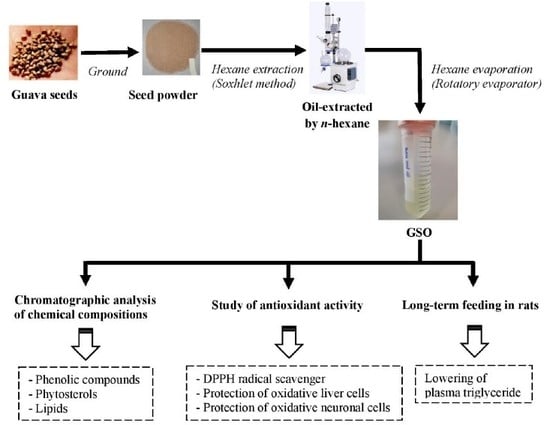
Phytosterol, Lipid and Phenolic Composition, and Biological Activities of Guava Seed Oil
Abstract
:1. Introduction
2. Results
2.1. Identification of Lipids
2.2. Identification of Phytosterols
2.3. Liquid Chromatographic Analysis of Phenolic Compounds
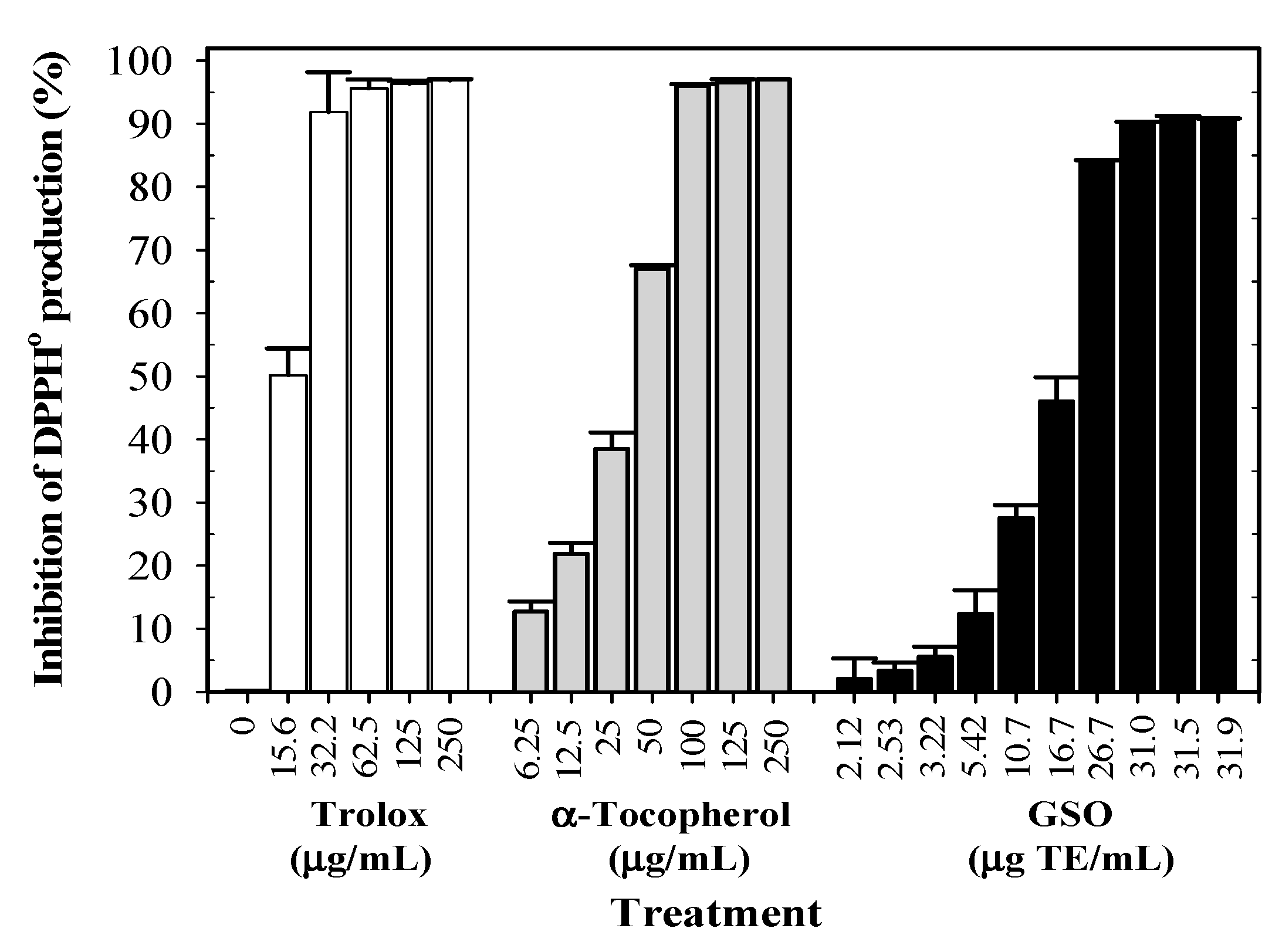
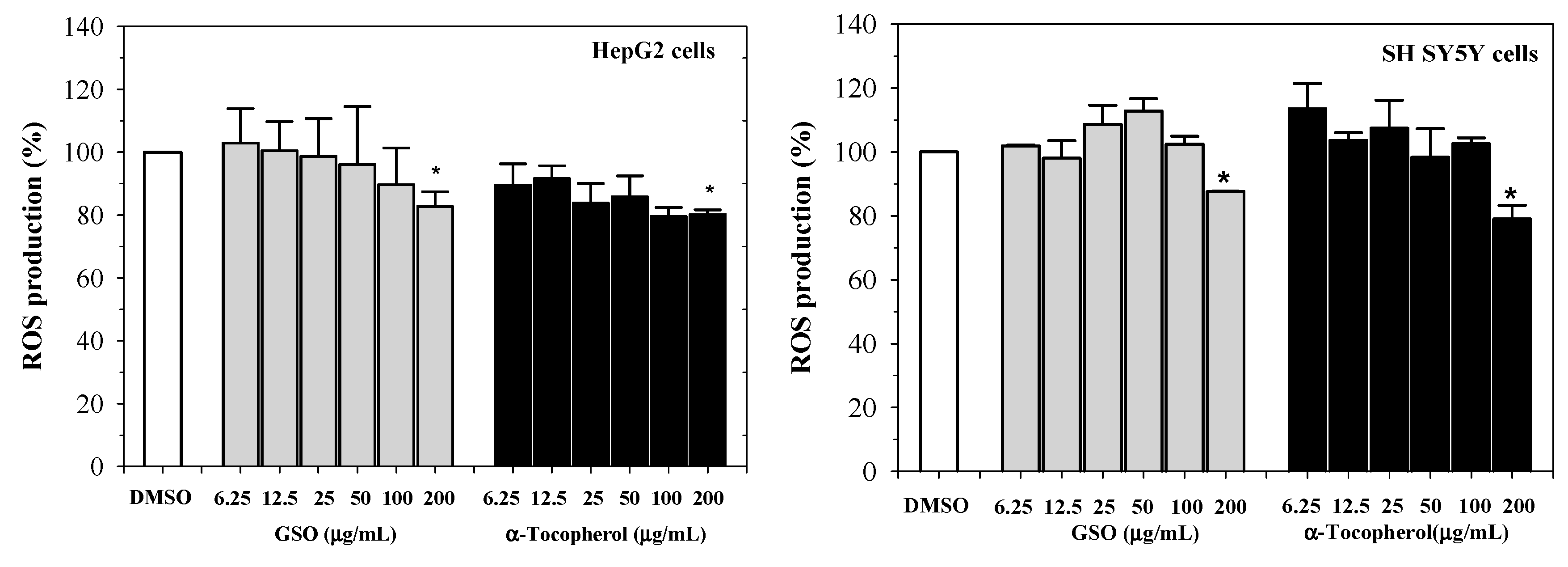
2.4. Free-Radical Scavenging Activity
2.5. Bioavailability of Serum Lipids in GSO-Fed Rats
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of GSO Extract
4.3. High-Performance Liquid Chromatography-Electrospray Ionization-Quadrupole Time-of-Flight/Mass Spectrometry for Lipids
4.4. Trimethylsilylation Derivatization-Gas Chromatography/Mass Spectrometry of Phytosterols
4.4.1. Derivatization
4.4.2. Gas Chromatography/Mass Spectrometry
4.5. High-Performance Liquid Chromatography-Electrospray Ionization/Mass Spectrometry of Phenolic Compounds
4.6. Determination of Free-Radical Scavenging Activity
4.7. Analysis Serum Lipids in Guava Seed Oil-Fed Rats
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | arachidonic acid |
| ALA | α-linolenic acid |
| BF3 | boron trifluoride |
| Br-MMC | 4-bromomethyl-7-methoxycoumarin |
| CO | corn oil |
| DCF | dichlorofluorescein |
| DCFH-DA | 2′,7′-dichlorodihydrofluorescein diacetate |
| DI | deionized water |
| DMEM | Dulbecco’s modified Eagle medium |
| DPPH | 1,1-diphenyl-2-picrylhydrazyl |
| EC50 | a half effective concentration |
| ESI | electrospray ionization |
| F | female |
| FBS | fetal bovine serum |
| FI | fluorescent intensity |
| GAE | gallic acid equivalent |
| GC/MS | gas chromatography/mass spectrometry |
| GSO | guava seed oil |
| HPLC-ESI/MS | high-performance liquid chromatography-electrospray ionization/mass spectrometry |
| HPLC-ESI-QTOF/MS | high-performance liquid chromatography-electrospray ionization-quadrupole time of flight/mass spectrometry |
| HepG2 | human hepatocellular carcinoma cells |
| HPLC/FLD | high-performance liquid chromatography/fluorescence detection |
| H2O2 | hydrogen peroxide |
| LA | linoleic acid |
| LAE | linoleic acid equivalent |
| LOD | limit of detection |
| LOQ | limit of quantitation |
| M | male |
| MMC-FA | methyl-7-methoxycoumarin fatty acid |
| MSTFA | N-methyl-N-trimethylsilyltrifluoroacetamide |
| m/z | mass to charge ratio |
| ND | not determined |
| OA | oleic acid |
| OD | optical density |
| P. | Psidium |
| P-12 | nepheochromocytoma cell |
| PA | palmitic acid |
| PDA | photodiode array |
| PLA | palmitoleic acid |
| Ppm | part per million |
| PUFA | polyunsaturated fatty acid |
| ROS | reactive oxygen species |
| SA | stearic acid |
| SD | standard deviation |
| SEM | standard error of mean |
| SH-SY5Y and SK-N-SH | human neuroblastoma cells |
| TC | total cholesterol |
| TE | Trolox equivalent |
| TG | triglyceride |
| TIC | total ion chromatogram |
| TMS | trimethylsilyl |
| Trolox | 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid |
| trypsin-EDTA | trypsin-ethylene diamine tetraacetic acid |
| TR | retention time |
References
- Gutierrez, R.M.; Mitchell, S.; Solis, R.V. Psidium guajava: A review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2008, 117, 1–27. [Google Scholar] [CrossRef]
- Morais-Braga, M.F.B.; Carneiro, J.N.P.; Machado, A.J.T.; dos Santos, A.T.L.; Sales, D.L.; Lima, L.F.; Figueredo, F.G.; Coutinho, H.D.M. Psidium guajava L., from ethnobiology to scientific evaluation: Elucidating bioactivity against pathogenic microorganisms. J. Ethnopharmacol. 2016, 194, 1140–1152. [Google Scholar] [CrossRef] [PubMed]
