Identification of Bioactive Phytochemicals in Leaf Protein Concentrate of Jerusalem Artichoke (Helianthus tuberosus L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Installation
2.2. Harvest of Above-Ground Biomass
2.3. Fractionation of Harvested Green Biomass
2.4. Biochemical Composition of JAPC
2.4.1. Crude Protein Content
2.4.2. Quantification of Amino Acid Composition in JAPC Using an Amino Acid Analyzer
2.4.3. Determination of Fatty Acid Composition in JAPC Using Gas Chromatography
2.5. Screening of Phytochemicals in JAPC by UHPLC-ESI-ORBITRAP-MS/MS
2.5.1. Sample Preparation
2.5.2. UHPLC-ESI-ORBITRAP-MS/MS Analysis
2.5.3. Mass Spectrometry Conditions
2.6. Quality Assurance of Results
2.7. Statistical Analysis
3. Results
3.1. Green Biomass of Jerusalem Artichoke Clones
3.2. JAPC Yield
3.3. Total Protein Content of JAPC
3.4. Amino Acid Composition of JAPC
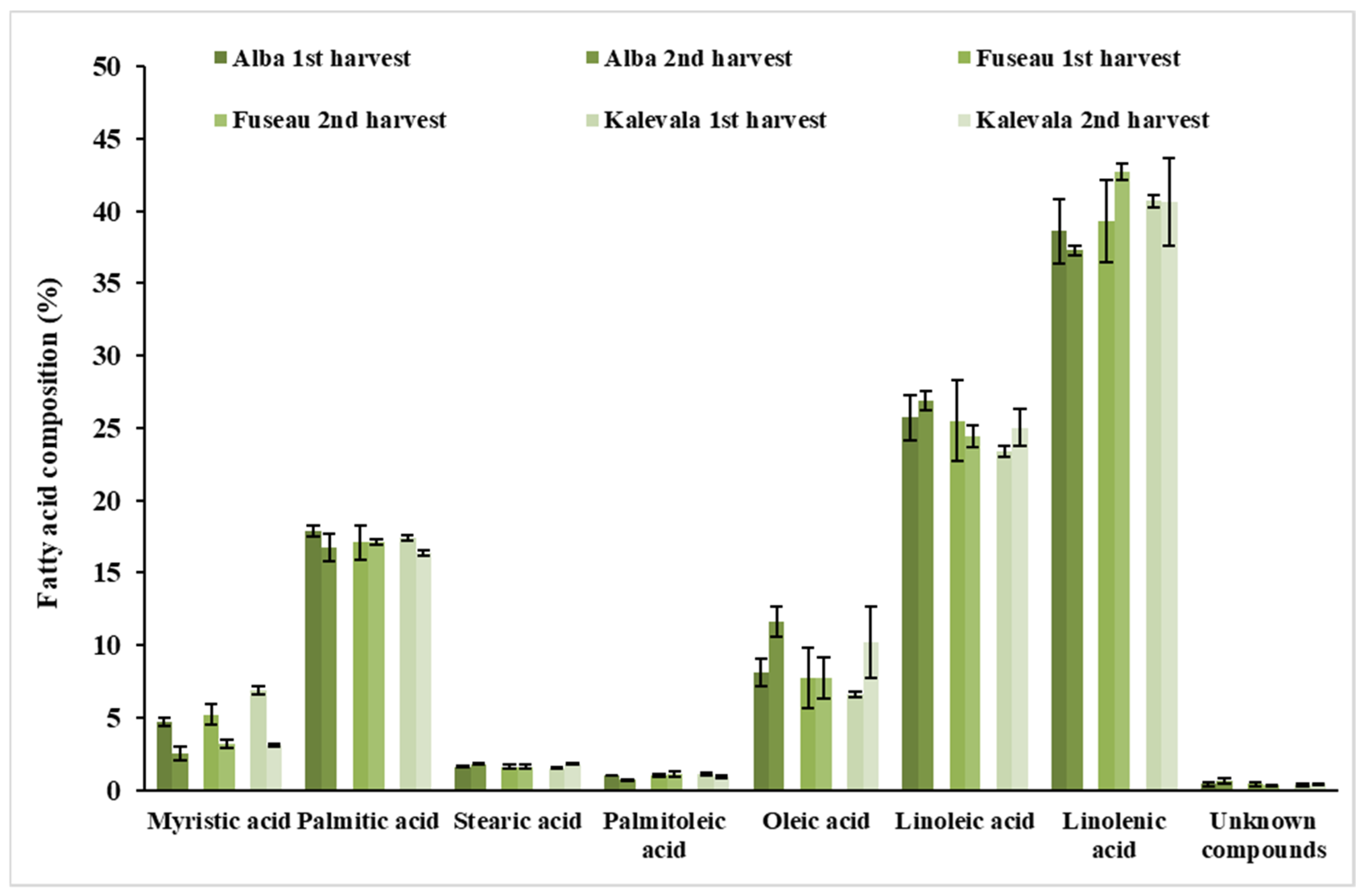
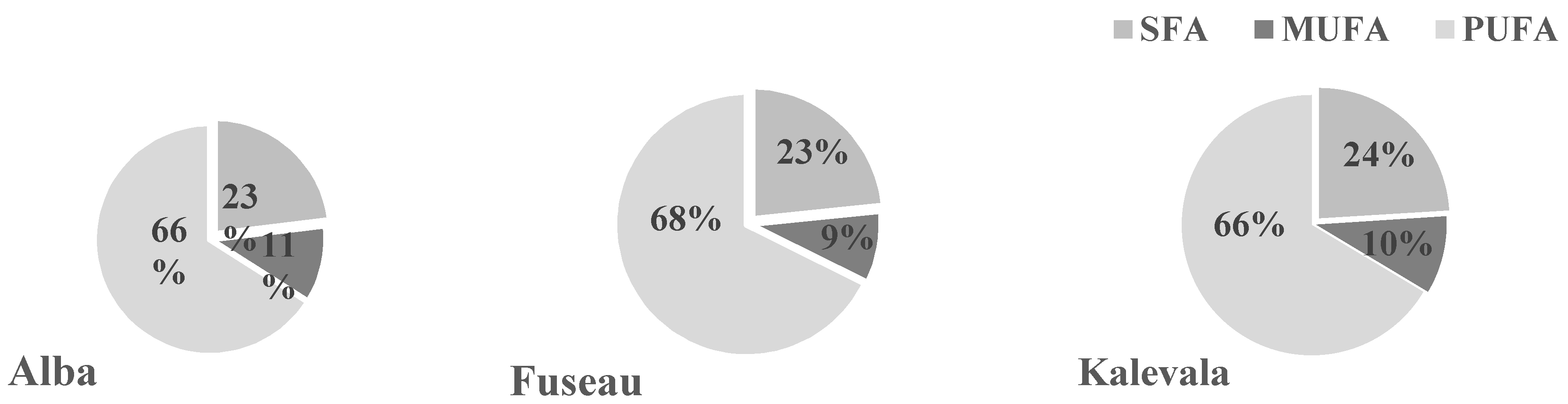
3.5. Qualitative Analysis of JAPC Fatty Acid Composition
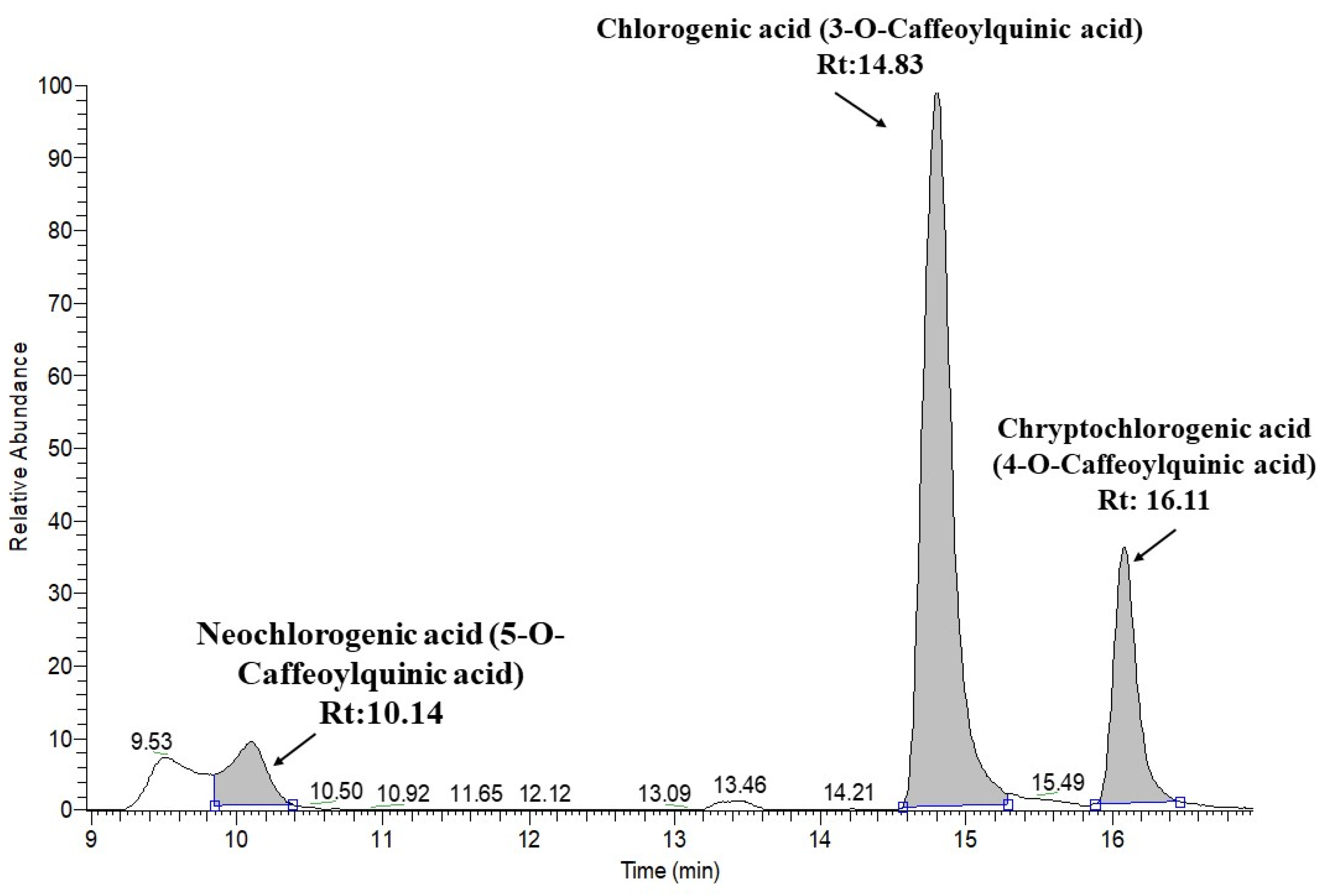
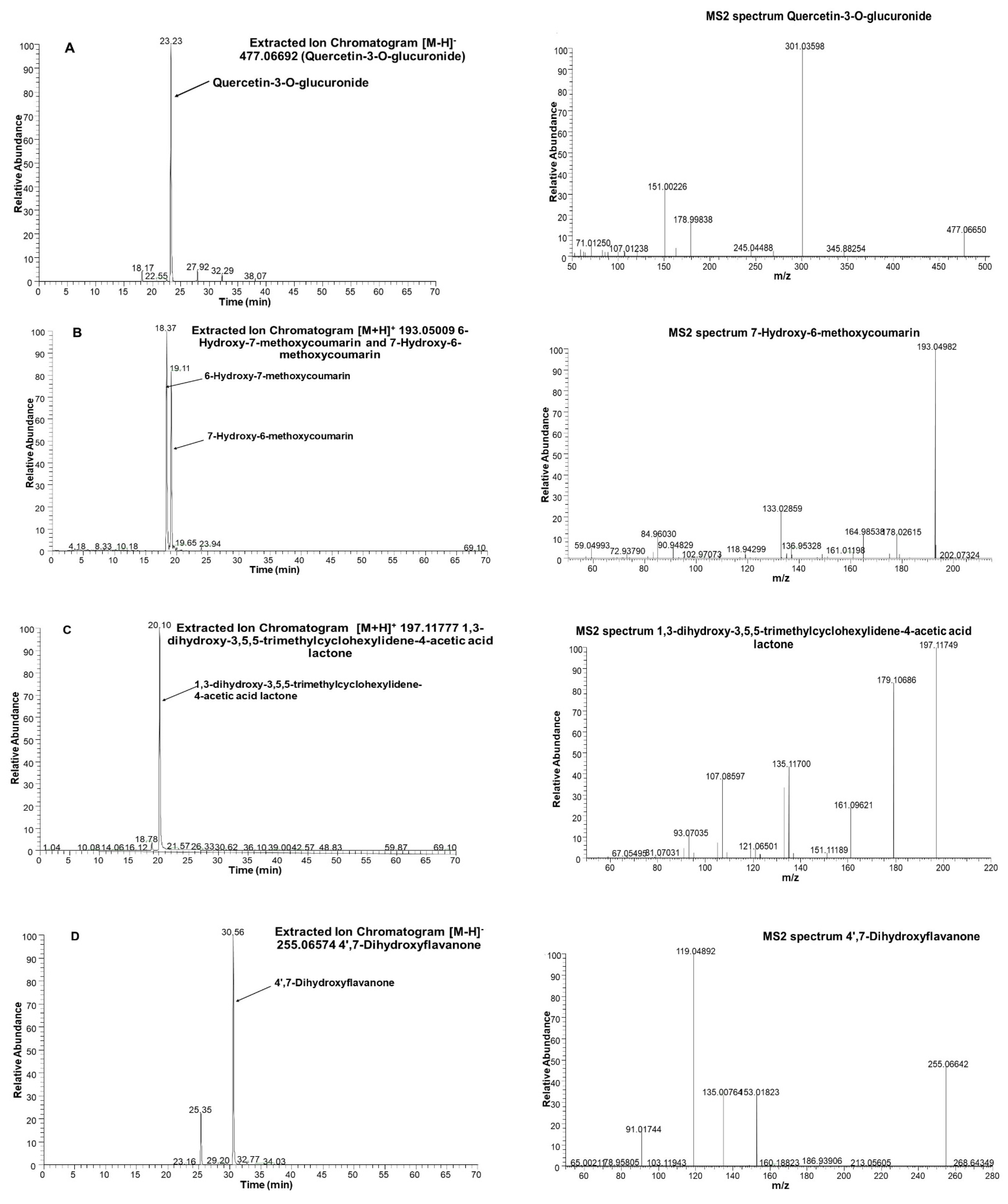
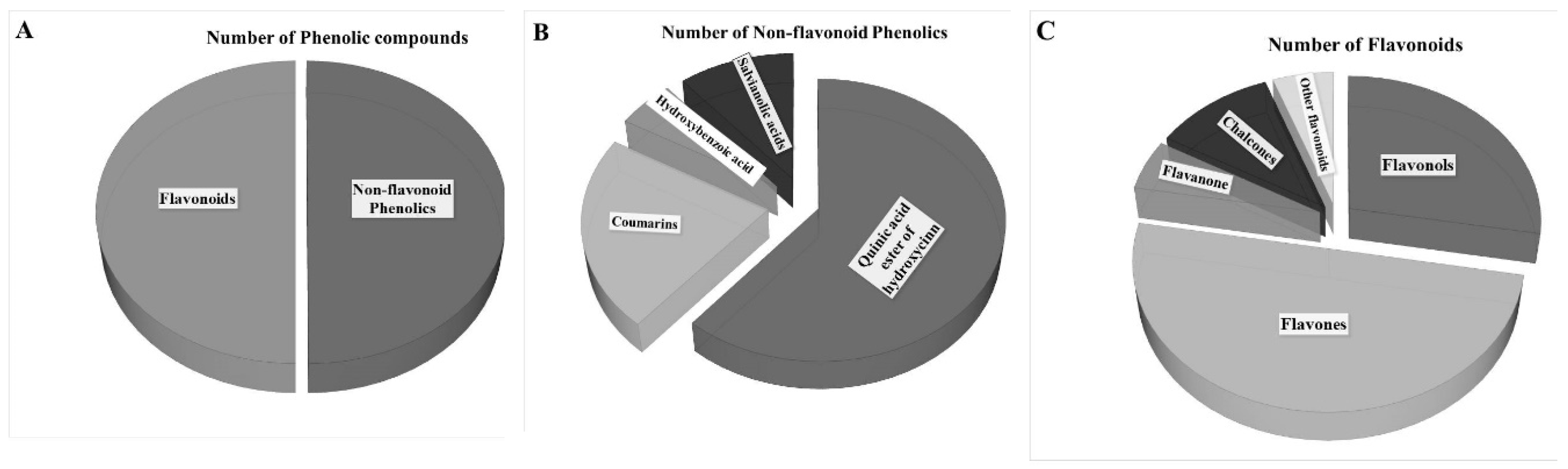
3.6. Screening JAPC Phytochemicals Using UHPLC-ESI-ORBITRAP-MS/MS
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- La Cour, R.; Schjoerring, J.K.; Jørgensen, H. Enhancing Protein Recovery in Green Biorefineries by Lignosulfonate-Assisted Precipitation. Front. Sustain. Food Syst. 2019, 3, 112. [Google Scholar] [CrossRef] [Green Version]
- Kamm, B.; Schönicke, P.; Hille, C.H. Green biorefinery–industrial implementation. Food Chem. 2016, 197, 1341–1345. [Google Scholar] [CrossRef] [PubMed]
