Ecdysteroid Content and Therapeutic Activity in Elicited Spinach Accessions
Abstract
:1. Introduction
1.1. Spinach
1.2. Ecdysteroids
1.3. Ecdysteroids in Spinach
2. Results
2.1. Ecdysteroid Content
2.2. Anabolic Activity
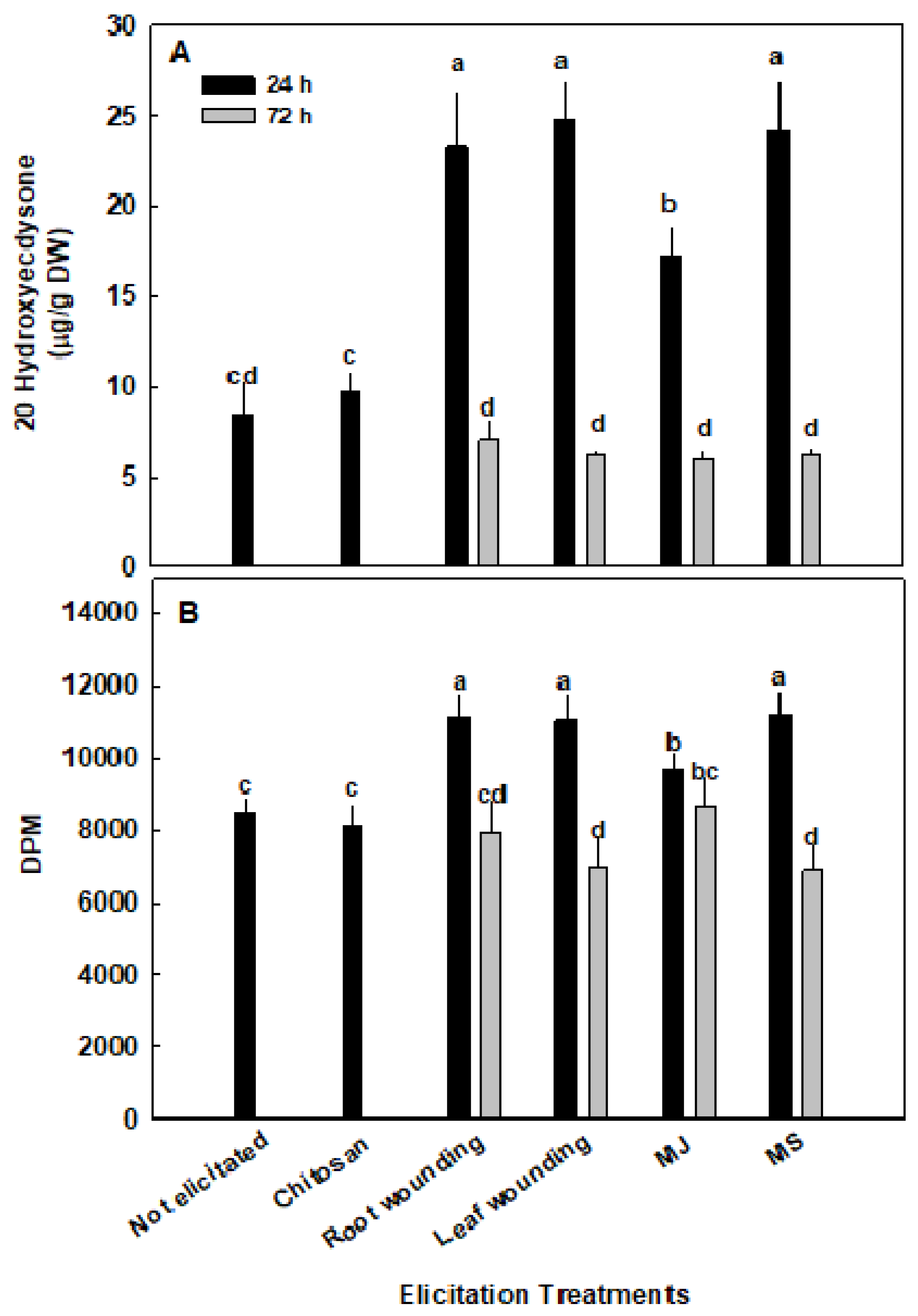
2.3. Elicitation on Ecdysteroid Content
2.4. Elicitation and Anabolic Activity
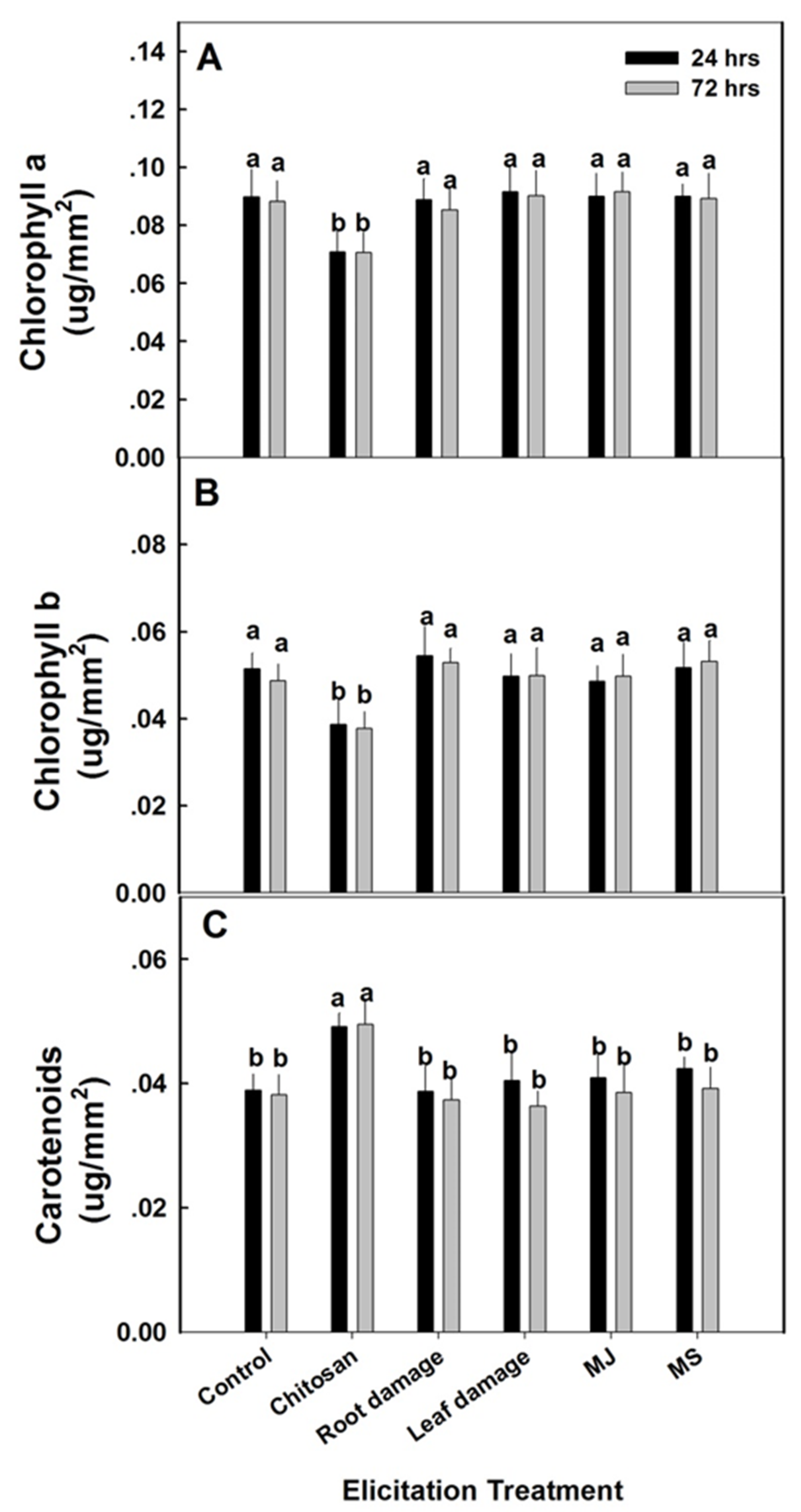
2.5. Physiological Response to Elicitation
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Growing Conditions
4.3. Elicitation
4.4. Membrane Leakage
4.5. Osmotic Potential
4.6. Chlorophyll and Carotenoids Content
4.7. Ecdysteroid Extraction and Quantification
4.8. Protein Synthesis Activity
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Roberts, J.L.; Moreau, R. Functional properties of spinach (Spinacia oleracea L.) phytochemicals and bioactives. Food Funct. 2016, 7, 3337–3353. [Google Scholar] [CrossRef] [PubMed]
- Lomnitski, L.; Bergman, M.; Nyska, A.; Ben-Shaul, V.; Grossman, S. Composition, Efficacy, and Safety of Spinach Extracts. Nutr. Cancer 2003. [Google Scholar] [CrossRef] [PubMed]