- Conde Garcia, E.A.; Nascimento, V.T.; Santiago Santos, A.B. Inotropic effects of extracts of Psidium guajava L. (guava) leaves on the guinea pig atrium. Braz. J. Med. Biol. Res. 2003, 36, 661–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ojewole, J.A.O. Hypoglycemic and hypotensive effects of Psidium guajava Linn. (Myrtaceae) leaf aqueous extract. Methods Find. Exp. Clin. Pharm. 2006, 27, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Ojewole, J.A.O.; Awe, E.O.; Chiwororo, W.D.H. Antidiarrhoeal activity of Psidium guajava Linn. (Myrtaceae) leaf aqueous extract in rodents. J. Smooth Muscle Res. 2008, 44, 195–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deguchi, Y.; Miyazaki, K. Anti-hyperglycemic and anti-hyperlipidemic effects of guava leaf extract. Nutr. Metab. 2010, 7, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adesida, A.; Farombi, E.O. Free radical scavenging activities of guava extract in vitro. Afr. J. Med. Med. Sci. 2012, 41, 81–90. [Google Scholar]
- Sen, S.S.; Sukumaran, V.; Giri, S.S.; Park, S.C. Flavonoid fraction of guava leaf extract attenuates lipopolysaccharide-induced inflammatory response via blocking of NF-kappaB signalling pathway in Labeo rohita macrophages. Fish. Shellfish Immunol. 2015, 47, 85–92. [Google Scholar] [CrossRef]
- Bontempo, P.; Doto, A.; Miceli, M.; Mita, L.; Benedetti, R.; Nebbioso, A.; Veglione, M.; Rigano, D.; Cioffi, M.; Sica, V.; et al. Psidium guajava L. anti-neoplastic effects: Induction of apoptosis and cell differentiation. Cell Prolif. 2012, 45, 22–31. [Google Scholar] [CrossRef]
- Nair, S.; Nagar, R.; Gupta, R. Antioxidant phenolics and flavonoids in common Indian foods. J. Assoc. Physicians India 1998, 46, 708–710. [Google Scholar]
- Opute, F.I. The component fatty acids of Psidium guajava seed fats. J. Sci. Food Agric. 1978, 29, 737–738. [Google Scholar] [CrossRef]
- Bernardino-Nicanor, A.; Anon, M.C.; Scilingo, A.A.; Davila-Ortiz, G. Functional properties of guava seed glutelins. J. Agric. Food Chem. 2005, 53, 3613–3617. [Google Scholar] [CrossRef]
- Chang, Y.P.; Tan, M.P.; Lok, W.L.; Pakianathan, S.; Supramaniam, Y. Making use of guava seed (Psidium guajava L): The effects of pre-treatments on its chemical composition. Plant. Foods Hum. Nutr. 2014, 69, 43–49. [Google Scholar] [CrossRef]
- Michael, H.N.; Salib, J.Y.; Ishak, M.S. Acylated flavonol glycoside from Psidium gauijava L. seeds. Die. Pharm. 2002, 57, 859–860. [Google Scholar]
- Biegelmeyer, R.; Andrade, J.M.M.; Aboy, A.L.; Apel, M.A.; Dresch, R.R.; Marin, R.; Raseira, M.d.C.B.; Henriques, A.T. Comparative analysis of the chemical composition and antioxidant activity of red (Psidium cattleianum) and yellow (Psidium cattleianum var. lucidum) strawberry guava fruit. J. Food Sci. 2011, 76, C991–C996. [Google Scholar] [CrossRef] [PubMed]
- Salib, J.Y.; Michael, H.N. Cytotoxic phenylethanol glycosides from Psidium guaijava seeds. Phytochem 2004, 65, 2091–2093. [Google Scholar] [CrossRef] [PubMed]
- Malacrida, C.R.; Jorge, N. Fatty acids and some antioxidant compounds of Psidium guaijava seed oil. Acta Aliment. 2013, 42, 371–378. [Google Scholar] [CrossRef]
- Arain, A.; Sherazi, S.T.H.; Mahesar, S.A.; Sirajuddin. Spectroscopic and chromatographic evaluation of solvent extracted guava seed oil. Int. J. Food Prop. 2017, 20, S556–S563. [Google Scholar] [CrossRef] [Green Version]
- Garg, S.; Talwar, G.P.; Upadhyay, S.N. Comparison of extraction procedures on the immunocontraceptive activity of neem seed extracts. J. Ethnopharmacol. 1994, 44, 87–92. [Google Scholar] [CrossRef]
- Adeleke, M.A.; Popoola, S.A.; Agbaje, W.B.; Adewale, B.; Adeoye, M.D.; Jimoh, W.A. Larvicidal efficacy of seed oils of Pterocarpus santalinoides and tropical Manihot species against Aedes aegypti and effects on aquatic fauna. Tanzan J. Health Res. 2009, 11, 250–252. [Google Scholar] [CrossRef] [Green Version]
- Hossain, S.J.; Islam, M.R.; Pervin, T.; Iftekharuzzaman, M.; Hamdi, O.A.A.; Mubassara, S.; Saifuzzaman, M.; Shilpi, J.A. Antibacterial, anti-diarrhoeal, analgesic, cytotoxic activities, and GC-MS profiling of Sonneratia apetala (Buch.-Ham.) Seed. Prev. Nutr. Food Sci. 2017, 22, 157–165. [Google Scholar]