- Grela, E.R.; Pietrzak, K. Production technology, chemical composition and use of alfalfa protein xanthophyll concentrate as dietary supplement. J. Food Process. Technol. 2014, 5, 10. [Google Scholar]
- European Food Safety Authority, EFSA. Scientific Opinion of the Panel on Dietetic Products Nutrition and Allergies on a request from the European Commission on the safety of ‘alfalfa protein concentrate’ as food. EFSA J. 2009, 997, 1–19. [Google Scholar]
- Tenorio, A.T.; Kyriakopoulou, K.E.; Suarez-Garcia, E.; van den Berg, C.; van der Goot, A.J. Understanding differences in protein fractionation from conventional crops, and herbaceous and aquatic biomass-consequences for industrial use. Trends Food Sci. Technol. 2018, 71, 235–245. [Google Scholar] [CrossRef]
- Gunnarsson, I.B.; Svensson, S.-E.; Johansson, E.; Karakashev, D.; Angelidaki, I. Potential of Jerusalem artichoke (Helianthus tuberosus L.) as a biorefinery crop. Ind. Crops Prod. 2014, 56, 231–240. [Google Scholar] [CrossRef]
- Fang, Y.R.; Liu, J.A.; Steinberger, Y.; Xie, G.H. Energy use efficiency and economic feasibility of Jerusalem artichoke production on arid and coastal saline lands. Ind. Crops Prod. 2018, 117, 131–139. [Google Scholar] [CrossRef]
- Kaszás, L.; Kovács, Z.; Nagy, E.; Elhawat, N.; Abdalla, N.; Domokos-Szabolcsy, E. Jerusalem artichoke (Helianthus tuberosus L.) as a potential chlorophyll source for humans and animals nutrition. Environ. Biodivers. Soil Secur. 2018, 2, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Razmkhah, M.; Rezaei, J.; Fazaeli, H. Use of Jerusalem artichoke tops silage to replace corn silage in sheep diet. Anim. Feed Sci. Technol. 2017, 228, 168–177. [Google Scholar] [CrossRef]
