Hindering of Cariogenic Streptococcus mutans Biofilm by Fatty Acid Array Derived from an Endophytic Arthrographis kalrae Strain
Abstract
:1. Introduction
2. Material and Methods
2.1. Isolation of Endophytic Fungus from the Collected Plant Samples
2.2. Selection and Identification of an Endophytic Isolate
2.3. Extraction and Analysis of the Fatty Acids from the Identified Endophytic Isolate
2.4. Antivirulence Activity of Arthrographis kalrae Fatty Acids (AKFAs) against S. mutans
2.4.1. Antibiofilm Activity
2.4.2. Inhibition of S. mutans Biofilm’s Water Insoluble EPS Assay
2.4.3. Acidogenesis- Mitigating Assay
2.5. Bacterial Viability Assay
2.6. Antivirulence Activity of AKFAs on S. mutans Biofilm Using an In Vitro Tooth Model
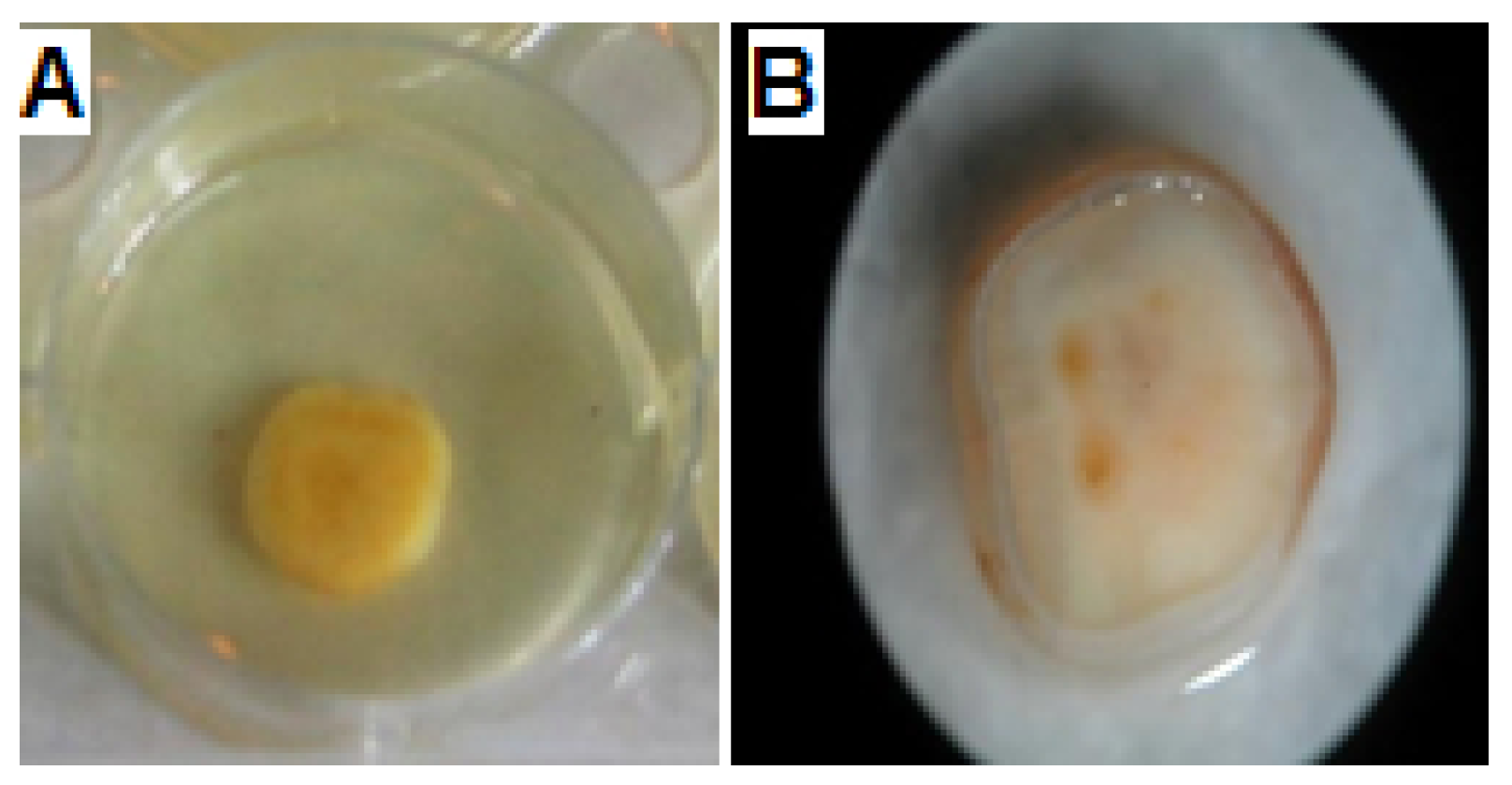
2.6.1. Saliva-Coated Hydroxyapatite (S-H) Disc Assay
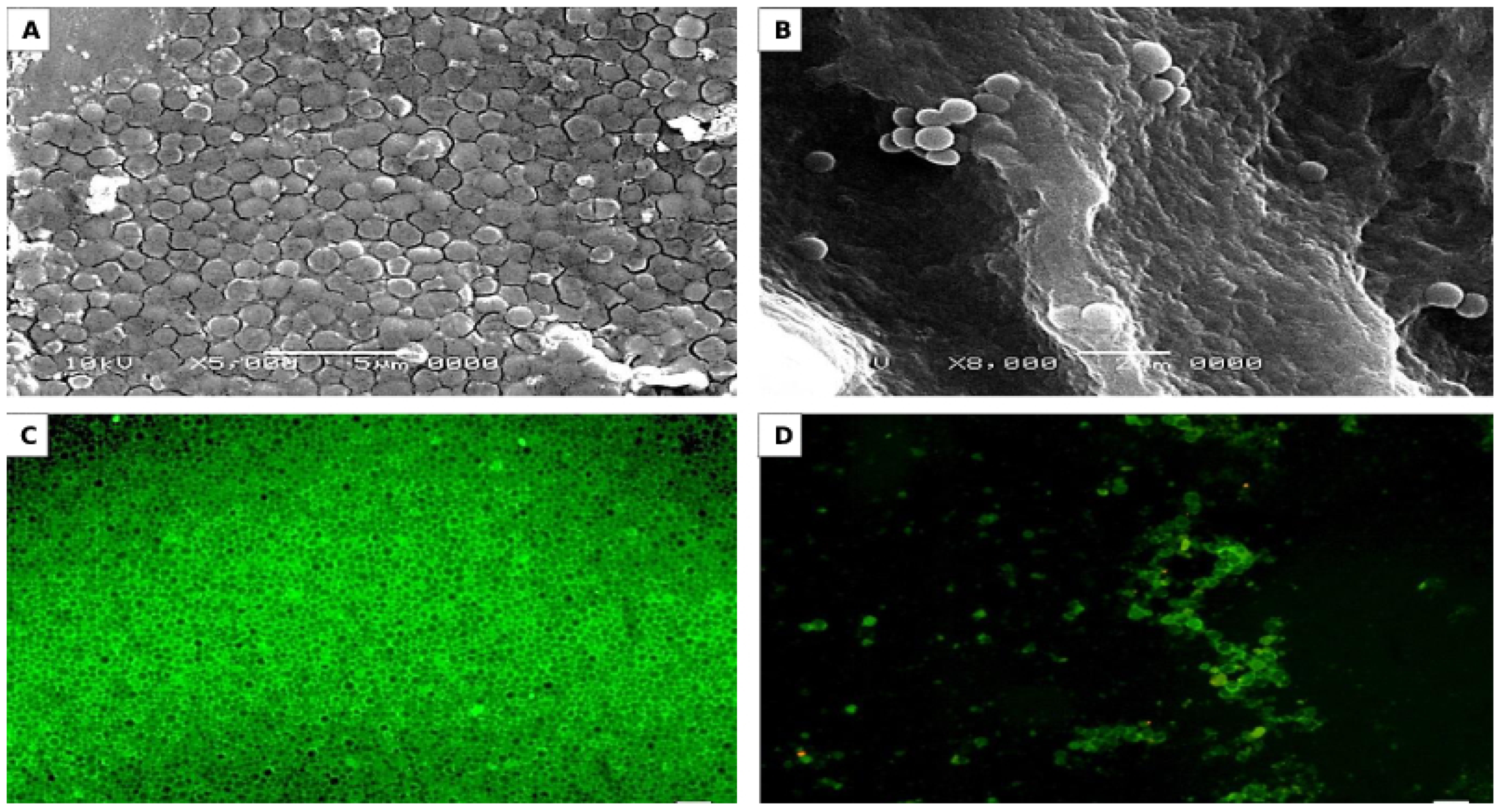
2.6.2. Microscopic Observations of S-H Discs Treated with MBIC50 of AKFAs
2.7. Oral Fibroblast Viability Assay
2.8. Statistical Analysis
3. Results
3.1. Isolation and Identification of the Selected Endophytic Isolate