- Cartford, M.C.; Gemma, C.; Bickford, P.C. Eighteen-month-old Fischer 344 rats fed a spinach-enriched diet show improved delay classical eyeblink conditioning and reduced expression of tumor necrosis factor α (TNFα) and TNFβ in the cerebellum. J. Neurosci. 2002. [Google Scholar] [CrossRef] [Green Version]
- Grebenok, R.J.; Ripa, P.V.; Adler, J.H. Occurrence and levels of ecdysteroids in spinach. Lipids 1991. [Google Scholar] [CrossRef]
- Lafont, R.; Koolman, J. Diversity of Ecdysteroids in Animal Species. In Ecdysone: Structures and Functions; Springer: Berlin, Germany, 2009. [Google Scholar]
- Dinan, L.; Savchenko, T.; Whiting, P. On the distribution of phytoecdysteroids in plants. Cell. Mol. Life Sci. 2001. [Google Scholar] [CrossRef]
- Soriano, I.R.; Riley, I.T.; Potter, M.J.; Bowers, W.S. Phytoecdysteroids: A novel defense against plant-parasitic nematodes. J. Chem. Ecol. 2004. [Google Scholar] [CrossRef]
- Couderc, J.-L. Ecdysone: From Metabolism to Regulation of Gene Expression. Papers from the Seventh Ecdysone Workshop, Edinburgh, UK, March 31-April 3, 1985. Mary Bownes. Q. Rev. Biol. 1987. [Google Scholar] [CrossRef]
- Vanyolos, A.; Bathori, M. New Perspectives in the Analysis of Ecdysteroids: A Promising Group of Biologically Active Compounds. Curr. Pharm. Anal. 2008. [Google Scholar] [CrossRef]
