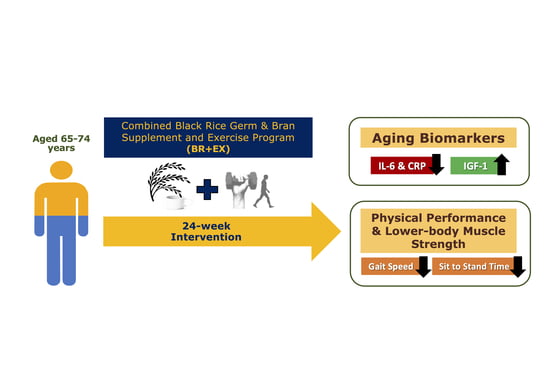
Combined Black Rice Germ, Bran Supplement and Exercise Intervention Modulate Aging Biomarkers and Improve Physical Performance and Lower-Body Muscle Strength Parameters in Aging Population
Abstract
:1. Introduction
2. Materials and Methods
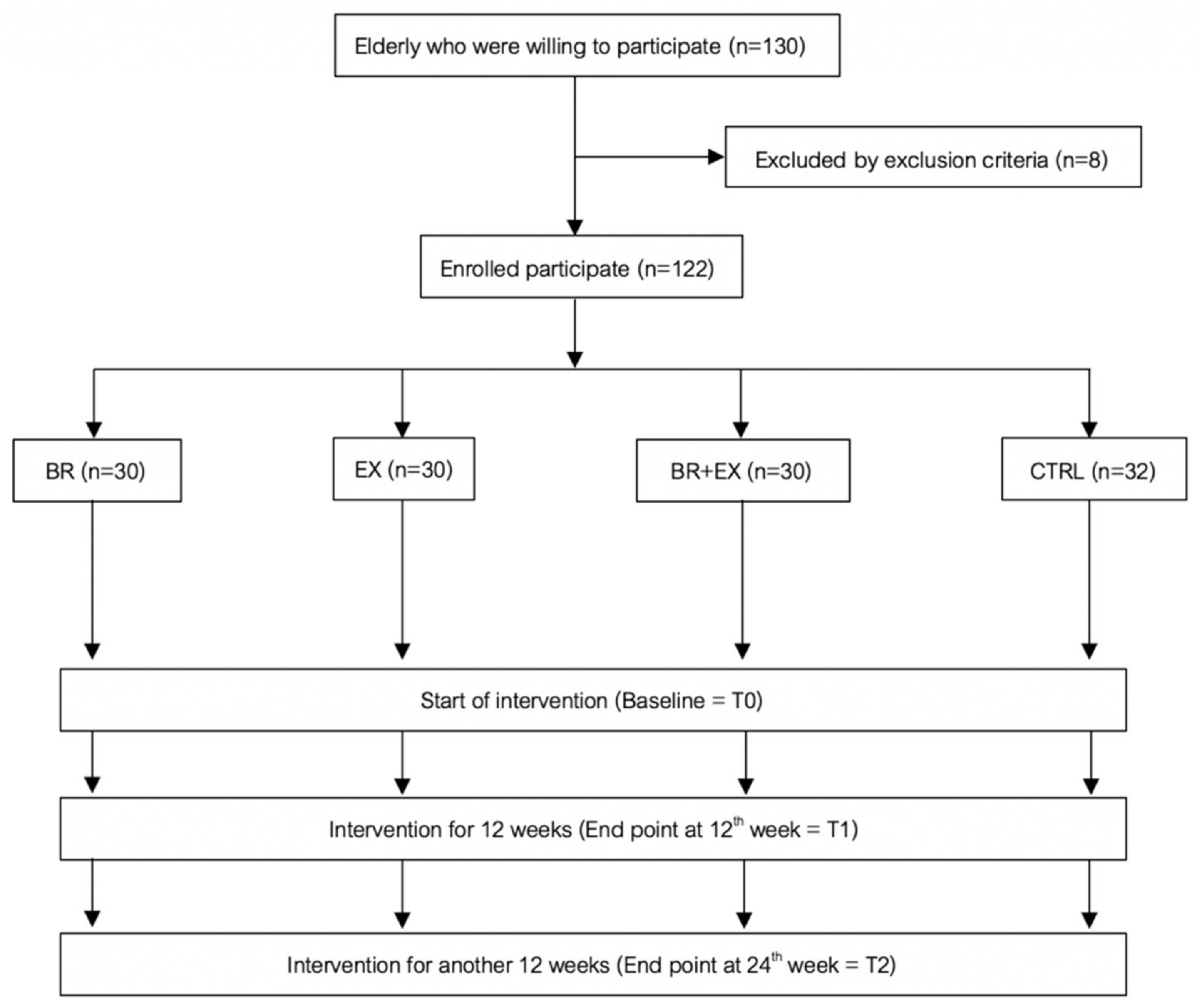
2.1. Study Design and Participants
2.2. Intervention Programs
2.3. Outcome Measurements
2.4. Sample Size Calculation and Statistical Analysis
2.5. Ethical Consideration
3. Results
3.1. Baseline Descriptive Data of Sociodemographic Characteristics and Health Profile of the Aging Population
3.2. Changes in Blood-Based Health Profile and Aging Biomarkers during 24-Week Intervention Period
3.2.1. Changes in Blood-Based Health Profile
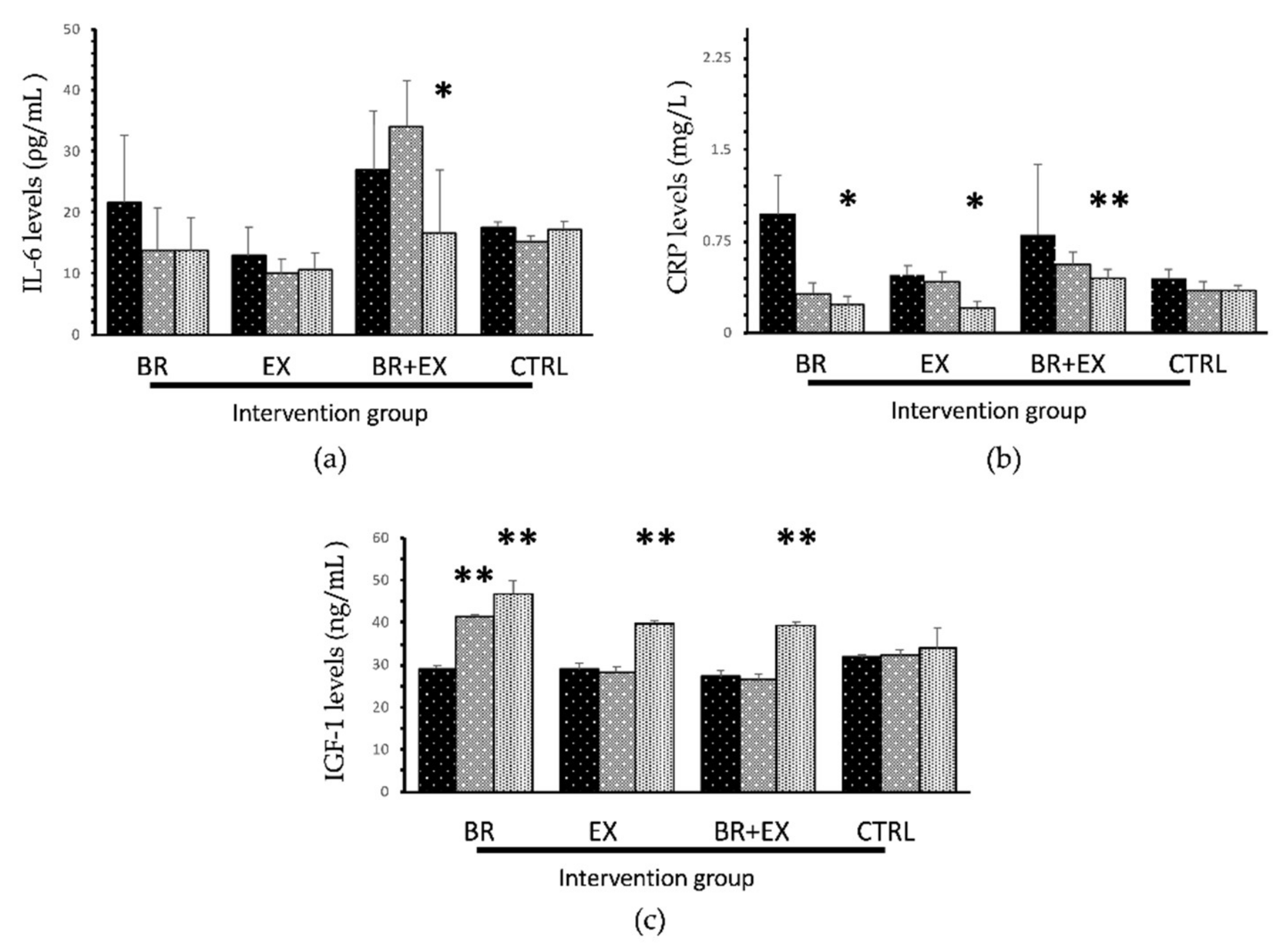
3.2.2. Changes in Aging Biomarkers
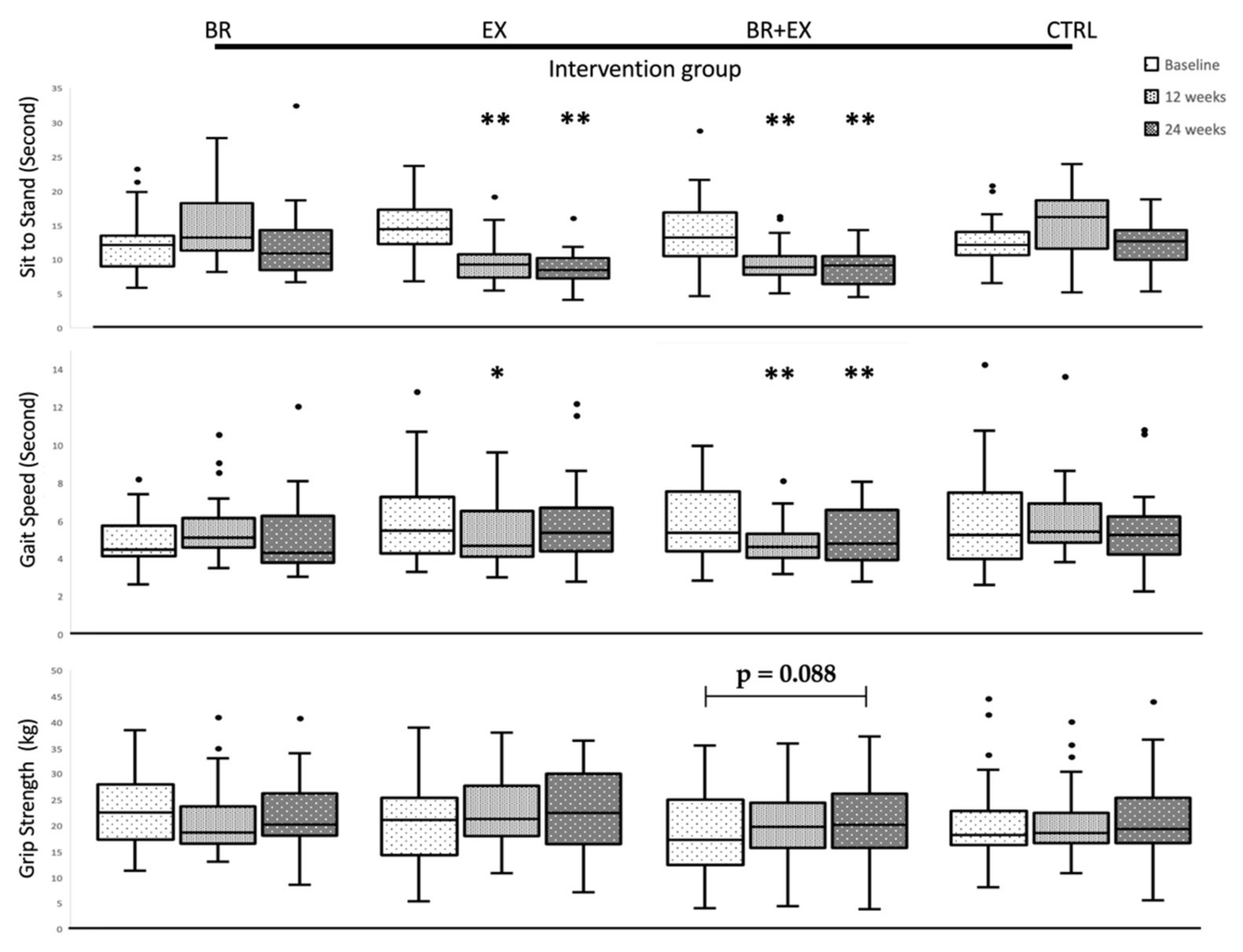
3.3. Changes in Physical Performance and Muscle Strength during 24-Week Intervention Period
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- United Nations Department for Economic and Social Affairs. World Population Prospects 2019: Highlights; United Nations Department for Economic and Social Affairs: New York, NY, USA, 2019. [Google Scholar]
- Institute for Population and Social Research MU. Situation of Thai Elderly 2015; Foundation of Thai Gerontology Research and Development Institute: Bangkok, Thailand, 2015; p. 132. [Google Scholar]
- Fulop, T.; Larbi, A.; Dupuis, G.; Le Page, A.; Frost, E.H.; Cohen, A.A.; Witkowski, J.M.; Franceschi, C. Immunosenescence and inflamm-aging as two sides of the same coin: Friends or foes? Front. Immunol. 2018, 8, 1960. [Google Scholar] [CrossRef] [Green Version]
- Franceschi, C.; Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2014, 69 (Suppl. 1), S4–S9. [Google Scholar] [CrossRef]
- Janssen, I.; Heymsfield, S.B.; Wang, Z.; Ross, R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J. Appl. Physiol. 2000, 89, 81–88. [Google Scholar] [CrossRef] [Green Version]
- Janssen, I.; Heymsfield, S.B.; Ross, R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J. Am. Geriatr. Soc. 2002, 50, 889–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szulc, P.; Beck, T.J.; Marchand, F.; Delmas, P.D. Low skeletal muscle mass is associated with poor structural parameters of bone and impaired balance in elderly men—The MINOS study. J. Bone Miner. Res. 2005, 20, 721–729. [Google Scholar] [CrossRef]
- Baumann, C.W.; Kwak, D.; Liu, H.M.; Thompson, L.V. Age-induced oxidative stress: How does it influence skeletal muscle quantity and quality? J. Appl. Physiol. 2016, 121, 1047–1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newman, A.B.; Kupelian, V.; Visser, M.; Simonsick, E.M.; Goodpaster, B.H.; Kritchevsky, S.B.; Tylavsky, F.A.; Rubin, S.M.; Harris, T.B. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2006, 61, 72–77. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [Green Version]