- Huang, H.Y.; Chang, C.K.; Tso, T.K.; Huang, J.J.; Chang, W.W.; Tsai, Y.C. Antioxidant activities of various fruits and vegetables produced in Taiwan. Int. J. Food Sci. Nutr. 2004, 55, 423–429. [Google Scholar] [CrossRef]
- Pelegrini, P.B.; Murad, A.M.; Silva, L.P.; dos Santos, R.C.P.; Costa, F.T.; Tagliari, P.D.; Bloch Jr, C.; Noronha, E.F.; Miller, R.N.G.; Franco, O.L. Identification of a novel storage glycine-rich peptide from guava (Psidium guajava) seeds with activity against Gram-negative bacteria. Peptides 2008, 29, 1271–1279. [Google Scholar] [CrossRef]
- Prommaban, A.; Utama-Ang, N.; Chaikitwattana, A.; Uthaipibull, C.; Srichairatanakool, S. Linoleic acid-rich guava seed oil: Safety and bioactivity. Phytother. Res. 2019, 33, 2749–2764. [Google Scholar] [CrossRef]
- Chen, H.J.; Inbaraj, B.S.; Chen, B.H. Determination of phenolic acids and flavonoids in Taraxacum formosanum Kitam by liquid chromatography-tandem mass spectrometry coupled with a post-column derivatization technique. Int. J. Mol. Sci. 2012, 13, 260–285. [Google Scholar] [CrossRef] [Green Version]
- Yu, F.; Qian, H.; Zhang, J.; Sun, J.; Ma, Z. Simultaneous quantification of eight organic acid components in Artemisia capillaris Thunb (Yinchen) extract using high-performance liquid chromatography coupled with diode array detection and high-resolution mass spectrometry. J. Food Drug Anal. 2018, 26, 788–795. [Google Scholar] [CrossRef]
- Baeza, G.; Sarria, B.; Bravo, L.; Mateos, R. Exhaustive qualitative LC-DAD-MSn analysis of arabica green coffee beans: Cinnamoyl-glycosides and cinnamoylshikimic acids as new polyphenols in green coffee. J. Agric. Food Chem. 2016, 64, 9663–9674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plazonic, A.; Bucar, F.; Males, Z.; Mornar, A.; Nigovic, B.; Kujundzic, N. Identification and quantification of flavonoids and phenolic acids in burr parsley (Caucalis platycarpos L.), using high-performance liquid chromatography with diode array detection and electrospray ionization mass spectrometry. Molecules 2009, 14, 2466–2490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, J.Y.; Zhou, X.; Fu, J.; Hu, T.; Or, P.M.; Feng, R.; He, C.Y.; Chen, W.J.; Zhang, X.; Chen, Y.; et al. Metabolite profiling analysis of FR429, an ellagitannin purified from Polygonum capitatum, in rat and human liver microsomes, cytosol and rat primary hepatocytes in vitro. Chem. Biol. Interact. 2014, 220, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.Y.; Zhou, X.; Fu, J.; He, C.Y.; Feng, R.; Huang, M.; Shou, J.W.; Zhao, Z.X.; Li, X.Y.; Zhang, L.; et al. In vivo metabolite profiling of a purified ellagitannin isolated from polygonum capitatum in rats. Molecules 2016, 21, 1110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simirgiotis, M.J.; Benites, J.; Areche, C.; Sepulveda, B. Antioxidant capacities and analysis of phenolic compounds in three endemic Nolana species by HPLC-PDA-ESI-MS. Molecules 2015, 20, 11490–11507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, J.X.; Zhao, B.B.; Zan, J.F.; Wang, P.; Chen, L.L. Simultaneous determination of phenolic acids and flavonoids in Artemisiae Argyi Folium by HPLC-MS/MS and discovery of antioxidant ingredients based on relevance analysis. J. Pharm. Biomed. Anal. 2019, 175, 112734. [Google Scholar] [CrossRef]
- Hossain, M.B.; Rai, D.K.; Brunton, N.P.; Martin-Diana, A.B.; Barry-Ryan, C. Characterization of phenolic composition in Lamiaceae spices by LC-ESI-MS/MS. J. Agric. Food Chem. 2010, 58, 10576–10581. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF/MS characterization of phenolic compounds from medicinal plants (hops and juniper berries) and their antioxidant activity. Foods 2020, 9, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, C.; Howell, K.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF/MS characterisation of phenolic acids and flavonoids in polyphenol-rich fruits and vegetables and their potential antioxidant activities. Antioxidants 2019, 8, 405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jimenez, C.; Crews, P. Novel marine sponge amino acids, 10. Xestoaminols from Xestospongia sp. J. Nat. Prod. 1990, 53, 978–982. [Google Scholar] [CrossRef] [PubMed]