- Niu, L.; Manxia, C.; Xiumei, G.; Xiaohua, L.; Hongbo, S.; Zhaopu, L.; Zed, R. Carbon sequestration and Jerusalem artichoke biomass under nitrogen applications in coastal saline zone in the northern region of Jiangsu, China. Sci. Total Environ. 2016, 568, 885–890. [Google Scholar] [CrossRef]
- Kays, S.J.; Nottingham, S.F. Biology and Chemistry of Jerusalem Artichoke: Helianthus tuberosus L.; CRC Press, Taylor & Francis Group: New York, NY, USA, 2008. [Google Scholar]
- Long, X.H.; Shao, H.B.; Liu, L.; Liu, L.P.; Liu, Z.P. Jerusalem artichoke: A sustainable biomass feedstock for biorefinery. Renew. Sustain. Energy Rev. 2016, 54, 1382–1388. [Google Scholar] [CrossRef]
- Johansson, E.; Prade, T.; Angelidaki, I.; Svensson, S.E.; Newson, W.R.; Gunnarsson, I.B.; Hovmalm, H.P. Economically viable components from Jerusalem artichoke (Helianthus tuberosus L.) in a biorefinery concept. Int. J. Mol. Sci. 2015, 16, 8997–9016. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Long, X.; Liu, Z.; Shao, H.; Liu, L. Analysis of phenolic acids of Jerusalem artichoke (Helianthus tuberosus L.) responding to salt-stress by Liquid chromatography/tandem mass spectrometry. Sci. World J. 2014, 2014, 568043. [Google Scholar] [CrossRef] [Green Version]
- Yuan, X.; Gao, M.; Xiao, H.; Tan, C.; Du, Y. Free radical scavenging activities and bioactive substances of Jerusalem artichoke (Helianthus tuberosus L.) leaves. Food Chem. 2012, 133, 10–14. [Google Scholar] [CrossRef]
- Chae, S.W.; Lee, S.H.; Kang, S.S.; Lee, H.J. Flavone glucosides from the leaves of Helianthus tuberosus. Nat. Prod. Sci. 2002, 8, 141–143. [Google Scholar]