- Linton, P.; Thoman, M.L. T cell senescence. Front. Biosci. J. Virtual Libr. 2001, 6, D248–D261. [Google Scholar] [CrossRef]
- Kirkwood, T. Understanding ageing from an evolutionary perspective. J. Intern. Med. 2008, 263, 117–127. [Google Scholar] [CrossRef]
- Spadaro, O.; Goldberg, E.L.; Camell, C.D.; Youm, Y.-H.; Kopchick, J.J.; Nguyen, K.Y.; Bartke, A.; Sun, L.Y.; Dixit, V.D. Growth hormone receptor deficiency protects against age-related NLRP3 inflammasome activation and immune senescence. Cell Rep. 2016, 14, 1571–1580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santy-Tomlinson, J.; Speerin, R.; Hertz, K.; Tochon-Laruaz, A.C.; van Oostwaard, M. Frailty, Sarcopenia and Falls. In Fragility Fracture Nursing; Hertz, K., Santy-Tomlinson, J., Eds.; Springer: Cham, Switzerland, 2018; pp. 15–26. [Google Scholar]
- Fulle, S.; Protasi, F.; Di Tano, G.; Pietrangelo, T.; Beltramin, A.; Boncompagni, S.; Vecchiet, L.; Fanò, G. The contribution of reactive oxygen species to sarcopenia and muscle ageing. Exp. Gerontol. 2004, 39, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Semba, R.D.; Ferrucci, L.; Sun, K.; Walston, J.; Varadhan, R.; Guralnik, J.M.; Fried, L.P. Oxidative stress and severe walking disability among older women. Am. J. Med. 2007, 120, 1084–1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semmarath, W.; Seesen, M.; Yodkeeree, S.; Sapbamrer, R.; Ayood, P.; Malasao, R.; Siviroj, P.; Limtrakul (Dejkriengkraikul), P. The Association between Frailty Indicators and Blood-Based Biomarkers in Early-Old Community Dwellers of Thailand. Int. J. Environ. Res. Public Health 2019, 16, 3457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabbri, E.; An, Y.; Zoli, M.; Simonsick, E.M.; Guralnik, J.M.; Bandinelli, S.; Boyd, C.M.; Ferrucci, L. Aging and the burden of multimorbidity: Associations with inflammatory and anabolic hormonal biomarkers. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2014, 70, 63–70. [Google Scholar] [CrossRef]
- Rajpathak, S.N.; McGinn, A.P.; Strickler, H.D.; Rohan, T.E.; Pollak, M.; Cappola, A.R.; Kuller, L.; Xue, X.; Newman, A.B.; Strotmeyer, E.S. Insulin-like growth factor-(IGF)-axis, inflammation, and glucose intolerance among older adults. Growth Horm. IGF Res. 2008, 18, 166–173. [Google Scholar] [CrossRef] [Green Version]
- Maggio, M.; Cattabiani, C.; Lauretani, F.; Bandinelli, S.; De Vita, F.; Dall’Aglio, E.; Corsonello, A.; Lattanzio, F.; Paolisso, G.; Ferrucci, L. Insulin-like growth factor-1 bioactivity plays a prosurvival role in older participants. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2013, 68, 1342–1350. [Google Scholar] [CrossRef] [Green Version]
- Garatachea, N.; Pareja-Galeano, H.; Sanchis-Gomar, F.; Santos-Lozano, A.; Fiuza-Luces, C.; Morán, M.; Emanuele, E.; Joyner, M.J.; Lucia, A. Exercise attenuates the major hallmarks of aging. Rejuvenation Res. 2015, 18, 57–89. [Google Scholar] [CrossRef] [Green Version]