- Phillip, D.; Hobe, S.; Paulsen, H.; Molnar, P.; Hashimoto, H.; Young, A.J. The binding of Xanthophylls to the bulk light-harvesting complex of photosystem II of higher plants. A specific requirement for carotenoids with a 3-hydroxy-beta-end group. J. Biol. Chem. 2002, 277, 25160–25169. [Google Scholar] [CrossRef] [Green Version]
- Burdi, D.K.; Samejo, M.Q.; Bhanger, M.I.; Khan, K.M. Fatty acid composition of Abies pindrow (west himalayan fir). Pak. J. Pharm. Sci. 2007, 20, 15–19. [Google Scholar]
- Mohamed, M.H.; Wilson, L.D.; Headley, J.V. Tunable polymeric sorbent materials for fractionation of model naphthenates. J. Phys. Chem. B 2013, 117, 3659–3666. [Google Scholar] [CrossRef]
- Nenoff, P.; Haustein, U.F. In vitro activity of phytosphingosines against Malassezia furfur and Candida albicans. Acta Derm. Venereol. 2002, 82, 170–173. [Google Scholar] [CrossRef] [Green Version]
- Oh, M.J.; Cho, Y.H.; Cha, S.Y.; Lee, E.O.; Kim, J.W.; Kim, S.K.; Park, C.S. Novel phytoceramides containing fatty acids of diverse chain lengths are better than a single C18-ceramide N-stearoyl phytosphingosine to improve the physiological properties of human stratum corneum. Clin. Cosmet. Investig. Derm. 2017, 10, 363–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dasyam, N.; Munkacsi, A.B.; Fadzilah, N.H.; Senanayake, D.S.; O’Toole, R.F.; Keyzers, R.A. Identification and bioactivity of 3-epi-xestoaminol C isolated from the New Zealand brown alga Xiphophora chondrophylla. J. Nat. Prod. 2014, 77, 1519–1523. [Google Scholar] [CrossRef]
- Hormaetxe, K.; Hernandez, A.; Becerril, J.M.; Garcia-Plazaola, J.I. Role of red carotenoids in photoprotection during winter acclimation in Buxus sempervirens leaves. Plant. Biol. 2004, 6, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.C.; Jorge, N. Bioactive compounds of oils extracted from fruits seeds obtained from agroindustrial waste. Eur. J. Lipid Sci. Tech. 2016, 118, 1600024. [Google Scholar] [CrossRef]
- Beveridge, T.H.; Li, T.S.; Drover, J.C. Phytosterol content in American ginseng seed oil. J. Agric. Food Chem. 2002, 50, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Hassan, E.M.; Matloub, A.A.; Aboutabl, M.E.; Ibrahim, N.A.; Mohamed, S.M. Assessment of anti-inflammatory, antinociceptive, immunomodulatory, and antioxidant activities of Cajanus cajan L. seeds cultivated in Egypt and its phytochemical composition. Pharm. Biol. 2015, 54, 1380–1391. [Google Scholar] [CrossRef] [Green Version]
- Hamedi, A.; Ghanbari, A.; Razavipour, R.; Saeidi, V.; Zarshenas, M.M.; Sohrabpour, M.; Azari, H. Alyssum homolocarpum seeds: Phytochemical analysis and effects of the seed oil on neural stem cell proliferation and differentiation. J. Nat. Med. 2015, 69, 387–396. [Google Scholar] [CrossRef]
- Kozlowska, M.; Gruczynska, E.; Scibisz, I.; Rudzinska, M. Fatty acids and sterols composition, and antioxidant activity of oils extracted from plant seeds. Food Chem. 2016, 213, 450–456. [Google Scholar] [CrossRef]
- Kalleli, F.; Bettaieb Rebey, I.; Wannes, W.A.; Boughalleb, F.; Hammami, M.; Saidani Tounsi, M.; M’Hamdi, M. Chemical composition and antioxidant potential of essential oil and methanol extract from Tunisian and French fennel (Foeniculum vulgare Mill.) seeds. J. Food Biochem. 2019, 43, e12935. [Google Scholar] [CrossRef]
- Kayano, S.; Yamada, N.F.; Suzuki, T.; Ikami, T.; Shioaki, K.; Kikuzaki, H.; Mitani, T.; Nakatani, N. Quantitative evaluation of antioxidant components in prunes (Prunus domestica L.). J. Agric. Food Chem. 2003, 51, 1480–1485. [Google Scholar] [CrossRef]
