Combined kinetic analysis of multistep processes of thermal decomposition of polydimethylsiloxane silicone. Polymer 2018, 153, 558–564. [Google Scholar] [CrossRef]
- Šesták, J.; Berggren, G. Study of the kinetics of the mechanism of solid-state reactions at increasing temperatures. Thermochim. Acta 1971, 3, 1–12. [Google Scholar] [CrossRef]
- Gotor, F.J.; Criado, J.M.; Malek, J.; Koga, N. Kinetic analysis of solid-state reactions: The universality of master plots for analyzing isothermal and nonisothermal experiments. J. Phys. Chem. A 2000, 104, 10777–10782. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Dollimore, D. Linear and nonlinear procedures in isoconversional computations of the activation energy of nonisothermal reactions in solids. J. Chem. Inf. Comput. Sci. 1996, 36, 42–45. [Google Scholar] [CrossRef]
- Vyazovkin, S. Evaluation of activation energy of thermally stimulated solid-state reactions under arbitrary variation of temperature. J. Comput. Chem. 1997, 18, 393–402. [Google Scholar] [CrossRef]
- Vyazovkin, S. Modification of the integral isoconversional method to account for variation in the activation energy. J. Comput. Chem. 2001, 22, 178–183. [Google Scholar] [CrossRef]

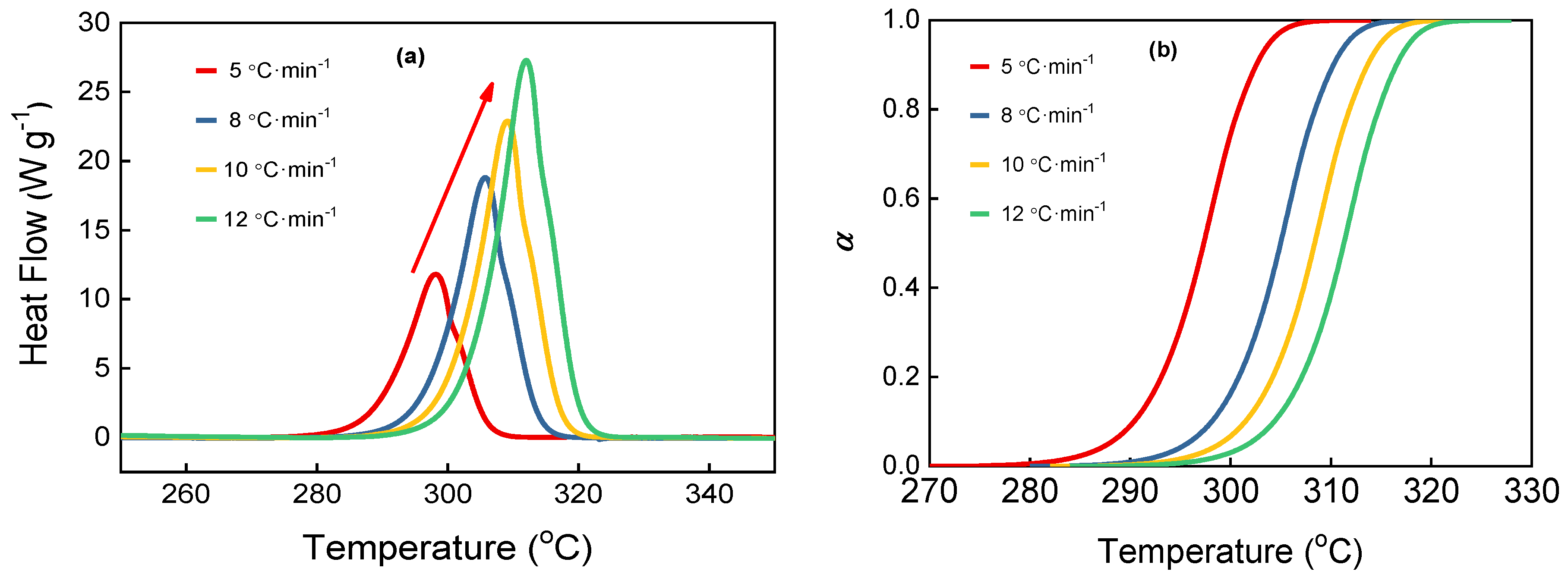

| β/°C·min−1 | Tonset/°C | Tp/°C | Tend/°C | ΔQ/J·g−1 | Combined Kinetic Analysis | ||||
|---|---|---|---|---|---|---|---|---|---|
| E/kJ·mol−1 | ln(cA/min−1) | m | n | r | |||||
| 5 | 289.2 | 298.2 | 303.5 | 1473 | 170.61 ± 0.65 | 36.46 ± 0.13 | 0.860 | 1.009 | 0.9977 |
| 8 | 297.0 | 305.7 | 311.2 | 1451 | |||||
| 10 | 300.5 | 309.2 | 314.5 | 1412 | |||||
| 12 | 303.6 | 312.0 | 313.8 | 1398 | |||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, E.; Xu, S.; Zhao, F.; Huang, T.; Li, H.; Zhao, N.; Yi, J.; Yang, Y.; Wang, C. Study on Thermal Decomposition Behavior, Gaseous Products, and Kinetic Analysis of Bis-(Dimethylglyoximato) Nickel(II) Complex Using TG-DSC-FTIR-MS Technique. Catalysts 2020, 10, 331. https://doi.org/10.3390/catal10030331
Yao E, Xu S, Zhao F, Huang T, Li H, Zhao N, Yi J, Yang Y, Wang C. Study on Thermal Decomposition Behavior, Gaseous Products, and Kinetic Analysis of Bis-(Dimethylglyoximato) Nickel(II) Complex Using TG-DSC-FTIR-MS Technique. Catalysts. 2020; 10(3):331. https://doi.org/10.3390/catal10030331
Chicago/Turabian StyleYao, Ergang, Siyu Xu, Fengqi Zhao, Taizhong Huang, Haijian Li, Ningning Zhao, Jianhua Yi, Yanjing Yang, and Changjian Wang. 2020. "Study on Thermal Decomposition Behavior, Gaseous Products, and Kinetic Analysis of Bis-(Dimethylglyoximato) Nickel(II) Complex Using TG-DSC-FTIR-MS Technique" Catalysts 10, no. 3: 331. https://doi.org/10.3390/catal10030331
APA StyleYao, E., Xu, S., Zhao, F., Huang, T., Li, H., Zhao, N., Yi, J., Yang, Y., & Wang, C. (2020). Study on Thermal Decomposition Behavior, Gaseous Products, and Kinetic Analysis of Bis-(Dimethylglyoximato) Nickel(II) Complex Using TG-DSC-FTIR-MS Technique. Catalysts, 10(3), 331. https://doi.org/10.3390/catal10030331