- Oleszek, M.; Kowalska, I.; Oleszek, W. Phytochemicals in bioenergy crops. Phytochem. Rev. 2019, 18, 893–927. [Google Scholar] [CrossRef] [Green Version]
- Pan, L.; Sinden, M.R.; Kennedy, A.H.; Chai, H.; Watson, L.E.; Graham, T.L.; Kinghorn, A.D. Bioactive constituents of Helianthus tuberosus (Jerusalem artichoke). Phytochem. Lett. 2009, 2, 15–18. [Google Scholar] [CrossRef]
- Showkat, M.M.; Falck-Ytter, A.B.; Strætkvern, K.O. Phenolic Acids in Jerusalem Artichoke (Helianthus tuberosus L.): Plant Organ Dependent Antioxidant Activity and Optimized Extraction from Leaves. Molecules 2019, 24, 3296. [Google Scholar] [CrossRef] [Green Version]
- Boisen, S.; Hvelplund, T.; Weisbjerg, M.R. Ideal amino acid profiles as a basis for feed protein evaluation. Livest. Prod. Sci. 2000, 64, 239–251. [Google Scholar] [CrossRef]
- Sparks, D.L.; Page, A.L.; Helmke, P.A.; Loppert, R.H. Methods of Soil Analysis: Chemical Methods, Part 3; ASA and SSSA: Madison, WI, USA, 1996. [Google Scholar]
- Duncan, D.B. Multiple range and multiple F-tests. Biometrics 1955, 11, 1–42. [Google Scholar] [CrossRef]
- Malmberg, A.; Theander, O. Differences in chemical composition of leaves and stem in Jerusalem artichoke and changes in low-molecular sugar and fructan content with time of harvest. Swed. J. Agric. Res. 1986, 16, 7–12. [Google Scholar]
- Lamsal, B.P.; Koegel, R.G.; Gunasekaran, S. Some physicochemical and functional properties of alfalfa soluble leaf proteins. LWT-Food Sci. Technol. 2007, 40, 1520–1526. [Google Scholar] [CrossRef]