3.2. Chemical Composition of Arthrographis kalrae’s AKFAs
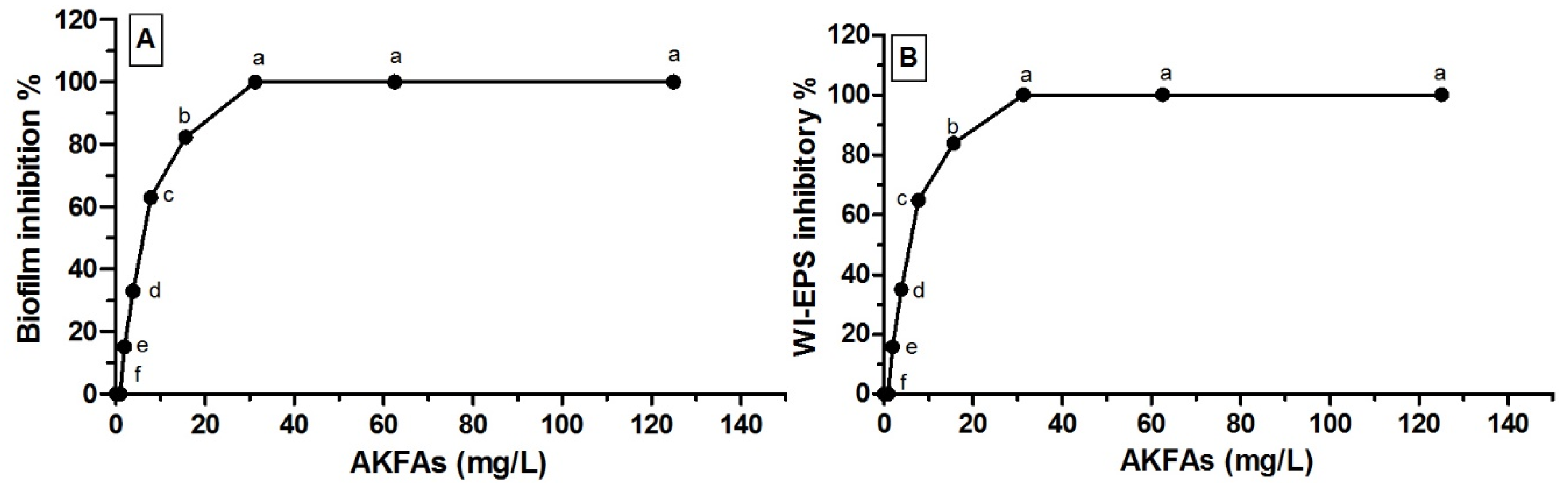
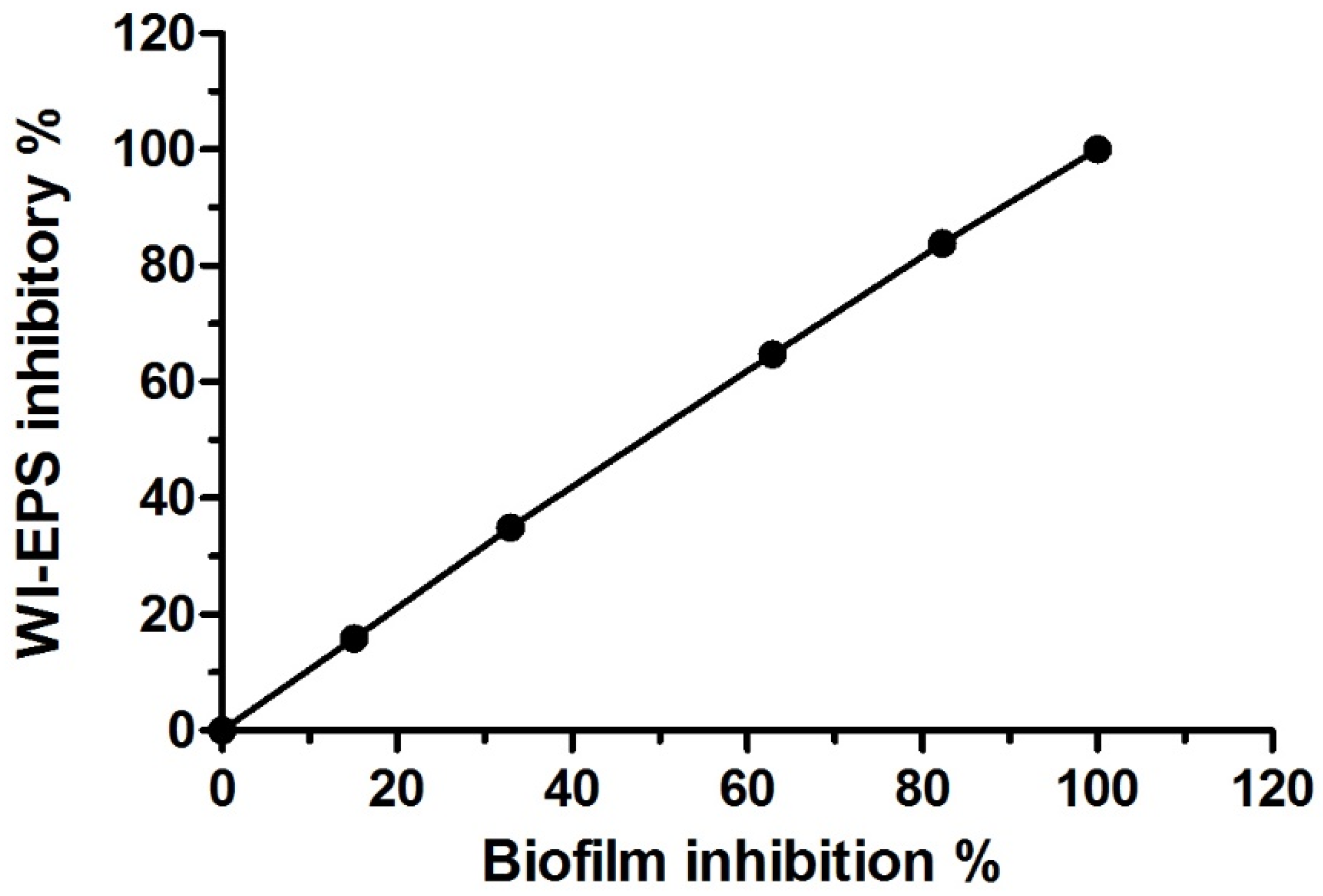
3.3. Antivirulence Activity of the Extracted AKFAs on S. mutans
3.4. Activity of AKFAs on S. mutans Biofilm Using an In Vitro Tooth Model
3.5. Cytotoxicity of the Extracted AKFAs in Human Oral Fibroblast Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Sugars and Dental Caries. 2017. Available online: https://apps.who.int/iris/handle/10665/259413 (accessed on 17 September 2017). License: CC BY-NC-SA 3.0 IGO.