- Gorelick-Feldman, J.; MacLean, D.; Ilic, N.; Poulev, A.; Lila, M.A.; Cheng, D.; Raskin, I. Phytoecdysteroids increase protein synthesis in skeletal muscle cells. J. Agric. Food Chem. 2008. [Google Scholar] [CrossRef]
- Lafont, R.; Dinan, L. Practical uses for ecdysteroids in mammals including humans: And update. J. Insect Sci. 2003. [Google Scholar] [CrossRef]
- Bajguz, A.; Bąkała, I.; Talarek, M. Ecdysteroids in plants and their pharmacological effects in vertebrates and humans. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Tavares, M.C.H.; Yariwake Vilegas, J.H.; Lanças, F.M. Phytoecdysteroid profiles in seeds of Sida spp. (Malvaceae). Phytochem. Anal. 2001. [Google Scholar] [CrossRef]
- Sarpong, F.M.; Armah, F.A.; Amponsah, I.K.; Atchoglo, P.K. Antinociceptive ecdysteroids and other constituents of palisota hirsuta k. schum (commelinaceae). J. Appl. Pharm. Sci. 2016. [Google Scholar] [CrossRef] [Green Version]
- Hunyadi, A.; Herke, I.; Lengyel, K.; Báthori, M.; Kele, Z.; Simon, A.; Tóth, G.; Szendrei, K. Ecdysteroid-containing food supplements from Cyanotis arachnoidea on the European market: Evidence for spinach product counterfeiting. Sci. Rep. 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Available online: www.ecdybase.org (accessed on 9 March 2020).