- Rashchenko, I.N. Biochemical investigations of the aerial parts of Jerusalem artichoke. Tr. Kazakh Sel’skokhoz Inst 1959, 6, 40–52. [Google Scholar]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; Xia, F.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Rawate, P.D.; Hill, R.M. Extraction of a high-protein isolate from Jerusalem artichoke (Helianthus tuberosus) tops and evaluation of its nutrition potential. J. Agric. Food Chem. 1985, 33, 29–31. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Role of chlorogenic acids in controlling oxidative and inflammatory stress conditions. Nutrients 2016, 8, 16. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.T.; Chang, C.Q.; Liu, Y.; Chen, Z.M. Effect of chlorogenic acid on disordered glucose and lipid metabolism in db/db mice and its mechanism. Zhongguo Yi Xue Ke Xue Yuan Xue Bao Acta Acad. Med. Sin. 2011, 33, 281–286. [Google Scholar]
- Cabello-Hurtado, F.; Durst, F.; Jorrin, J.V.; Werck-Reichhard, D. Coumarins in Helianthus tuberosus: Characterization, induced accumulation and biosynthesis. Phytochemistry 1998, 49, 1029–1036. [Google Scholar] [CrossRef]
- Venugopala, K.N.; Rashmi, V.; Odhav, B. Review on natural coumarin lead compounds for their pharmacological activity. BioMed Res. Int. 2013, 963248. [Google Scholar] [CrossRef] [Green Version]
- Docampo, M.; Olubu, A.; Wang, X.; Pasinetti, G.; Dixon, R.A. Glucuronidated flavonoids in neurological protection: Structural analysis and approaches for chemical and biological synthesis. J. Agric. Food Chem. 2017, 65, 7607–7623. [Google Scholar] [CrossRef]