- Bagramian, R.A.; Garcia-Godoy, F.; Volpe, A.R. The global increase in dental caries. A pending public health crisis. Am. J. Dent. 2009, 22, 3–8. [Google Scholar] [PubMed]
- Goc, A.; Sumera, W.; Niedzwiecki, A.; Rath, M. 10-undecynoic acid is a new anti-adherent agent killing biofilm of oral Streptococcus spp. PLoS ONE 2019, 14, e0214763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowen, W.H.; Koo, H. Biology of Streptococcus mutans-Derived Glucosyltransferases: Role in Extracellular Matrix Formation of Cariogenic Biofilms. Caries Res. 2011, 45, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Burne, R.A. Regulation of the gtfBC and ftf genes of Streptococcus mutans in biofilms in response to pH and carbohydrate. Microbiology 2001, 147, 2841–2848. [Google Scholar] [CrossRef] [Green Version]
- Forssten, S.D.; Björklund, M.; Ouwehand, A.C. Streptococcus mutans, Caries and Simulation Models. Nutrients 2010, 2, 290–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Ren, Z.; Zhou, X.; Zeng, J.; Zou, J.; Li, Y. Inhibition of Streptococcus mutans biofilm formation, extracellular polysaccharide production, and virulence by an oxazole derivative. Appl. Microbiol. Biotechnol. 2016, 100, 857–867. [Google Scholar] [CrossRef]
- Forbes, S.; Latimer, J.; Sreenivasan, P.K.; McBain, A.J. Simultaneous Assessment of Acidogenesis-Mitigation and Specific Bacterial Growth-Inhibition by Dentifrices. PLoS ONE 2016, 11, e0149390. [Google Scholar] [CrossRef] [Green Version]
- Haffajee, A.D.; Socransky, S.S.; Patel, M.R.; Song, X. Microbial complexes in supragingival plaque. Oral Microbiol. Immunol. 2008, 23, 196–205. [Google Scholar] [CrossRef]
- Koo, H.; Duarte, S.; Murata, R.M.; Scott-Anne, K.; Gregoire, S.; Watson, G.E.; Singh, A.P.; Vorsa, N. Influence of Cranberry Proanthocyanidins on Formation of Biofilms by Streptococcus mutans on Saliva-Coated Apatitic Surface and on Dental Caries Development in vivo. Caries Res. 2010, 44, 116–126. [Google Scholar] [CrossRef] [Green Version]
- Klein, M.I.; Hwang, G.; Santos, P.H.S.; Campanella, O.H.; Koo, H. Streptococcus mutans-derived extracellular matrix in cariogenic oral biofilms. Front. Cell. Infect. Microbiol. 2015, 5. [Google Scholar] [CrossRef] [Green Version]
- Emilson, C.G.; Lindquist, B.; Wennerholm, K. Recolonization of Human Tooth Surfaces by Streptococcus mutans after Suppression by Chlorhexidine Treatment. J. Dent. Res. 1987, 66, 1503–1508. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Worthington, R.J.; Melander, C.; Wu, H. A New Small Molecule Specifically Inhibits the Cariogenic Bacterium Streptococcus mutans in Multispecies Biofilms. Antimicrob. Agents Chemother. 2011, 55, 2679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laribi, B.; Kouki, K.; M’Hamdi, M.; Bettaieb, T. Coriander (Coriandrum sativum L.) and its bioactive constituents. Fitoterapia 2015, 103, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Khare, E.; Mishra, J.; Arora, N.K. Multifaceted Interactions Between Endophytes and Plant: Developments and Prospects. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Kaddes, A.; Fauconnier, M.-L.; Sassi, K.; Nasraoui, B.; Jijakli, M.-H. Endophytic Fungal Volatile Compounds as Solution for Sustainable Agriculture. Molecules 2019, 24, 1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez, R.J.; White, J.F., Jr.; Arnold, A.E.; Redman, A.R.A. Fungal endophytes: Diversity and functional roles: Tansley review. New Phytol. 2009, 182, 314–330. [Google Scholar] [CrossRef] [PubMed]