- Nicklas, B.J.; Brinkley, T.E. Exercise training as a treatment for chronic inflammation in the elderly. Exerc. Sport Sci. Rev. 2009, 37, 165–170. [Google Scholar] [CrossRef]
- Giné-Garriga, M.; Guerra, M.; Pagès, E.; Manini, T.M.; Jiménez, R.; Unnithan, V.B. The effect of functional circuit training on physical frailty in frail older adults: A randomized controlled trial. J. Aging Phys. Act. 2010, 18, 401–424. [Google Scholar] [CrossRef] [Green Version]
- Kidd, T.; Mold, F.; Jones, C.; Ream, E.; Grosvenor, W.; Sund-Levander, M.; Tingström, P.; Carey, N. What are the most effective interventions to improve physical performance in pre-frail and frail adults? A systematic review of randomised control trials. BMC Geriatr. 2019, 19, 184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Z.-B.; Maeda, A.; Shima, N.; Kurata, H.; Nishizono, H. The effect of a 12-week combined exercise intervention program on physical performance and gait kinematics in community-dwelling elderly women. J. Physiol. Anthropol. 2007, 26, 325–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, D.; Lin, Z.; Li, S.; Liu, S.-J. Effect of nutritional supplement combined with exercise intervention on sarcopenia in the elderly: A meta-analysis. Int. J. Nurs. Sci. 2017, 4, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Shahar, S.; Kamaruddin, N.S.; Badrasawi, M.; Sakian, N.I.M.; Manaf, Z.A.; Yassin, Z.; Joseph, L. Effectiveness of exercise and protein supplementation intervention on body composition, functional fitness, and oxidative stress among elderly Malays with sarcopenia. Clin. Interv. Aging 2013, 8, 1365–1375. [Google Scholar] [CrossRef] [Green Version]
- Mocchegiani, E.; Costarelli, L.; Giacconi, R.; Cipriano, C.; Muti, E.; Tesei, S.; Malavolta, M. Nutrient–gene interaction in ageing and successful ageing: A single nutrient (Zinc) and some target genes related to inflammatory/immune response. Mech. Ageing Dev. 2006, 127, 517–525. [Google Scholar] [CrossRef]
- Alarcon De La Lastra, C.; Villegas, I. Resveratrol as an anti-inflammatory and anti-aging agent: Mechanisms and clinical implications. Mol. Nutr. Food Res. 2005, 49, 405–430. [Google Scholar] [CrossRef]
- Olesen, J.; Ringholm, S.; Nielsen, M.M.; Brandt, C.T.; Pedersen, J.T.; Halling, J.F.; Goodyear, L.J.; Pilegaard, H. Role of PGC-1α in exercise training-and resveratrol-induced prevention of age-associated inflammation. Exp. Gerontol. 2013, 48, 1274–1284. [Google Scholar] [CrossRef] [Green Version]
- Wahab, A.; Gao, K.; Jia, C.; Zhang, F.; Tian, G.; Murtaza, G.; Chen, J. Significance of resveratrol in clinical management of chronic diseases. Molecules 2017, 22, 1329. [Google Scholar] [CrossRef] [Green Version]
- Bakhtiari, M.; Panahi, Y.; Ameli, J.; Darvishi, B. Protective effects of flavonoids against Alzheimer’s disease-related neural dysfunctions. Biomed. Pharmacother. 2017, 93, 218–229. [Google Scholar] [CrossRef]
- Xia, S.; Zhang, X.; Zheng, S.; Khanabdali, R.; Kalionis, B.; Wu, J.; Wan, W.; Tai, X. An update on inflamm-aging: Mechanisms, prevention, and treatment. J. Immunol. Res. 2016, 2016, 8426874. [Google Scholar] [CrossRef]