- Costa, F.; Jerz, G.; Figueiredo Fde, S.; Winterhalter, P.; Leitao, G.G. Solvent system selectivities in countercurrent chromatography using Salicornia gaudichaudiana metabolites as practical example with off-line electrospray mass-spectrometry injection profiling. J. Chromatogr. A 2015, 1385, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Cortazar, M.; Herrera-Ruiz, M.; Zamilpa, A.; Jimenez-Ferrer, E.; Marquina, S.; Alvarez, L.; Tortoriello, J. Anti-inflammatory activity and chemical profile of Galphimia glauca. Planta Med. 2013, 80, 90–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jho, E.H.; Kang, K.; Oidovsambuu, S.; Lee, E.H.; Jung, S.H.; Shin, I.S.; Nho, C.W. Gymnaster koraiensis and its major components, 3,5-di-O-caffeoylquinic acid and gymnasterkoreayne B, reduce oxidative damage induced by tert-butyl hydroperoxide or acetaminophen in HepG2 cells. BMB Rep. 2013, 46, 513–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.G.; Lee, H.; Nam, T.G.; Eom, S.H.; Heo, H.J.; Lee, C.Y.; Kim, D.O. Neuroprotective effect of caffeoylquinic acids from Artemisia princeps Pampanini against oxidative stress-induced toxicity in PC-12 cells. J. Food Sci. 2011, 76, C250–C256. [Google Scholar] [CrossRef] [PubMed]
- Kayano, S.; Kikuzaki, H.; Fukutsuka, N.; Mitani, T.; Nakatani, N. Antioxidant activity of prune (Prunus domestica L.) constituents and a new synergist. J. Agric. Food Chem. 2002, 50, 3708–3712. [Google Scholar] [CrossRef]
- Menichini, F.; Losi, L.; Bonesi, M.; Pugliese, A.; Loizzo, M.R.; Tundis, R. Chemical profiling and in vitro biological effects of Cardiospermum halicacabum L. (Sapindaceae) aerial parts and seeds for applications in neurodegenerative disorders. J. Enzym. Inhib. Med. Chem. 2013, 29, 677–685. [Google Scholar] [CrossRef]
- Kenny, O.; Smyth, T.J.; Hewage, C.M.; Brunton, N.P. Antioxidant properties and quantitative UPLC-MS analysis of phenolic compounds from extracts of fenugreek (Trigonella foenum-graecum) seeds and bitter melon (Momordica charantia) fruit. Food Chem. 2013, 141, 4295–4302. [Google Scholar] [CrossRef]
- Ablat, A.; Halabi, M.F.; Mohamad, J.; Hasnan, M.H.; Hazni, H.; Teh, S.H.; Shilpi, J.A.; Mohamed, Z.; Awang, K. Antidiabetic effects of Brucea javanica seeds in type 2 diabetic rats. BMC Complement. Altern. Med. 2017, 17, 94–107. [Google Scholar] [CrossRef] [Green Version]
- Arranz, S.; Cert, R.; Pérez-Jiménez, J.; Cert, A.; Saura-Calixto, F. Comparison between free radical scavenging capacity and oxidative stability of nut oils. Food Chem. 2008, 110, 985–990. [Google Scholar] [CrossRef] [Green Version]
- Gorinstein, S.; Martin-Belloso, O.; Katrich, E.; Lojek, A.; Ciz, M.; Gligelmo-Miguel, N.; Haruenkit, R.; Park, Y.S.; Jung, S.T.; Trakhtenberg, S. Comparison of the contents of the main biochemical compounds and the antioxidant activity of some Spanish olive oils as determined by four different radical scavenging tests. J. Nutr. Biochem. 2003, 14, 154–159. [Google Scholar] [CrossRef]
- Assiri, A.M.; Hassanien, M.F. Bioactive lipids, radical scavenging potential, and antimicrobial properties of cold pressed clove (Syzygium aromaticum) oil. J. Med. Food 2013, 16, 1046–1056. [Google Scholar] [CrossRef] [PubMed]
- Ali, Q.; Anwar, F.; Ashraf, M.; Saari, N.; Perveen, R. Ameliorating effects of exogenously applied proline on seed composition, seed oil quality and oil antioxidant activity of maize (Zea mays L.) under drought stress. Int. J. Mol. Sci. 2013, 14, 818–835. [Google Scholar] [CrossRef]