- Schneider, P.; Misiek, M.; Hoffmeister, D. In Vivo and In Vitro Production Options for Fungal Secondary Metabolites. Mol. Pharm. 2008, 5, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, M.A.; McGaw, L.J. Bioprospecting of South African Plants as a Unique Resource for Bioactive Endophytic Microbes. Front. Pharm. 2018, 9, 456. [Google Scholar] [CrossRef]
- Bioprospecting Potential of Endophytic Bacteria Isolated From Indigenous Plants of Ambala (Haryana, India) | International Journal of Pharmaceutical Sciences and Research. Available online: http://ijpsr.com/bft-article/bioprospecting-potential-of-endophytic-bacteria-isolated-from-indigenous-plants-of-ambala-haryana-india/?view=fulltext (accessed on 15 May 2020).
- Zin, W.W.M.; Buttachon, S.; Dethoup, T.; Pereira, J.A.; Gales, L.; Inacio, A.; Costa, P.M.; Lee, M.; Sekeroglu, N.; Silva, A.M. Antibacterial and antibiofilm activities of the metabolites isolated from the culture of the mangrove-derived endophytic fungus Eurotium chevalieri KUFA 0006. Phytochemistry 2017, 141, 86–97. [Google Scholar] [CrossRef]
- Meenambiga, S.S.; Rajagopal, K. Antibiofilm activity and molecular docking studies of bioactive secondary metabolites from endophytic fungus Aspergillus nidulans on oral Candida albicans. J. Appl. Pharm. Sci. 2018. [Google Scholar] [CrossRef]
- Nguyen, Q.-H.; Talou, T.; Cerny, M.; Evon, P.; Merah, O. Oil and fatty acid accumulation during coriander (Coriandrum sativum L.) fruit ripening under organic cultivation. Crop J. 2015, 3, 366–369. [Google Scholar] [CrossRef]
- Giacaman, R.A.; Jobet-Vila, P.; Muñoz-Sandoval, C. Fatty acid effect on sucrose-induced enamel demineralization and cariogenicity of an experimental biofilm–caries model. Odontology 2015, 103, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Francisco, M.R.; Saldanha, T.; Fraga, M.E. Fungi as an alternative to produce essential fatty acids. Científica 2017, 45, 123. [Google Scholar] [CrossRef] [Green Version]
- Nicol, M.; Alexandre, S.; Luizet, J.B.; Skogman, M.; Jouenne, T.; Salcedo, S.P.; Dé, E. Unsaturated Fatty Acids Affect Quorum Sensing Communication System and Inhibit Motility and Biofilm Formation of Acinetobacter baumannii. Int. J. Mol. Sci. 2018, 19, 214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hazalin, N.A.; Ramasamy, K.; Lim, S.S.M.; Wahab, I.A.; Cole, A.L.; Majeed, A.B.A. Cytotoxic and antibacterial activities of endophytic fungi isolated from plants at the National Park, Pahang, Malaysia. Bmc Complement Altern. Med. 2009, 9, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva-Hughes, A.F.; Wedge, D.E.; Cantrell, C.L.; Carvalho, C.R.; Pan, Z.; Moraes, R.M.; Madoxx, V.L.; Rosa, L.H. Diversity and antifungal activity of the endophytic fungi associated with the native medicinal cactus Opuntia humifusa (Cactaceae) from the United States. Microbiol. Res. 2015, 175, 67–77. [Google Scholar] [CrossRef]
- Abu-Elreesh, G.; Abd-El-Haleem, D. Promising oleaginous filamentous fungi as biodiesel feed stocks: Screening and identification. Eur. J. Exp. Biol. 2014, 4, 576–582. [Google Scholar]