- Samyor, D.; Das, A.B.; Deka, S.C. Pigmented rice a potential source of bioactive compounds: A review. Int. J. Food Sci. Technol. 2017, 52, 1073–1081. [Google Scholar] [CrossRef]
- Sohail, M.; Rakha, A.; Butt, M.S.; Iqbal, M.J.; Rashid, S. Rice bran nutraceutics: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2017, 57, 3771–3780. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Hu, Z.; Yu, Y.; Mou, R.; Zhu, Z.; Beta, T. Phenolic acids, anthocyanins, proanthocyanidins, antioxidant activity, minerals and their correlations in non-pigmented, red, and black rice. Food Chem. 2018, 239, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Limtrakul, P.; Semmarath, W.; Mapoung, S. Anthocyanins and Proanthocyanidins in Natural Pigmented Rice and Their Bioactivities. In Phytochemicals in Human Health; IntechOpen: London, UK, 2019; pp. 1–25. [Google Scholar]
- Moongngarm, A.; Daomukda, N.; Khumpika, S. Chemical compositions, phytochemicals, and antioxidant capacity of rice bran, rice bran layer, and rice germ. Apcbee Procedia 2012, 2, 73–79. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.W.; Zhang, R.F.; Zhang, F.X.; Liu, R.H. Phenolic profiles and antioxidant activity of black rice bran of different commercially available varieties. J. Agric. Food Chem. 2010, 58, 7580–7587. [Google Scholar] [CrossRef]
- Pojer, E.; Mattivi, F.; Johnson, D.; Stockley, C.S. The case for anthocyanin consumption to promote human health: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 483–508. [Google Scholar] [CrossRef]
- Dias, A.L.d.S.; Pachikian, B.; Larondelle, Y.; Quetin-Leclercq, J. Recent advances on bioactivities of black rice. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 470–476. [Google Scholar] [CrossRef]
- Limtrakul, P.; Yodkeeree, S.; Pitchakarn, P.; Punfa, W. Suppression of inflammatory responses by black rice extract in RAW 264.7 macrophage cells via downregulation of NF-kB and AP-1 signaling pathways. Asian Pac. J. Cancer Prev. 2015, 16, 4277–4283. [Google Scholar] [CrossRef] [Green Version]
- Ichikawa, H.; Ichiyanagi, T.; Xu, B.; Yoshii, Y.; Nakajima, M.; Konishi, T. Antioxidant activity of anthocyanin extract from purple black rice. J. Med. Food 2001, 4, 211–218. [Google Scholar] [CrossRef]
- Bhawamai, S.; Lin, S.-H.; Hou, Y.-Y.; Chen, Y.-H. Thermal cooking changes the profile of phenolic compounds, but does not attenuate the anti-inflammatory activities of black rice. Food Nutr. Res. 2016, 60, 32941. [Google Scholar] [CrossRef]
- Hiemori, M.; Koh, E.; Mitchell, A.E. Influence of cooking on anthocyanins in black rice (Oryza sativa L. japonica var. SBR). J. Agric. Food Chem. 2009, 57, 1908–1914. [Google Scholar] [CrossRef] [PubMed]
- National Statistical Office. Report on the 2017 Survey of the Older Persons in Thailand; Statistical Forecasting Division, National Statistical Office: Bangkok, Thailand, 2018; p. 266.