- Alu’datt, M.H.; Rababah, T.; Alhamad, M.N.; Al-Mahasneh, M.A.; Almajwal, A.; Gammoh, S.; Ereifej, K.; Johargy, A.; Alli, I. A review of phenolic compounds in oil-bearing plants: Distribution, identification and occurrence of phenolic compounds. Food Chem. 2017, 218, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Z.; Liu, R.H. Phenolic and carotenoid profiles and antiproliferative activity of foxtail millet. Food Chem. 2014, 174, 495–501. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, R.; Niu, W. Phytochemical and antiproliferative activity of proso millet. PLoS ONE 2014, 9, e104058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.S.; Park, R.Y.; Jeon, H.J.; Kwon, Y.S.; Chun, W. Neuroprotective effects of 3,5-dicaffeoylquinic acid on hydrogen peroxide-induced cell death in SH-SY5Y cells. Phytother. Res. 2005, 19, 243–245. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Burgos, E.; Liaudanskas, M.; Viskelis, J.; Zvikas, V.; Janulis, V.; Gomez-Serranillos, M.P. Antioxidant activity, neuroprotective properties and bioactive constituents analysis of varying polarity extracts from Eucalyptus globulus leaves. J. Food Drug Anal. 2018, 26, 1293–1302. [Google Scholar] [CrossRef] [PubMed]
- Shahat, A.A.; Hidayathulla, S.; Khan, A.A.; Alanazi, A.M.; Al Meanazel, O.T.; Alqahtani, A.S.; Alsaid, M.S.; Hussein, A.A. Phytochemical profiling, antioxidant and anticancer activities of Gastrocotyle hispida growing in Saudi Arabia. Acta Trop. 2019, 191, 243–247. [Google Scholar] [CrossRef]
- Chung, M.J.; Lee, S.; Park, Y.I.; Lee, J.; Kwon, K.H. Neuroprotective effects of phytosterols and flavonoids from Cirsium setidens and Aster scaber in human brain neuroblastoma SK-N-SH cells. Life Sci. 2016, 148, 173–182. [Google Scholar] [CrossRef]
- Asadi, F.; Shahriari, A.; Chahardah-Cheric, M. Effect of long-term optional ingestion of canola oil, grape seed oil, corn oil and yogurt butter on serum, muscle and liver cholesterol status in rats. Food Chem. Toxicol. 2010, 48, 2454–2457. [Google Scholar] [CrossRef]
- Mekonnen, Z.; Gebreselema, A.; Abere, Y. Effect of locally manufactured niger seed oil on lipid profile compared to imported palm and sunflower oils on rat models. J. Lipids 2018, 2018, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakono, M.; Yoshida, K.; Yahiro, M. Combined effects of dietary protein and fat on lipid metabolism in rats. J. Nutr. Sci. Vitam. 1993, 39, 335–343. [Google Scholar] [CrossRef]
- Jurgonski, A.; Juskiewicz, J.; Zdunczyk, Z.; Krol, B. Caffeoylquinic acid-rich extract from chicory seeds improves glycemia, atherogenic index, and antioxidant status in rats. Nutrition 2011, 28, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Pieszka, M.; Tombarkiewicz, B.; Roman, A.; Migdal, W.; Niedziolka, J. Effect of bioactive substances found in rapeseed, raspberry and strawberry seed oils on blood lipid profile and selected parameters of oxidative status in rats. Environ. Toxicol. Pharm. 2013, 36, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Zahir, A.A.; Rahuman, A.A.; Kamaraj, C.; Bagavan, A.; Elango, G.; Sangaran, A.; Kumar, B.S. Laboratory determination of efficacy of indigenous plant extracts for parasites control. Parasitol. Res. 2009, 105, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Park, N.H.; Lee, W.; Kim, E.H.; Jin, Y.H.; Seo, E.K.; Hong, J. Comprehensive chemical profiling of Pinellia species tuber and processed Pinellia tuber by gas chromatography-mass spectrometry and liquid chromatography-atmospheric pressure chemical ionization-tandem mass spectrometry. J. Chromatogr. A 2016, 1471, 164–177. [Google Scholar] [CrossRef]
- Cuyckens, F.; Claeys, M. Optimization of a liquid chromatography method based on simultaneous electrospray ionization mass spectrometric and ultraviolet photodiode array detection for analysis of flavonoid glycosides. Rapid Commun. Mass Spectrom. 2002, 16, 2341–2348. [Google Scholar] [CrossRef]
- Kano, M.; Takayanagi, T.; Harada, K.; Makino, K.; Ishikawa, F. Antioxidative activity of anthocyanins from purple sweet potato, Ipomoera batatas cultivar Ayamurasaki. Biosci. Biotechnol. Biochem. 2005, 69, 979–988. [Google Scholar] [CrossRef] [Green Version]
- Pangjit, K.; Udomsuk, L.; Upanan, S.; Pongjanta, A.; Chansiw, N.; Srichairatanakool, S. Iron-chelating and anti-hemolytic properties of ethanolic extract of lotus (Nelumbonucifera gaertn) leaves. J. Med. Assoc. Thai. 2016, 99, S58–S66. [Google Scholar]