- Ho, L.; Ferruzzi, M.G.; Janle, E.M.; Wang, J.; Gong, B.; Chen, T.Y.; Lobo, J.; Cooper, B.; Wu, Q.L.; Talcott, S.T.; et al. Identification of brain-targeted bioactive dietary quercetin-3-O-glucuronide as a novel intervention for Alzheimer’s disease. FASEB J. 2013, 27, 769–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, C.S.; Wu, J.C.; Ho, C.T.; Pan, M.H. Disease chemopreventive effects and molecular mechanisms of hydroxylated polymethoxyflavones. BioFactors 2015, 41, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.Y.; Hu, D.N.; Lin, I.C.; Liu, F.S. Butein Shows Cytotoxic Effects and Induces Apoptosis in Human Ovarian Cancer Cells. Am. J. Chin. Med. 2015, 43, 769–782. [Google Scholar] [CrossRef]
- Mersereau, J.E.; Levy, N.; Staub, R.E.; Baggett, S.; Zogovic, T.; Chow, S.; Ricke, W.A.; Tagliaferri, M.; Cohen, I.; Bjeldanes, L.F.; et al. Liquiritigenin is a plant-derived highly selective estrogen receptor beta agonist. Mol. Cell. Endocrinol. 2008, 283, 49–57. [Google Scholar] [CrossRef] [Green Version]
- Seiler, G.J. Nitrogen and mineral content of selected wild and cultivated genotypes of Jerusalem artichoke. Agron. J. 1988, 80, 681–687. [Google Scholar] [CrossRef]
- Murata, M.; Nakai, Y.; Kawazu, K.; Ishizaka, M.; Kajiwara, H.; Abe, H.; Takeuchi, K.; Ichiro, M.; Mochizuki, A.; Seo, S. Loliolide, a carotenoid metabolite, is a potential endogenous inducer of herbivore resistance. Plant Physiol. 2019, 179, 1822–1833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, N.; Kang, J.W.; Lee, S.M. Protective effects of chlorogenic acid against ischemia/reperfusion injury in rat liver: Molecular evidence of its antioxidant and anti-inflammatory properties. J. Nutr. Biochem. 2012, 23, 1249–1255. [Google Scholar] [CrossRef]