- Karlsen, A.; Retterstøl, L.; Laake, P.; Paur, I.; Kjølsrud-Bøhn, S.; Sandvik, L.; Blomhoff, R. Anthocyanins inhibit nuclear factor-κ B activation in monocytes and reduce plasma concentrations of pro-inflammatory mediators in healthy adults. J. Nutr. 2007, 137, 1951–1954. [Google Scholar] [CrossRef] [PubMed]
- Paw, M.J.C.A.; Chin, A.; van Uffelen, J.G.; Riphagen, I.; van Mechelen, W. The functional effects of physical exercise training in frail older people. Sports Med. 2008, 38, 781–793. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Park, I.; Joo Lee, H.; Lee, O. The reliability and validity of gait speed with different walking pace and distances against general health, physical function, and chronic disease in aged adults. J. Exerc. Nutr. Biochem. 2016, 20, 46–50. [Google Scholar] [CrossRef]
- Desrosiers, J.; Bravo, G.; Hebert, R.; Dutil, E. Normative data for grip strength of elderly men and women. Am. J. Occup. Ther. 1995, 49, 637–644. [Google Scholar] [CrossRef] [Green Version]
- Fox, B.; Henwood, T.; Schaap, L.; Bruyère, O.; Reginster, J.-Y.; Beaudart, C.; Buckinx, F.; Roberts, H.; Cooper, C.; Cherubini, A. Adherence to a standardized protocol for measuring grip strength and appropriate cut-off values in adults over 65 years with sarcopenia: A systematic review protocol. JBI Database Syst. Rev. Implement. Rep. 2015, 13, 50–59. [Google Scholar] [CrossRef]
- Whitney, S.L.; Wrisley, D.M.; Marchetti, G.F.; Gee, M.A.; Redfern, M.S.; Furman, J.M. Clinical measurement of sit-to-stand performance in people with balance disorders: Validity of data for the Fives-Times-Sit-to-Stand Test. Phys. Ther. 2005, 85, 1034–1045. [Google Scholar] [CrossRef] [Green Version]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G. Frailty in older adults: Evidence for a phenotype. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M146–M157. [Google Scholar] [CrossRef]
- Silpakit, O.; Silpakit, C.; Pukdeenaul, P. A comparison study of cognitive impairment screening tools: CDT, IQCODE VS MMSE. Siriraj Med. J. 2007, 59, 361–363. [Google Scholar]
- Tracy, R. Emerging relationships of inflammation, cardiovascular disease and chronic diseases of aging. Int. J. Obes. 2003, 27, S29–S34. [Google Scholar] [CrossRef] [Green Version]
- Bektas, A.; Schurman, S.H.; Sen, R.; Ferrucci, L. Aging, inflammation and the environment. Exp. Gerontol. 2018, 105, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Freund, A.; Orjalo, A.V.; Desprez, P.-Y.; Campisi, J. Inflammatory networks during cellular senescence: Causes and consequences. Trends Mol. Med. 2010, 16, 238–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, H.Y.; Cesari, M.; Anton, S.; Marzetti, E.; Giovannini, S.; Seo, A.Y.; Carter, C.; Yu, B.P.; Leeuwenburgh, C. Molecular inflammation: Underpinnings of aging and age-related diseases. Ageing Res. Rev. 2009, 8, 18–30. [Google Scholar] [CrossRef] [Green Version]
- Elis, S.; Wu, Y.; Courtland, H.W.; Sun, H.; Rosen, C.J.; Adamo, M.L.; Yakar, S. Increased serum IGF-1 levels protect the musculoskeletal system but are associated with elevated oxidative stress markers and increased mortality independent of tissue igf1 gene expression. Aging Cell 2011, 10, 547–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging: An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, S.; Walston, J.D. Frailty in older adults: Insights and interventions. Clevel. Clin. J. Med. 2005, 72, 1105–1112. [Google Scholar] [CrossRef]
- Roubenoff, R. Sarcopenia: A major modifiable cause of frailty in the elderly. J. Nutr. Health Aging 2000, 4, 140–142. [Google Scholar]
- Giovannini, S.; Marzetti, E.; Borst, S.E.; Leeuwenburgh, C. Modulation of GH/IGF-1 axis: Potential strategies to counteract sarcopenia in older adults. Mech. Ageing Dev. 2008, 129, 593–601. [Google Scholar] [CrossRef]
- Taekema, D.G.; Ling, C.H.; Blauw, G.J.; Meskers, C.G.; Westendorp, R.G.; De Craen, A.J.; Maier, A.B. Circulating levels of IGF1 are associated with muscle strength in middle-aged-and oldest-old women. Eur. J. Endocrinol. 2011, 164, 189–196. [Google Scholar] [CrossRef] [Green Version]
- Leng, S.X.; Cappola, A.R.; Andersen, R.E.; Blackman, M.R.; Koenig, K.; Blair, M.; Walston, J.D. Serum levels of insulin-like growth factor-I (IGF-I) and dehydroepiandrosterone sulfate (DHEA-S), and their relationships with serum interleukin-6, in the geriatric syndrome of frailty. Aging Clin. Exp. Res. 2004, 16, 153–157. [Google Scholar] [CrossRef]