- Bathori, M.; Mathe, I., Jr.; Solymosi, P.; Szendrei, K. Phytoecdysteroids in some species of Caryophyllaceae and Chenopodiaceae. Acta Bot. Hung. 1987, 33, 377–385. [Google Scholar]
- Dinan, L.; Savchenko, T.; Whiting, P. Phytoecdysteroids in the genus Asparagus (Asparagaceae). Phytochemistry 2001. [Google Scholar] [CrossRef]
- Bakrim, A.; Maria, A.; Sayah, F.; Lafont, R.; Takvorian, N. Ecdysteroids in spinach (Spinacia oleracea L.): Biosynthesis, transport and regulation of levels. Plant Physiol. Biochem. 2008. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.M.; Yousef, G.G.; Lila, M.A. Variation in phytoecdysteroid accumulation in seeds and shoots of Spinacia oleracea L. accessions. HortScience 2010, 45, 1634–1638. [Google Scholar] [CrossRef] [Green Version]
- Schmelz, E.A.; Grebenok, R.J.; Ohnmeiss, T.E.; Bowers, W.S. Interactions between Spinacia oleracea and Bradysia impatiens: A role for phytoecdysteroids. Arch. Insect Biochem. Physiol. 2002. [Google Scholar] [CrossRef]
- Schmelz, E.A.; Grebenok, R.J.; Galbraith, D.W.; Bowers, W.S. Damage-induced accumulation of phytoecdysteroids in spinach: A rapid root response involving the octadecanoic acid pathway. J. Chem. Ecol. 1998. [Google Scholar] [CrossRef]
- Ament, K.; Krasikov, V.; Allmann, S.; Rep, M.; Takken, F.L.W.; Schuurink, R.C. Methyl salicylate production in tomato affects biotic interactions. Plant J. 2010. [Google Scholar] [CrossRef]
- El Hadrami, A.; Adam, L.R.; El Hadrami, I.; Daayf, F. Chitosan in plant protection. Mar. Drugs 2010, 8, 968–987. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.; Chaturvedi, R.; Chowdhury, Z.; Venables, B.; Petros, R.A. Signaling by small metabolites in systemic acquired resistance. Plant J. 2014. [Google Scholar] [CrossRef] [PubMed]
- Hadwiger, L.A. Plant science review: Multiple effects of chitosan on plant systems: Solid science or hype. Plant Sci. 2013, 208, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Gorelick, J.; Bernstein, N. Elicitation: An underutilized tool in the development of medicinal plants as a source of therapeutic secondary metabolites. Adv. Agron. 2014. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A.; González-Aguëro, M.; Cisneros-Zevallos, L. Cross-talk between signaling pathways: The link between plant secondary metabolite production and wounding stress response. Sci. Rep. 2015. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, R.C.; Kumaraswamy, R.V.; Kumari, S.; Sharma, S.S.; Pal, A.; Raliya, R.; Biswas, P.; Saharan, V. Cu-chitosan nanoparticle boost defense responses and plant growth in maize (Zea mays L.). Sci. Rep. 2017, 7, 9754. [Google Scholar] [CrossRef]
- Abu-Muriefah, S.S. Effect of chitosan on common bean (Phaseolus vulgaris L.) plants grown under water stress conditions. Int. Res. J. Agric. Sci. Soil Sci. 2013, 3, 192–199. [Google Scholar]
- Moharekar, S.T.; Lokhande, S.D.; Hara, T.; Tanaka, R.; Tanaka, A.; Chavan, P.D. Effect of salicylic acid on chlorophyll and carotenoid contents of wheat and moong seedlings. Photosynthetica 2003. [Google Scholar] [CrossRef]
- Ahmed, F.; Fanning, K.; Netzel, M.; Schenk, P.M. Induced carotenoid accumulation in Dunaliella salina and Tetraselmis suecica by plant hormones and UV-C radiation. Appl. Microbiol. Biotechnol. 2015. [Google Scholar] [CrossRef]