- Islam, M.S.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Isolation and identification of two potential phytotoxic substances from the aquatic fern Marsilea crenata. J. Plant Biol. 2017, 60, 75–81. [Google Scholar] [CrossRef]
- Chernenko, T.V.; Glushenkova, A.I.; Rakhimov, D.A. Lipids of Helianthus tuberosus tubers. Chem. Nat. Compd. 2008, 44, 1–2. [Google Scholar] [CrossRef]
- Kaszás, L.; Alshaal, T.; Kovács, Z.; Koroknai, J.; Elhawat, N.; Nagy, É.; El-Ramady, H.; Fári1, M.; Domokos-Szabolcsy, É. Refining high-quality leaf protein and valuable co-products from green biomass of Jerusalem artichoke (Helianthus tuberosus L.) for sustainable protein supply. Biomass Convers. Biorefinery 2020. [Google Scholar] [CrossRef] [Green Version]
- Simopoulos, A.P. Evolutionary aspects of diet, the omega-6/omega-3 ratioand genetic variation: Nutritional implications for chronic diseases. Biomed. Pharmacother. 2006, 60, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Barrero-Sicilia, C.; Silvestre, S.; Haslam, R.P.; Michaelson, L.V. Lipid remodelling: Unraveling the response to cold stress in Arabidopsis and its extremophile relative Eutrema salsugineum. Plant Sci. 2017, 263, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Sadali, N.M.; Sowden, R.G.; Ling, Q.; Jarvis, R.P. Differentiation of chromoplasts and other plastids in plants. Plant Cell Rep. 2019, 38, 803–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Clones | Fresh Biomass Yield (kg m−2) | JAPC (g kg−1 Fresh Biomass) | Total Protein % | |||
|---|---|---|---|---|---|---|
| 1st Harvest | 2nd Harvest | 1st Harvest | 2nd Harvest | 1st Harvest | 2nd Harvest | |
| Alba | 5.0 ± 0.43 a | 1.8 ± 0.22 b | 31.9 ± 0.63 a | 30.4 ± 0.59 a | 35.3 ± 0.8 a | 31.6 ± 0.8 b |