- Roubenoff, R.; Parise, H.; Payette, H.A.; Abad, L.W.; D’Agostino, R.; Jacques, P.F.; Wilson, P.W.; Dinarello, C.A.; Harris, T.B. Cytokines, insulin-like growth factor 1, sarcopenia, and mortality in very old community-dwelling men and women: The Framingham Heart Study. Am. J. Med. 2003, 115, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Theou, O.; Stathokostas, L.; Roland, K.P.; Jakobi, J.M.; Patterson, C.; Vandervoort, A.A.; Jones, G.R. The effectiveness of exercise interventions for the management of frailty: A systematic review. J. Aging Res. 2011, 2011, 569194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forbes, S.C.; Little, J.P.; Candow, D.G. Exercise and nutritional interventions for improving aging muscle health. Endocrine 2012, 42, 29–38. [Google Scholar] [CrossRef]
- Candow, D.G.; Chilibeck, P.D.; Abeysekara, S.; Zello, G.A. Short-term heavy resistance training eliminates age-related deficits in muscle mass and strength in healthy older males. J. Strength Cond. Res. 2011, 25, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Sung, B.; Aggarwal, B.B. Age-associated chronic diseases require age-old medicine: Role of chronic inflammation. Prev. Med. 2012, 54, S29–S37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imai, T.; Nakamura, M.; Ando, F.; Shimokata, H. Dietary supplement use by community-living population in Japan: Data from the National Institute for Longevity Sciences Longitudinal Study of Aging (NILS-LSA). J. Epidemiol. 2006, 16, 249–260. [Google Scholar] [CrossRef] [Green Version]
- Martucci, M.; Ostan, R.; Biondi, F.; Bellavista, E.; Fabbri, C.; Bertarelli, C.; Salvioli, S.; Capri, M.; Franceschi, C.; Santoro, A. Mediterranean diet and inflammaging within the hormesis paradigm. Nutr. Rev. 2017, 75, 442–455. [Google Scholar] [CrossRef] [Green Version]
- Novotny, J.A.; Baer, D.J.; Khoo, C.; Gebauer, S.K.; Charron, C.S. Cranberry juice consumption lowers markers of cardiometabolic risk, including blood pressure and circulating C-reactive protein, triglyceride, and glucose concentrations in adults. J. Nutr. 2015, 145, 1185–1193. [Google Scholar] [CrossRef] [Green Version]
- Shukitt-Hale, B.; Bielinski, D.F.; Lau, F.C.; Willis, L.M.; Carey, A.N.; Joseph, J.A. The beneficial effects of berries on cognition, motor behaviour and neuronal function in ageing. Br. J. Nutr. 2015, 114, 1542–1549. [Google Scholar] [CrossRef] [Green Version]
- Peng, C.-H.; Liu, L.-K.; Chuang, C.-M.; Chyau, C.-C.; Huang, C.-N.; Wang, C.-J. Mulberry water extracts possess an anti-obesity effect and ability to inhibit hepatic lipogenesis and promote lipolysis. J. Agric. Food Chem. 2011, 59, 2663–2671. [Google Scholar] [CrossRef]
- Xia, X.; Ling, W.; Ma, J.; Xia, M.; Hou, M.; Wang, Q.; Zhu, H.; Tang, Z. An anthocyanin-rich extract from black rice enhances atherosclerotic plaque stabilization in apolipoprotein E–deficient mice. J. Nutr. 2006, 136, 2220–2225. [Google Scholar] [CrossRef] [PubMed]
- Boirie, Y.; Morio, B.; Caumon, E.; Cano, N.J. Nutrition and protein energy homeostasis in elderly. Mech. Ageing Dev. 2014, 136, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Solerte, S.B.; Gazzaruso, C.; Bonacasa, R.; Rondanelli, M.; Zamboni, M.; Basso, C.; Locatelli, E.; Schifino, N.; Giustina, A.; Fioravanti, M. Nutritional supplements with oral amino acid mixtures increases whole-body lean mass and insulin sensitivity in elderly subjects with sarcopenia. Am. J. Cardiol. 2008, 101, S69–S77. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.M.; Verlaan, S.; Bautmans, I.; Brandt, K.; Donini, L.M.; Maggio, M.; McMurdo, M.E.; Mets, T.; Seal, C.; Wijers, S.L. Effects of a vitamin D and leucine-enriched whey protein nutritional supplement on measures of sarcopenia in older adults, the PROVIDE study: A randomized, double-blind, placebo-controlled trial. J. Am. Med. Dir. Assoc. 2015, 16, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Aizawa, J.; Nagasawa, H.; Gomi, I.; Kugota, H.; Nanjo, K.; Jinno, T.; Masuda, T.; Morita, S. Effects and feasibility of exercise therapy combined with branched-chain amino acid supplementation on muscle strengthening in frail and pre-frail elderly people requiring long-term care: A crossover trial. Appl. Physiol. Nutr. Metab. 2016, 41, 438–445. [Google Scholar] [CrossRef]
- Rondanelli, M.; Klersy, C.; Terracol, G.; Talluri, J.; Maugeri, R.; Guido, D.; Faliva, M.A.; Solerte, B.S.; Fioravanti, M.; Lukaski, H. Whey protein, amino acids, and vitamin D supplementation with physical activity increases fat-free mass and strength, functionality, and quality of life and decreases inflammation in sarcopenic elderly. Am. J. Clin. Nutr. 2016, 103, 830–840. [Google Scholar] [CrossRef]
- Hartati, F.K.; Widjanarko, S.B.; Widyaningsih, T.D.; Rifa’i, M. Anti-Inflammatory evaluation of black rice extract inhibits TNF-α, IFN-γ and IL-6 cytokines produced by immunocompetent cells. Food Agric. Immunol. 2017, 28, 1116–1125. [Google Scholar] [CrossRef]