- Pedneault, K.; Léonhart, S.; Gosselin, A.; Angers, P.; Papadopoulos, A.P.; Dorais, M. Variations in concentration of active compounds in four hydroponically- and field-grown medicinal plant species. In IV International ISHS Symposium on Artificial Lighting; International Society for Horticultural Science (ISHS): Leuven, Belgium, 2002; Volume 580, pp. 255–262. [Google Scholar]
- Gobbo-Neto, L.; Lopes, N.P. Medicinal plants: Factors of influence on the content of secondary metabolites | Plantas medicinais: Fatores de influência no conteúdo de metabólitos secundários. Quim. Nova 2007, 30, S0100–S40422007000200026. [Google Scholar] [CrossRef]
- Towler, M.J.; Weathers, P.J. Variations in key artemisinic and other metabolites throughout plant development in Artemisia annua L. for potential therapeutic use. Ind. Crops Prod. 2015, 67, 185–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mou, B. Leafminer resistance in spinach. HortScience 2008, 43, 1716–1719. [Google Scholar] [CrossRef] [Green Version]
- Bernstein, N.; Lauchli, A.; Silk, K. Kinematics and Dynamics of Sorghum ( Sorghum bicolor 1.) Leaf Development at Various Na/Ca Salinities. Plant Physiol. 1993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernstein, N.; Kravchik, M.; Dudai, N. Salinity-induced changes in essential oil, pigments and salts accumulation in sweet basil (Ocimum basilicum) in relation to alterations of morphological development. Ann. Appl. Biol. 2010. [Google Scholar] [CrossRef]
- Gorelick, J.; Rosenberg, R.; Smotrich, A.; Hanuš, L.; Bernstein, N. Hypoglycemic activity of withanolides and elicitated Withania somnifera. Phytochemistry 2015, 116, 283–289. [Google Scholar] [CrossRef]
- Bernstein, N.; Shoresh, M.; Xu, Y.; Huang, B. Involvement of the plant antioxidative response in the differential growth sensitivity to salinity of leaves vs roots during cell development. Free Radic. Biol. Med. 2010. [Google Scholar] [CrossRef]
- Lu, S.; Wang, Z.; Niu, Y.; Guo, Z.; Huang, B. Antioxidant responses of radiation-induced dwarf mutants of bermudagrass to drought stress. J. Am. Soc. Hortic. Sci. 2008. [Google Scholar] [CrossRef] [Green Version]
- Shoresh, M.; Spivak, M.; Bernstein, N. Involvement of calcium-mediated effects on ROS metabolism in the regulation of growth improvement under salinity. Free Radic. Biol. Med. 2011. [Google Scholar] [CrossRef]
- Lichtenthaler, H.; Wellburn, A. Determinations of total carotenoids and chlorophylls b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983. [Google Scholar] [CrossRef] [Green Version]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorelick, J.; Iraqi, R.H.; Bernstein, N. Ecdysteroid Content and Therapeutic Activity in Elicited Spinach Accessions. Plants 2020, 9, 727. https://doi.org/10.3390/plants9060727
Gorelick J, Iraqi RH, Bernstein N. Ecdysteroid Content and Therapeutic Activity in Elicited Spinach Accessions. Plants. 2020; 9(6):727. https://doi.org/10.3390/plants9060727
Chicago/Turabian StyleGorelick, Jonathan, Rona Hacohen Iraqi, and Nirit Bernstein. 2020. "Ecdysteroid Content and Therapeutic Activity in Elicited Spinach Accessions" Plants 9, no. 6: 727. https://doi.org/10.3390/plants9060727