- Agarwala, S.; Rao B., N.; Mudholkar, K.; Bhuwania, R.; Satish Rao, B.S. Mangiferin, a dietary xanthone protects against mercury-induced toxicity in HepG2 cells. Env. Toxicol. 2012, 27, 117–127. [Google Scholar] [CrossRef] [Green Version]
- Shen, L.; Zhao, H.Y.; Du, J.; Wang, F. Anti-tumor activities of four chelating agents against human neuroblastoma cells. In Vivo 2005, 19, 233–236. [Google Scholar] [PubMed]
- Puttmann, M.; Krug, H.; von Ochsenstein, E.; Kattermann, R. Fast HPLC determination of serum free fatty acids in the picomole range. Clin. Chem. 1993, 39, 825–832. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples are available from the authors. |


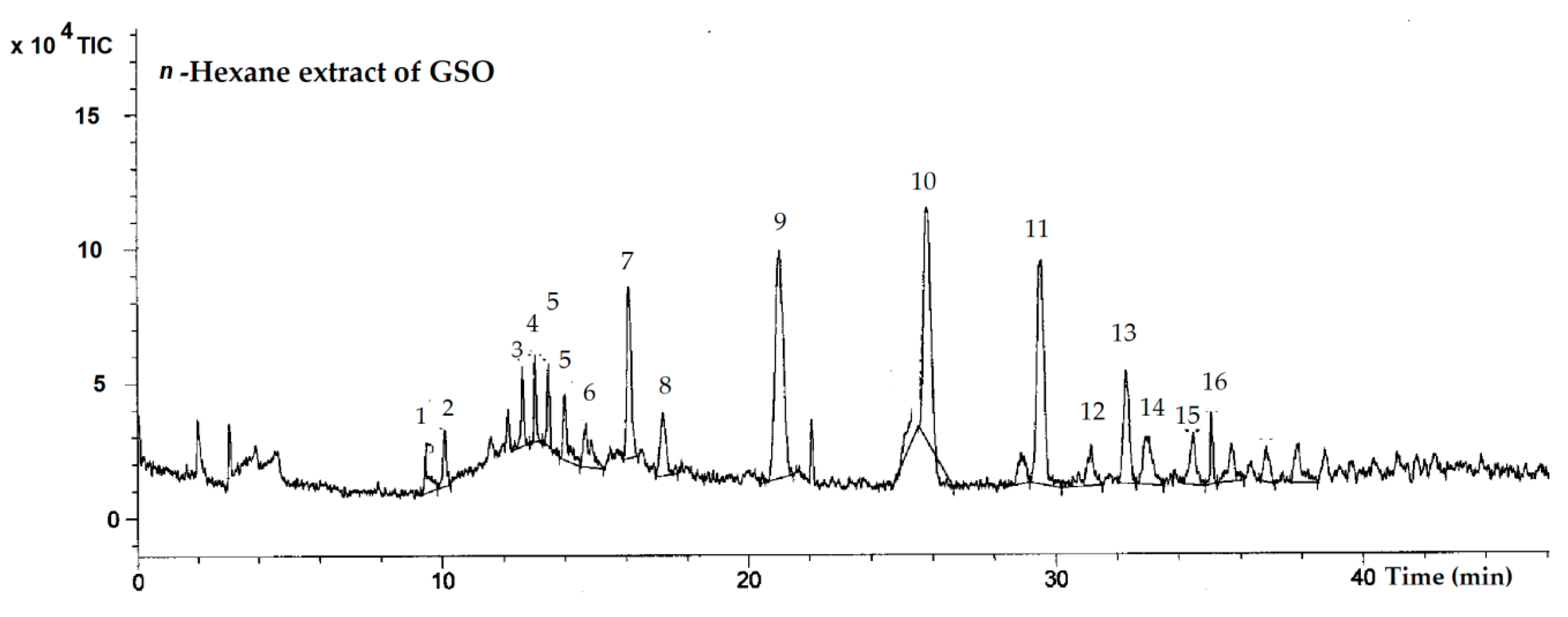
| Peak | TR | Target | Mass Error | Molecular Formula | Exact Mass | Observed Mass (m/z) | Identification | ||
|---|---|---|---|---|---|---|---|---|---|
| No. | (min) | Score | (ppm) | (g/mol) | [M + H]+ | [M + NH4]+ | [M + Na]+ | ||
| 1 | 0.576 | 96.14 | 3.86 | C16H32O2 | 256.24 | 257.24 | 274.27 | 279.22 | 4-Hexyl-decanoic acid |
| 96.14 | 3.54 | C16H35NO2 | 273.27 | 274.27 | 291.30 | 296.25 | Sphinganine | ||
| 2 | 1.051 | 82.26 | 1.82 | C20H34O8 | 402.23 | 403.23 | 420.26 | 425.21 | 5S-Hydroxyeicosatetraenoyl di-endoperoxide |
| 89.23 | −3.36 | C22H32O8 | 424.21 | 425.21 | 442.24 | 447.20 | Didrovaltratum | ||
| 3 | 1.066 | 95.18 | 1.45 | C20 H39NO6 | 389.28 | 390.28 | 407.31 | 412.27 | Sphingofungin B |
| 4 | 1.094 | 96.85 | 2.19 | C20 H36O6 | 372.25 | 373.25 | 390.28 | 399.25 | 13,14-Dihydro-19(R)-hydroxyprostaglandin E1 |
| 5 | 1.177 | 52.09 | −1.18 | C40H54O2 | 566.41 | 567.41 | 585.44 | 591.42 | Eschscholtzxanthin |
| 6 | 21.93 | 98.82 | 2.03 | C14H28O | 212.21 | 213.22 | 230.24 | 235.20 | Tetradecan-3-one |
| 98.82 | 1.88 | C14H31NO | 229.24 | 230.24 | 247.27 | 252.22 | Xestoaminol C | ||
| Peak No. | TR (min) | TIC | Exact Mass (g/mol) | Molecular Formula | Observed Mass (m/z) | Error (%) | Identification |
|---|---|---|---|---|---|---|---|
| 1 | 13.25 | 2277755 | 284.5 | C18H36O2 | 284 | −0.18 | Ethyl palmitate |
| 2 | 14.83 | 2423828 | 308.5 | C20H36O2 | 308 | −0.16 | Ethyll linolenate |
| 3 | 14.89 | 5411461 | 310.5 | C20H38O2 | 310 | −0.16 | Ethyl linoleate |
| 4 | 15.12 | 359612 | 312.5 | C20H40O2 | 312 | −0.16 | Ethyl stearate |
| 5 | 17.77 | 3836542 | 352.6 | C18H32O2 | 353 | 0.11 | Linoleic acid TMS |
| 6 | 18.10 | 17168 | 350.6 | C18H30O2 | 352 | 0.4 | Linolenic acid TMS |
| 7 | 20.41 | 18788155 | 372.7 | C27H48 | 372 | −0.19 | Cholestane |
| 8 | 23.44 | 452445 | 486.9 | C29H50O | 484 | −0.6 | β-Sitosterol TMS |
| 9 | 23.70 | 770310 | 485.8 | C29H48O | 485 | −0.16 | Stigmasterol TMS |
| 10 | 24.37 | 12299199 | 472.9 | C28H48O | 472 | −0.19 | Campesterol TMS |
| 11 | 25.99 | 324939 | - | - | - | - | Unknown |
| Peak No. | TR (min) | TIC | Exact Mass (g/mol) | Molecular Formula | Observed Mass (m/z) | Error (%) | Possible Constituents/Compounds | References |
|---|---|---|---|---|---|---|---|---|
| 1 | 9.47 | 153000 | 192.1 | C7H12O6 | [M − H]+ 194.1 | 1.03 | Quinic acid | [25] |
| 2 | 10.11 | 174000 | 354.3 | C16H18O9 | [M − H]+ 354.9 | 0.17 | O-Caffeoylquinic acid | [25,26] |
| 3 | 12.64 | 185000 | 290.3 | C15H14O6 | [M − H]+ 298.9 | 2.88 | Catechin | Authentic standard |
| 4 | 13.48 | 152000 | 432.1 | C21H20O10 | [M − H]+ 433.3 | 0.23 | Apigenin-4-O-glycoside | [27,28] |
| 5 | 14.02 | 254000 | 496.2 | C21H21O14 | [M − H]+ 497.2 | 0.2 | Ellagic acid-O-methoxyglucoside | [29,30] |
| 6 | 14.69 | 254000 | 342.3 | C18H14O7 | [M − H]+ 342.9 | 0.15 | Dicaffeic acid | [26,28] |
| 7 | 16.11 | 649000 | 464.1 | C15H10O7 | [M − H]+ 454.3 | 2.15 | isoquercetin | Authentic standard |
| 8 | 17.22 | 318000 | Unknown | Unknown | ND | ND | Unknown | - |
| 9 | 21.04 | 160000 | 452.1 | Unknown | [M − H]+ 453.2 | 0.27 | O-Caffeoylquinic acid derivative | [25] |
| 10 | 25.84 | 129000 | Unknown | Unknown | [M − H]+ ND | ND | Unknown | - |
| 11 | 29.53 | 138000 | 302.2 | C14H6O8 | [M − H]+ 302.5 | 0.1 | Ellagic acid | [31,32] |