- Lim, S.H.; Ming, H.; Park, E.Y.; Choi, J.S. Improvement of Riboflavin Production Using Mineral Support in the Culture of Ashbya gossypii. Food Technol. Biotechnol. 2003, 41, 137–144. [Google Scholar]
- Mirhendi, H.; Ghiasian, A.; Vismer, H.F.; Asgary, M.R.; Jalalizand, N.; Arendrup, M.C.; Makimura, K. Preliminary Identification and Typing of Pathogenic and Toxigenic Fusarium Species Using Restriction Digestion of ITS1-5.8S rDNA-ITS2 Region. Iran. J. Public Health 2010, 39, 35. [Google Scholar]
- Hamzah, T.N.T.; Lee, S.Y.; Hidayat, A.; Terhem, R.; Faridah-Hanum, I.; Mohamed, R. Diversity and Characterization of Endophytic Fungi Isolated From the Tropical Mangrove Species, Rhizophora mucronata, and Identification of Potential Antagonists Against the Soil-Borne Fungus, Fusarium solani. Front. Microbiol. 2018, 9, 1707. [Google Scholar] [CrossRef]
- BLAST: Basic Local Alignment Search Too. Available online: https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 21 May 2020).
- Grantina-Ievina, L.; Berzina, A.; Nikolajeva, V.; Mekss, P.; Muiznieks, I. Production of fatty acids by Mortierella and Umbelopsis species isolated from temperate climate soils. Environ. Exp. Bio. 2014, 12, 15–27. [Google Scholar]
- Qureshi, M.N.; Siddique, M.; Kanwal, I.-R.; Kanwal, F. Analytical Characterization of Fatty Acids Composition of Datura alba Seed Oil by Gas Chromatography Mass Spectrometry. Jnl Chin. Chem. Soc 2011, 58, 236–240. [Google Scholar] [CrossRef]
- Stein, S. Mass spectral reference libraries: An ever-expanding resource for chemical identification. Anal. Chem. 2012, 84, 7274–7282. [Google Scholar] [CrossRef] [PubMed]
- Dasu, T.; Johnson, T. Exploratory Data Mining and Data Cleaning; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Merritt, J.H.; Kadouri, D.E.; O’Toole, G.A. Growing and Analyzing Static Biofilms. Curr. Protoc. Microbiol. 2011, 22, 1B.1.1–1B.1.18. [Google Scholar] [CrossRef] [Green Version]
- Gopal, R.; Lee, J.; Kim, M.-S.; Seo, C.; Park, Y. Anti-Microbial, Anti-Biofilm Activities and Cell Selectivity of the NRC-16 Peptide Derived from Withc Flounder, Glyptocephalus cynoglossus. Marine Drugs 2013, 11, 1836–1852. [Google Scholar] [CrossRef] [Green Version]
- Sadovskaya, I.; Vinogradov, E.; Li, J.; Jabbouri, S. Structural elucidation of the extracellular and cell-wall teichoic acids of Staphylococcus epidermidis RP62A, a reference biofilm-positive strain. Carbohydr. Res. 2004, 339, 1467–1473. [Google Scholar] [CrossRef]
- Ansari, J.M.; Abraham, N.M.; Massaro, J.; Murphy, K.; Smith-Carpenter, J.; Fikrig, E. Anti-Biofilm Activity of a Self-Aggregating Peptide against Streptococcus mutans. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef]
- Lemos, J.A.; Abranches, J.; Koo, H.; Marquis, R.E.; Burne, R.A. Protocols to Study the Physiology of Oral Biofilms. In Oral Biology; 666; Seymour, G.J., Cullinan, M., Heng, N.C.K., Eds.; Humana Press: Totowa, NJ, USA, 2010; pp. 87–102. [Google Scholar]
- Mai, J.; Tian, X.L.; Gallant, J.W.; Merkley, N.; Biswas, Z.; Syvitski, R.; Douglas, S.E.; Ling, J.; Li, Y.H. A Novel Target-Specific, Salt-Resistant Antimicrobial Peptide against the Cariogenic Pathogen Streptococcus mutans. Antimicrob. Agents Chemother. 2011, 55, 5205–5213. [Google Scholar] [CrossRef] [Green Version]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, T.; Samaranayake, Y.H.; Fang, H.H.P.; Yip, H.K.; Samaranayake, L. The use of new probes and stains for improved assessment of cell viability and extracellular polymeric substances in Candida albicans biofilms. Mycopathologia 2005, 159, 353–360. [Google Scholar] [CrossRef]