- Bruun, J.M.; Helge, J.W.; Richelsen, B.; Stallknecht, B. Diet and exercise reduce low-grade inflammation and macrophage infiltration in adipose tissue but not in skeletal muscle in severely obese subjects. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E961–E967. [Google Scholar] [CrossRef]
- Pawelec, G.; Adibzadeh, M.; Pohla, H.; Schaudt, K. Immunosenescence: Ageing of the immune system. Immunol. Today 1995, 16, 420–422. [Google Scholar] [CrossRef]
- Correa, B.L.; Ornaghi, A.P.; Muller, G.C.; Engroff, P.; Lopes, R.P.; da Silva Filho, I.G.; Bosch, J.A.; Bonorino, C.; Bauer, M.E. The inverted CD4: CD8 ratio is associated with cytomegalovirus, poor cognitive and functional states in older adults. Neuroimmunomodulation 2014, 21, 206–212. [Google Scholar] [CrossRef]

| Average Nutrition Information | 100 g |
|---|---|
| Energy (Calories) | 300 Kcal. |
| Total Fat | 0 g |
| Cholesterol | 0 g |
| Total Carbohydrate | 80 g |
| Dietary Fiber | 8 g |
| Protein | 10 g |
| Sodium | 0.015 g |
| Vitamin B1 | 2 g |
| Iron | 1.5 g |
| Bioactive ingredients | g/100 g |
| Phenolic compounds | 5.21 ± 0.13 |
| Total flavonoids | 5.14 ± 0.06 |
| Tocopherol | 0.013 ± 0.002 |
| Anthocyanins | 3.2 ± 0.07 |
| Characteristics | BR Group (n = 30) | EX Group (n = 30) | BR + EX Group (n = 30) | CTRL Group (n = 32) | p-Value |
|---|---|---|---|---|---|
| Gender, n (%) | 0.653 | ||||
| Male | 11 (36.7) | 14 (46.7) | 12 (40) | 10 (31.3) | |
| Female | 19 (63.3) | 16 (53.3) | 18 (60) | 22 (68.8) | |
| Age, mean ± SD | 68.80 ± 2.82 | 68.17 ± 2.65 | 68.20 ± 2.41 | 69.06 ± 2.74 | 0.464 |
| Marital status, n (%) | 0.759 | ||||
| Single | 3 (10) | 2 (6.7) | 2 (6.7) | 5 (15.6) | |
| Married | 19 (63.3) | 21 (70) | 17 (56.7) | 22 (68.8) | |
| Separate/ divorce/ widow | 8 (26.7) | 7 (23.3) | 11 (36.7) | 5 (15.6) | |
| Number of comorbidities (mean ± SD) | 1.10 ± 0.88 | 0.96 ± 0.85 | 1.10 ± 1.14 | 1.0 ± 1.27 | 0.942 |
| Alcohol at present, n (%) | 3 (10) | 3 (10) | 4 (13.3) | 5 (15.6) | 0.883 |
| Smoking at present, n (%) | 2 (6.7) | 3 (10) | 4 (13.30 | 3 (9.4) | 0.863 |
| Depression score > 7, n (%) | 0 | 3 (10) | 3 (10) | 3 (9.4) | 0.365 |
| Frailty N (%) | 14 (46.7) | 14 (46.7) | 15 (50) | 16 (50) | 0.988 |
| Frailty score, mean ± SD | 1.43 ± 1.56 | 1.60 ± 1.77 | 1.66 ± 1.72 | 1.71 ± 1.80 | 0.239 |
| Parameter | Mean (SD) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BR Group (n = 30) | EX Group (n =30) | BR + EX Group (n =28) | CTRL Group (n = 32) | |||||||||||||
| T0 | T1 | T2 | p-Value | T0 | T1 | T2 | p-Value | T0 | T1 | T2 | p-Value | T0 | T1 | T2 | p-Value | |
| BUN (mg/dL) | 14.11 (5.15) | 14.11 (5.32) | 15.09 (5.46) | 0.329 | 15.30 (4.86) | 15.68 (4.58) | 16.34 (6.27) | 0.545 | 13.40 (4.67) | 13.91 (5.56) | 14.49 (4.57) | 0.240 | 13.60 (3.06) | 13.55 (3.87) | 14.84 (4.56) | 0.167 |
| Creatinine (mg/dL) | 0.92 (0.28) | 0.89 (0.23) | 0.87 (0.21) | 0.054 | 0.91 (0.24) | 0.90 (0.23) | 0.95 (0.31) | 0.052 | 0.89 (0.31) | 0.88 (0.27) | 0.89 (0.30) | 0.355 | 0.87 (0.22) | 0.86 (0.22) | 0.85 (0.22) | 0.108 |
| AST (U/L) | 23.67 (11.5) | 24.40 (14.3) | 24.10 (9.0) | 0.526 | 22.97 (10.7) | 22.60 (7.8) | 24.60 (11.0) | 0.156 | 22.86 (7.7) | 24.18 (10.2) | 23.32 (8.4) | 0.681 | 21.32 (5.3) | 21.19 (5.7) | 22.23 (7.6) | 0.732 |
| ALT (U/L) | 20.17 (14.0) | 21.43 (17.0) | 19.87 (10.4) | 0.514 | 19.00 (12.8) | 18.13 (10.0) | 16.60 (10.6) | 0.101 | 16.71 (7.5) | 18.00 (9.6) | 14.71 (8.0) | 0.206 | 17.52 (6.8) | 18.61 (8.1) | 17.84 (8.9) | 0.704 |
| FBS (mg/dL) | 101.6 (18.6) | 111.9 (38.0) | 104.3 (23.9) | 0.104 | 113.4 (36.2) | 122.5 (47.5) | 111.7 (29.1) | 0.169 | 100.0 (19.7) | 94.0 * (16.1) | 92.6 * (16.7) | 0.001 | 101.9 (26.3) | 108.6 (40.0) | 102.6 (30.2) | 0.072 |
| Parameter | Mean (SD) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BR Group (n = 30) | EX Group (n =30) | BR + EX Group (n =28) | CTRL Group (n = 32) | |||||||||||||
| T0 | T1 | T2 | p-Value | T0 | T1 | T2 | p-Value | T0 | T1 | T2 | p-Value | T0 | T1 | T2 | p-Value | |
| Total Cholesterol | 184.9 (42.6) | 194.7 (42.1) | 179.7 * (36.6) | 0.001 | 207.7 (42.0) | 209.1 (40.7) | 205.2 (35.1) | 0.664 | 210.6 (49.5) | 211.8 (56.4) | 200.4 (57.9) | 0.088 | 201.9 (44.4) | 212.1 (47.1) | 197.8 (44.4) | 0.151 |
| Triglycerides (mg/dL) | 115.7 (47.9) | 106.3 (37.2) | 95.8 * (29.7) | 0.020 | 147.5 (101.6) | 157.0 (97.2) | 145.4 (93.7) | 0.688 | 133.2 (62.0) | 140.6 (72.2) | 123.5 (52.0) | 0.288 | 126.0 (54.6) | 124.6 (45.3) | 111.2 (57.7) | 0.111 |
| HDL-C (mg/dL) | 52.13 (12.2) | 54.53 (12.0) | 52.17 (11.6) | 0.109 | 49.63 (11.1) | 49.73 (12.4) | 50.50 (12.0) | 0.714 | 55.86 (9.8) | 55.79 (11.6) | 53.93 (11.5) | 0.295 | 52.06 (10.6) | 56.16 (11.8) | 54.6 (10.7) | 0.811 |