| 12 | 31.15 | 306000 | 288.25 | C15H12O6 | [M − H]+ 297.0 | 2.95 | Eriodictyol | Authentic standard |
| 13 | 32.32 | 543000 | 594.5 | C27H30O15 | [M − H]+ 595.5 | 0.17 | Luteolin-7-O-rutinoside | [24] |
| 14 | 32.99 | 379000 | 302.2 | C15H10O7 | [M − K]+ 340.3 | 0.26 | Quercetin | Authentic standard |
| 15 | 34.47 | 261000 | 382.0 | C17H18O10 | [M − H]+ 383.3 | 0.34 | Caffeoyl-glycosides or cinnamoyl glycosides | [26] |
| 16 | 35.09 | 180000 | 516.45 | C₂₅H₂₄O₁₂ | [M − H]+ 517.3 | 0.16 | di-O-Caffeoyquinic acid | [26] |
| Serum Lipids | Time | DI | CO (30 g LAE/kg) | GSO (6 g LAE/kg) | GSO (30 g LAE/kg) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 M | 5 F | 5 M, 5 F | 5 M | 5 F | 5 M, 5 F | 5 M | 5 F | 5 M, 5 F | 5 M | 5 F | 5 M, 5 F | ||
| Total cholesterol (mg/dL) | Day 0 | 64.4 ± 8.0 | 61.8 ± 11.5 | 63.1 ± 9.4 | 65.6 ± 10.7 | 66.2 ± 6.9 | 65.9 ± 8.5 | 67.4 ± 11.2 | 72.6 ± 11.6 | 70.0 ± 11.1 | 62.4 ± 4.2 | 69.2 ± 12.7 | 65.8 ± 9.6 |
| Day 90 | 66.8 ± 13.5 | 51.8 ± 8.5 | 59.3 ± 13.3 | 62.2 ± 4.8 | 65.4 ± 6.1 | 63.8 ± 5.5 | 72.8 ± 16.4 | 62.2 ± 4.8 | 67.5 ± 12.7 | 74.0 ± 6.5 | 62.6 ± 10.1 | 68.3 ± 10.0 | |
| Triglyceride (mg/dL) | Day 0 | 87.0 ± 24.2 | 33.2 ± 8.2 | 60.1 ± 33.1 | 100.4 ± 24.6 | 44.8 ± 4.3 | 72.6 ± 33.7 | 70.0 ± 17.4 | 41.8 ± 10.0 | 55.9 ± 20.0 | 89.4 ± 34.2 | 60.8 ± 9.9 | 75.1 ± 28.1 |
| Day 90 | 66.2 ± 19.4 | 38.8 ± 11.7 | 52.5 ± 20.9 | 39.8 ± 6.4 | 48.4 ± 7.9 | 44.1 ± 8.1 & | 118.6 ± 45.1 | 39.8 ± 6.4 | 79.2 ± 51.4 | 77.4 ± 20.2 | 39.8 ± 9.8 | 58.6 ± 24.8 & | |
| Fatty Acid Levels | DI | CO (30 g LAE/kg) | GSO (6 g LAE/kg) | GSO (30 g LAE/kg) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 M | 5 F | 5 M, 5 F | 5 M | 5 F | 5 M, 5 F | 5 M | 5 F | 5 M, 5 F | 5 M | 5 F | 5 M, 5 F | |
| Palmitoleic acid (mg/dL) | 0.72 ± 0.29 | ND | 0.72 ± 0.29 | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Oleic acid (mg/dL) | 1.79 ± 0.85 | 1.00 ± 0.35 | 1.39 ± 0.74 | 0.57 ± 0.19 | 0.75 ± 0.19 | 0.66 ± 0.20 * | 1.04 ± 0.37 | 0.61 ± 0.16 | 0.82 ± 0.35 * | 0.54 ± 0.17 | 0.62 ± 0.07 | 0.58 ± 0.13 * |
| Stearic acid (mg/dL) | 4.39 ± 1.38 | 2.55 ± 0.77 | 3.47 ± 1.44 | 1.57 ± 0.65 | 1.84 ± 0.37 | 1.71 ± 0.52 * | 2.74 ± 0.43 | 1.38 ± 0.25 | 2.06 ± 0.79 * | 1.37 ± 0.22 | 1.33 ± 0.15 | 1.35 ± 0.18 *,# |
| Linoleic acid (mg/dL) | 1.70 ± 0.66 | 0.90 ± 0.31 | 1.30 ± 0.64 | 0.97 ± 0.41 | 1.41 ± 0.37 | 1.19 ± 0.43 | 1.05 ± 0.35 | 0.59 ± 0.15 | 0.82 ± 0.35 | 0.82 ± 0.24 | 0.78 ± 0.11 | 0.80 ± 0.18 |
| α-Linolenic acid (mg/dL) | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Arachidonic acid (mg/dL) | 0.74 ± 0.20 | 0.55 ± 0.13 | 0.64 ± 0.19 | 0.34 ± 0.13 | 0.40 ± 0.06 | 0.37 ± 0.10 * | 0.48 ± 0.10 | 0.40 ± 0.04 | 0.44 ± 0.08 * | 0.35 ± 0.05 | 0.43 ± 0.13 | 0.39 ± 0.10 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prommaban, A.; Utama-ang, N.; Chaikitwattana, A.; Uthaipibull, C.; Porter, J.B.; Srichairatanakool, S. Phytosterol, Lipid and Phenolic Composition, and Biological Activities of Guava Seed Oil. Molecules 2020, 25, 2474. https://doi.org/10.3390/molecules25112474
Prommaban A, Utama-ang N, Chaikitwattana A, Uthaipibull C, Porter JB, Srichairatanakool S. Phytosterol, Lipid and Phenolic Composition, and Biological Activities of Guava Seed Oil. Molecules. 2020; 25(11):2474. https://doi.org/10.3390/molecules25112474
Chicago/Turabian StylePrommaban, Adchara, Niramon Utama-ang, Anan Chaikitwattana, Chairat Uthaipibull, John B. Porter, and Somdet Srichairatanakool. 2020. "Phytosterol, Lipid and Phenolic Composition, and Biological Activities of Guava Seed Oil" Molecules 25, no. 11: 2474. https://doi.org/10.3390/molecules25112474
APA StylePrommaban, A., Utama-ang, N., Chaikitwattana, A., Uthaipibull, C., Porter, J. B., & Srichairatanakool, S. (2020). Phytosterol, Lipid and Phenolic Composition, and Biological Activities of Guava Seed Oil. Molecules, 25(11), 2474. https://doi.org/10.3390/molecules25112474