- Min, K.R.; Galvis, A.; Williams, B.; Rayala, R.; Cudic, P.; Ajdic, D. Antibacterial and Antibiofilm Activities of a Novel Synthetic Cyclic Lipopeptide against Cariogenic Streptococcus mutans UA159. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayer, F.L.; Kronstad, J.W. Disarming Fungal Pathogens: Bacillus safensis Inhibits Virulence Factor Production and Biofilm Formation by Cryptococcus neoformans and Candida albicans. Am. Soc. Microbiol. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Arai, M.; Niikawa, H.; Kobayashi, M. Marine-derived fungal sesterterpenes, ophiobolins, inhibit biofilm formation of Mycobacterium species. J. Nat. Med. 2013, 67, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Scopel, M.; Abraham, W.-R.; Antunes, A.L.; Henriques, A.; Macedo, A.J. Mevalonolactone: An Inhibitor of Staphylococcus Epidermidis Adherence and Biofilm Formation. Med. Chem. 2014, 10, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Bhagobaty, R.K.; Nihalani, M.C.; Joshi, S.R. Prospective oleaginous endophytic fungi isolated from biodiesel plants: An assessment of diversity and lipid content. Microbiology 2017, 49, 15–21. [Google Scholar]
- Peng, X.-W.; Chen, H.-Z. Microbial oil accumulation and cellulase secretion of the endophytic fungi from oleaginous plants. Ann. Microbiol. 2007, 57, 239–242. [Google Scholar] [CrossRef]
- Vadivelan, G.; Venkateswaran, G. Production and Enhancement of Omega-3 Fatty Acid from Mortierella alpina CFR-GV15: Its Food and Therapeutic Application. Biomed Res. Int. 2014, 2014, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Kaushik, N. Endophytic Fungi Isolated from Oil-Seed Crop Jatropha curcas Produces Oil and Exhibit Antifungal Activity. PLoS ONE 2013, 8, e56202. [Google Scholar] [CrossRef] [Green Version]
- Fakas, S.; Čertik, M.; Papanikolaou, S.; Aggelis, G.; Komaitis, M.; Galiotou-Panayotou, M. γ-Linolenic acid production by Cunninghamella echinulata growing on complex organic nitrogen sources. Bioresour. Technol. 2008, 99, 5986–5990. [Google Scholar] [CrossRef]
- Kolouchová, I.; Maťátková, O.; Sigler, K.; Masák, J.; Řezanka, T. Production of Palmitoleic and Linoleic Acid in Oleaginous and Nonoleaginous Yeast Biomass. Int. J. Anal. Chem. 2016, 2016, 7583684. [Google Scholar] [CrossRef] [Green Version]
- Stenz, L.; François, P.; Fischer, A.; Huyghe, A.; Tangomo, M.; Hernandez, D.; Cassat, J.; Linder, P.; Schrenzel, J. Impact of oleic acid (cis -9-octadecenoic acid) on bacterial viability and biofilm production in Staphylococcus aureus. Fems Microbiol. Lett. 2008, 287, 149–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chanda, W.; Joseph, T.P.; Padhiar, A.A.; Guo, X.; Min, L.; Wang, W.; Lolokote, S.; Ning, A.; Cao, J.; Huang, M.; et al. Combined effect of linolenic acid and tobramycin on Pseudomonas aeruginosa biofilm formation and quorum sensing. Exp. Ther. Med. 2017. [Google Scholar] [CrossRef] [PubMed]
- Osawa, K.; Miyazaki, K.; Shimura, S.; Okuda, J.; Matsumoto, M.; Ooshima, T. Identification of Cariostatic Substances in the Cacao Bean Husk: Their Anti-glucosyltransferase and Antibacterial Activities. J. Dent. Res. 2001, 80, 2000–2004. [Google Scholar] [CrossRef] [PubMed]