| LDL-C (mg/dL) | 109.6 (35.0) | 108.9 (37.0) | 108.4 (32.0) | 1.000 | 128.6 (30.8) | 127.9 (35.0) | 125.6 (32.4) | 0.742 | 128.1 (45.5) | 127.8 (50.4) | 121.8 (53.3) | 0.434 | 124.6 (38.4) | 131.0 (41.4) | 120.9 (38.3) | 0.092 |
| LDL: HDL Ratio | 2.12 (0.58) | 2.14 (0.67) | 2.14 (0.64) | 0.346 | 2.11 (0.89) | 2.14 (1.09) | 2.16 (0.93) | 0.279 | 2.15 (0.82) | 2.16 (0.93) | 2.20 (0.95) | 0.813 | 2.13 (0.73) | 2.18 (0.80) | 2.24 * (0.68) | 0.007 |
| TC: HDL Ratio | 3.61 (0.66) | 3.66 (0.79) | 3.53 (0.72) | 0.154 | 4.38 (1.38) | 4.26 (1.51) | 4.28 (1.27) | 0.375 | 3.67 (1.01) | 3.71 (1.16) | 3.69 (1.03) | 0.541 | 3.68 (0.82) | 3.85 (0.91) | 3.95 * (0.91) | 0.006 |
| Parameter | Mean (SE) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BR Group (n = 30) | EX Group (n = 30) | BR + EX Group (n = 28) | CTRL Group (n = 32) | |||||||||||||
| T0 | T1 | T2 | p-Value | T0 | T1 | T2 | p-Value | T0 | T1 | T2 | p-Value | T0 | T1 | T2 | p-Value | |
| IL-6 (ρg/mL) | 21.67 (11.03) | 13.79 (7.00) | 13.71 (5.50) | 0.058 | 12.89 (4.76) | 10.00 (2.40) | 10.73 (2.70) | 0.604 | 27.02 (9.66) | 34.11 (7.52) | 16.79 * (10.21) | 0.038 | 17.60 (0.88) | 15.25 (0.97) | 17.39 (1.25) | 0.641 |
| CRP (mg/L) | 0.97 (0.32) | 0.32 (0.09) | 0.23 * (0.07) | 0.037 | 0.47 (0.08) | 0.42 (0.08) | 0.21 * (0.05) | 0.04 | 0.80 (0.58) | 0.56 * (0.10) | 0.44 * (0.08) | <0.001 | 0.44 (0.08) | 0.35 (0.07) | 0.34 (0.05) | 0.167 |
| IGF-1 (ng/mL) | 29.17 (0.66) | 41.56 * (0.39) | 46.93 * (3.01) | <0.001 | 28.85 (1.59) | 28.41 (1.24) | 39.89 * (0.63) | <0.001 | 27.22 (1.39) | 26.59 (1.29) | 39.28 * (0.78) | <0.001 | 31.75 (0.66) | 32.24 (1.40) | 33.81 (4.98) | 0.294 |
| %CD4 T cell | 35.39 (1.24) | 34.58 (1.33) | 35.73 (1.44) | 0.291 | 37.69 (1.64) | 35.85 (1.43) | 37.11 (1.43) | 0.076 | 31.91 (1.84) | 33.48 (1.79) | 34.43 * (1.75) | 0.034 | 36.05 (1.52) | 34.55 (1.24) | 36.78 (1.43) | 0.118 |
| %CD8 T cell | 18.09 (1.40) | 17.85 (1.34) | 19.12 (1.59) | 0.103 | 20.34 (1.18) | 20.21 (1.37) | 20.59 (1.44) | 0.699 | 19.67 (1.21) | 19.38 (1.07) | 19.61 (1.17) | 0.800 | 18.96 (1.16) | 18.43 (1.12) | 19.33 (1.16) | 0.124 |
| CD4:CD8 Ratio | 2.33 (0.20) | 2.29 (0.21) | 2.24 (0.20) | 0.654 | 2.09 (0.19) | 2.07 (0.18) | 2.10 (0.18) | 0.872 | 1.97 (0.23) | 2.06 (0.27) | 2.06 (0.27) | 0.402 | 2.24 (0.22) | 2.17 (0.19) | 2.22 (0.21) | 0.645 |
| Parameter | Median [Q1, Q3] | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BR Group (n = 30) | EX Group (n = 30) | BR + EX Group (n = 28) | CTRL Group (n = 32) | |||||||||
| T0 | T1 | T2 | T0 | T1 | T2 | T0 | T1 | T2 | T0 | T1 | T2 | |
| Sit to stand, Second (s) | 11.93 [8.8, 13.3] | 13.08 [11.1, 18.0] | 10.77 [8.2, 14.2] | 14.43 [12.2, 17.2] | 9.26 ** [7.3, 10.7] | 8.40 ** [7.2, 10.1] | 13.89 [10.7, 16.9] | 8.99 ** [7.8, 10.6] | 9.23 ** [7.0, 9.2] | 12.12 [10.6, 13.9] | 16.15 [11.6, 18.7] | 12.66 [9.9, 14.3] |
| Gait speed, Second (s) | 4.52 [4.2, 5.8] | 5.18 [4.7, 6.2] | 4.37 [3.9, 6.3] | 5.43 [4.2, 7.2] | 4.67 * [4.1, 6.5] | 5.34 [4.4, 6.7] | 5.35 [4.4, 7.5] | 4.60 * [4.0, 5.3] | 4.76 * [3.9, 6.6] | 5.23 [4.0, 7.5] | 5.38 [4.8, 6.9] | 5.23 [4.2, 6.1] |
| Grip strength,(kg) | 22.31 [15.9, 27.3] | 18.43 [16.0, 23.7] | 19.83 [17.7, 25.3] | 21.13 [14.4, 25.5] | 21.26 [18.0, 27.7] | 22.45 [16.5, 30.2] | 18.95 [13.8, 26.7] | 20.75 [17.7, 26.0] | 20.70 [16.3, 26.7] | 18.33 [16.3, 22.9] | 18.58 [16.8, 22.4] | 19.46 [16.6, 25.5] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seesen, M.; Semmarath, W.; Yodkeeree, S.; Sapbamrer, R.; Ayood, P.; Malasao, R.; Ongprasert, K.; Chittrakul, J.; Siviroj, P.; Limtrakul, P. Combined Black Rice Germ, Bran Supplement and Exercise Intervention Modulate Aging Biomarkers and Improve Physical Performance and Lower-Body Muscle Strength Parameters in Aging Population. Int. J. Environ. Res. Public Health 2020, 17, 2931. https://doi.org/10.3390/ijerph17082931
Seesen M, Semmarath W, Yodkeeree S, Sapbamrer R, Ayood P, Malasao R, Ongprasert K, Chittrakul J, Siviroj P, Limtrakul P. Combined Black Rice Germ, Bran Supplement and Exercise Intervention Modulate Aging Biomarkers and Improve Physical Performance and Lower-Body Muscle Strength Parameters in Aging Population. International Journal of Environmental Research and Public Health. 2020; 17(8):2931. https://doi.org/10.3390/ijerph17082931
Chicago/Turabian StyleSeesen, Mathuramat, Warathit Semmarath, Supachai Yodkeeree, Ratana Sapbamrer, Pisittawoot Ayood, Rungnapa Malasao, Krongporn Ongprasert, Jiraporn Chittrakul, Penprapa Siviroj, and Pornngarm Limtrakul (Dejkriengkraikul). 2020. "Combined Black Rice Germ, Bran Supplement and Exercise Intervention Modulate Aging Biomarkers and Improve Physical Performance and Lower-Body Muscle Strength Parameters in Aging Population" International Journal of Environmental Research and Public Health 17, no. 8: 2931. https://doi.org/10.3390/ijerph17082931