- Overhage, J.; Campisano, A.; Bains, M.; Torfs, E.C.W.; Rehm, B.H.A.; Hancock, R.E.W. Human Host Defense Peptide LL-37 Prevents Bacterial Biofilm Formation. Infect. Immun. 2008, 76, 4176–4182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flemming, H.-C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Leme, A.F.P.; Koo, H.; Bellato, C.M.; Bedi, G.; Cury, J.A. The Role of Sucrose in Cariogenic Dental Biofilm Formation—New Insight. J. Dent. Res. 2006, 85, 878–887. [Google Scholar] [CrossRef]
- Williams, K.A.; Schemehorn, B.R.; McDonald, J.L.; Stookey, G.K.; Katz, S. Influence of selected fatty acids upon plaque formation and caries in the rat. Arch. Oral Biol. 1982, 27, 1027–1031. [Google Scholar] [CrossRef]
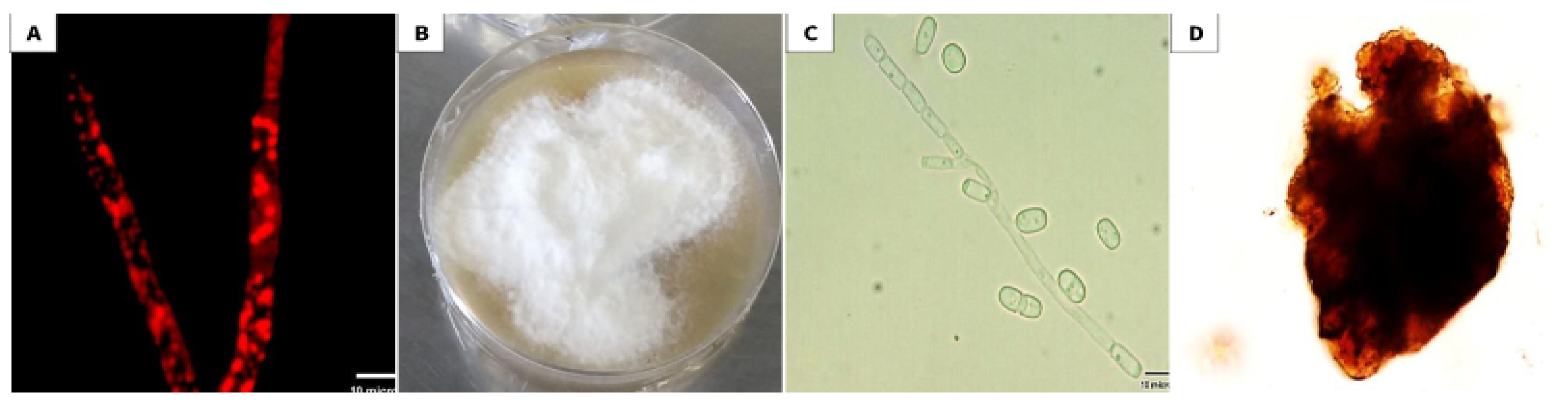
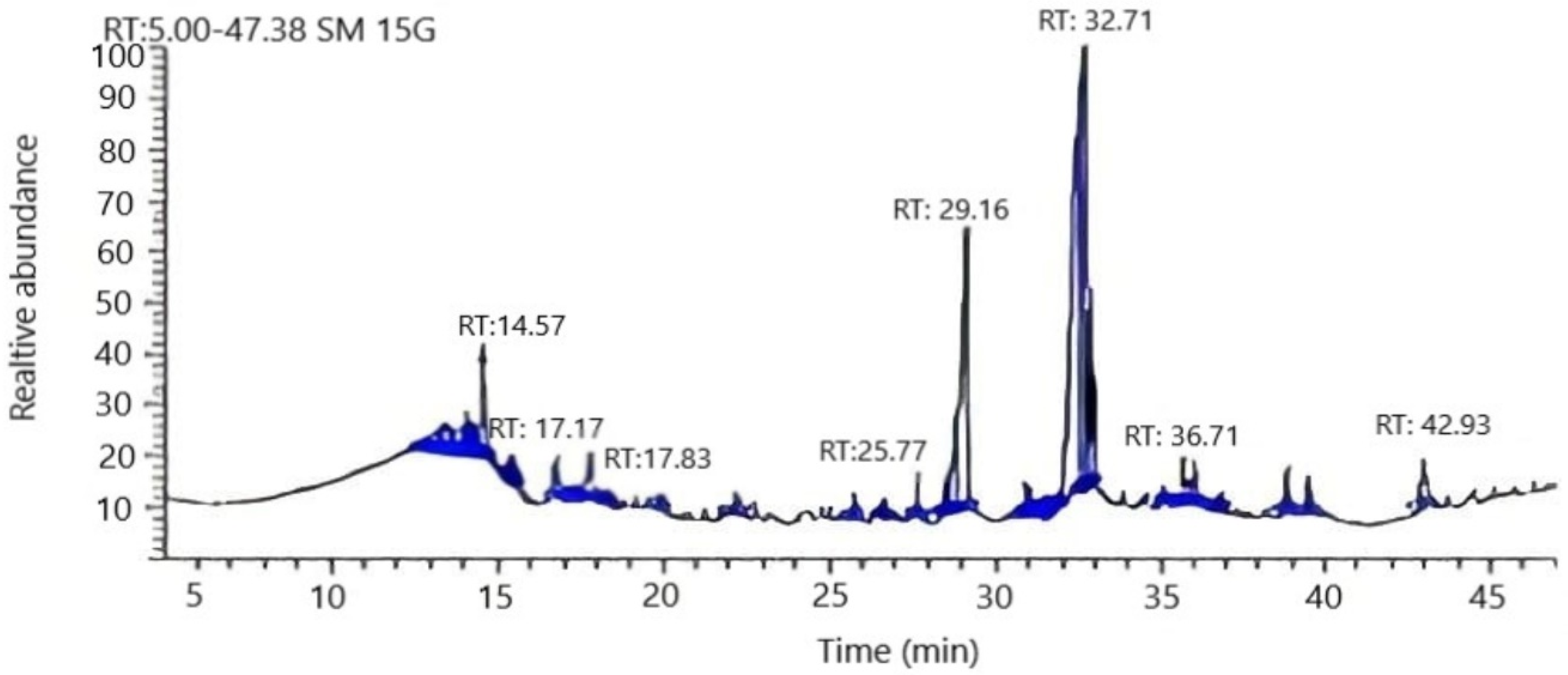
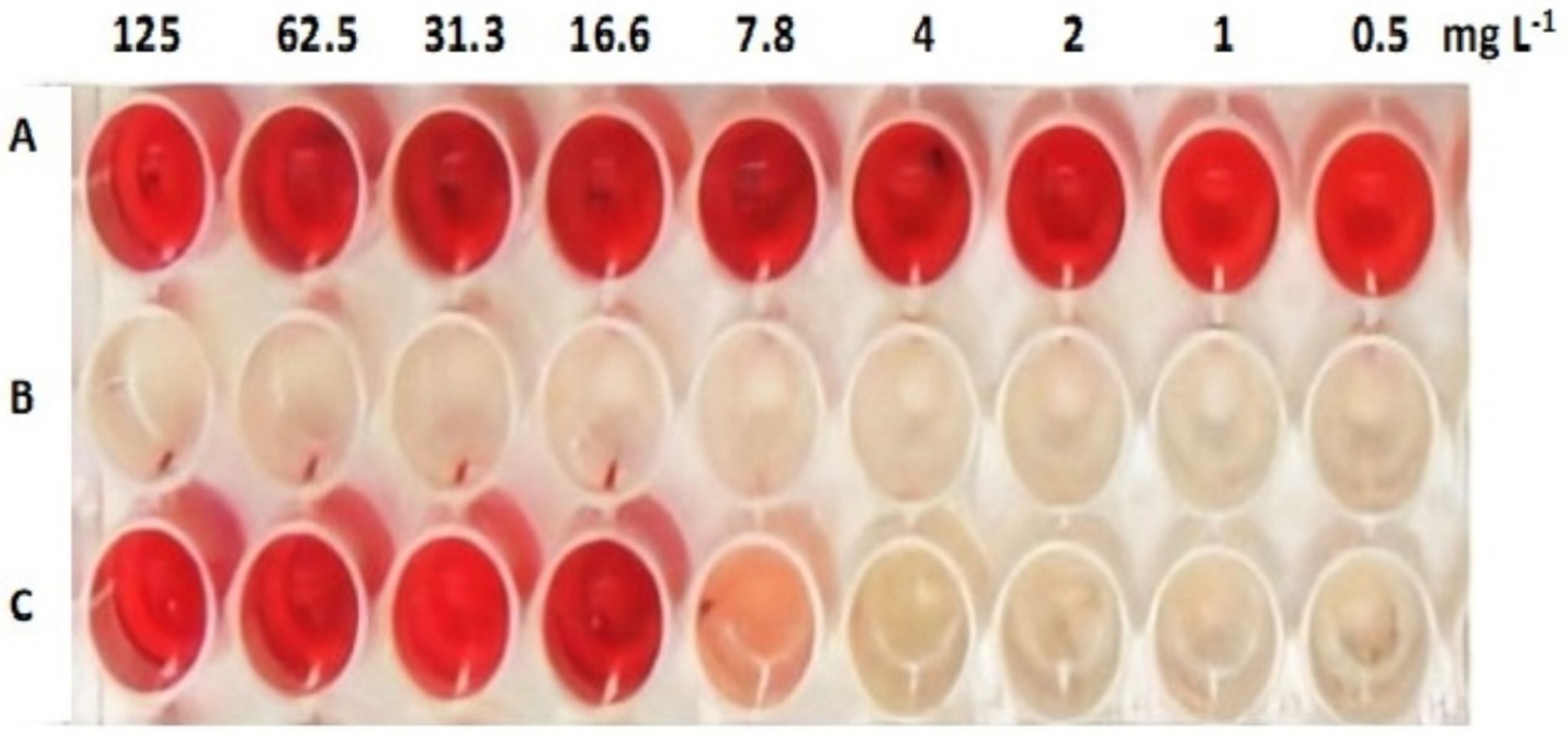

| tR a | Compound b | Peak Area (%) | Molecular Formula | Fragmentation Ion |
|---|---|---|---|---|
| Saturated fatty acids | 5.8 | |||
| 17.8 | Palmitic | 1.2 | C16H32O2 | 73, 93,256 |
| 25.8 | Margaric | 1.4 | C17H34O2 | 74, 129, 270 |
| 35.7 | Stearic | 2.0 | C18H36O2 | 74, 87, 298 |
| 39.3 | Arachidic | 1.2 | C20H40O2 | 74, 91, 295 |
| Unsaturated fatty acids | 93.8 | |||
| 14.6 | Myristoleic | 4.1 | C14H26O2 | 41,55, 226 |
| 17.2 | Palmitoleic | 0.2 | C16H30O2 | 55, 263,268 |
| 29.2 | Linolenic | 23.9 | C18H30O2 | 67, 81,292 |
| 32.7 | Oleic | 61.0 | C18H34O2 | 264,55,44 |
| 42.9 | Docosahexaenoic acid | 4.6 | C22H32O2 | 131, 91, 79 |
| Total | 99.6 |
| ACFAs (mg L−1) | Mean Biofilm Inhibitory % ± SD | Mean WIEPS Inhibitory % ± SD |
|---|---|---|
| 0.00 | 0.00 f ± 0.00 | 0.00 f ± 0.00 |
| 1 | 0.00 f ± 0.00 | 0.00 f ± 0.00 |
| 2 | 15.11 e ± 0.19 | 15.84 e ± 0.18 |
| 3.9 | 32.98 d ± 0.85 | 34.92 d ± 0.27 |
| 7.8 | 62.89 c ± 0.64 | 64.78 c ± 0.57 |
| 15.6 | 82.27 b ± 0.32 | 83.89 b ± 0.7 |
| 31.3 | 100.0 a ± 0.00 | 100.0 a ± 0.00 |
| 62.5 | 100.0 a ± 0.00 | 100.0 a ± 0.00 |
| 125 | 100.0 a ± 0.00 | 100.0 a ± 0.00 |
| MBIC50 = 6.1 mg L−1 | MEPSIC50 = 5.9 mg L−1 | |
| MBIC100 = 31.3 mg L−1 | MEPSIC100 = 31.3 mg L−1 | |
| p < 0.0001*** | p < 0.0001*** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel-Aziz, M.M.; Emam, T.M.; Raafat, M.M. Hindering of Cariogenic Streptococcus mutans Biofilm by Fatty Acid Array Derived from an Endophytic Arthrographis kalrae Strain. Biomolecules 2020, 10, 811. https://doi.org/10.3390/biom10050811
Abdel-Aziz MM, Emam TM, Raafat MM. Hindering of Cariogenic Streptococcus mutans Biofilm by Fatty Acid Array Derived from an Endophytic Arthrographis kalrae Strain. Biomolecules. 2020; 10(5):811. https://doi.org/10.3390/biom10050811
Chicago/Turabian StyleAbdel-Aziz, Marwa M., Tamer M. Emam, and Marwa M. Raafat. 2020. "Hindering of Cariogenic Streptococcus mutans Biofilm by Fatty Acid Array Derived from an Endophytic Arthrographis kalrae Strain" Biomolecules 10, no. 5: 811. https://doi.org/10.3390/biom10050811
APA StyleAbdel-Aziz, M. M., Emam, T. M., & Raafat, M. M. (2020). Hindering of Cariogenic Streptococcus mutans Biofilm by Fatty Acid Array Derived from an Endophytic Arthrographis kalrae Strain. Biomolecules, 10(5), 811. https://doi.org/10.3390/biom10050811