| Fuseau | 5.2 ± 0.28 a | 2.6 ± 0.19 ab | 28.3 ± 0.04 a | 28.8 ± 0.25 a | 33.3 ± 0.9 a | 35.2 ± 0.8 a |
| Kalevala | 5.6 ± 0.65 a | 2.8 ± 0.57 a | 32.3 ± 0.53 a | 28.0 ± 0.13 a | 33.8 ± 0.7 a | 33.4 ± 0.7 ab |
| Amino Acid | 1st Harvest | 2nd Harvest | ||||
|---|---|---|---|---|---|---|
| Alba | Fuseau | Kalevala | Alba | Fuseau | Kalevala | |
| Lysine | 2.32 ± 0.02 ‡ a | 2.19 ± 0.02 c | 2.25 ± 0.02 b | 2.35 ± 0.03 c | 2.54 ± 0.01 a | 2.46 ± 0.02 b |
| Histidine | 0.80 ± 0.20 a | 0.71 ± 0.01 b | 0.83 ± 0.03 a | 0.72 ± 0.02 c | 0.76 ± 0.02 b | 0.82 ± 0.02 a |
| Isoleucine | 1.72 ± 0.03 a | 1.64 ± 0.02 b | 1.77 ± 0.02 a | 1.72 ± 0.02 bc | 1.86 ± 0.02 a | 1.78 ± 0.02 ab |
| Leucine | 3.25 ± 0.05 b | 3.08 ± 0.02 c | 3.31 ± 0.01 a | 3.19 ± 0.02 b | 2.46 ± 0.02 c | 3.30 ± 0.10 a |
| Phenylalanine | 2.12 ± 0.02 b | 1.96 ± 0.02 c | 2.19 ± 0.01 a | 2.03 ± 0.03 b | 2.20 ± 0.10 a | 2.18 ± 0.02 a |
| Methionine | 0.87 ± 0.03 a | 0.84 ± 0.02 a | 0.79 ± 0.03 b | 0.82 ± 0.02 b | 0.95 ± 0.01 a | 0.77 ± 0.02 c |
| Threonine | 1.96 ± 0.01 b | 1.87 ± 0.02 c | 2.33 ± 0.03 a | 1.95 ± 0.02 c | 2.12 ± 0.02 b | 2.33±0.03 a |
| Valine | 2.05 ± 0.05 a | 2.02 ± 0.02 a | 2.06 ± 0.02 a | 2.10 ± 0.02 b | 2.34 ± 0.01 a | 2.09 ± 0.01 b |
| Alanine | 2.36 ± 0.05 a | 2.20 ± 0.10 b | 2.35 ± 0.02 a | 2.32 ± 0.02 b | 2.47 ± 0.02 a | 2.34 ± 0.02 b |
| Arginine | 2.08 ± 0.04 a | 1.88 ± 0.02 b | 1.86 ± 0.01 b | 1.87 ± 0.02 c | 1.97 ± 0.02 b | 2.21 ± 0.01 a |
| Aspartic acid | 3.81 ± 0.01 b | 3.63 ± 0.03 c | 4.23 ± 0.03 a | 3.89 ± 0.02 b | 4.23 ± 0.03 a | 4.24 ± 0.04 a |
| Cysteine | 0.24 ± 0.02 a | 0.22 ± 0.02 a | 0.22 ± 0.02 a | 0.24 ± 0.02 ab | 0.26 ± 0.02 a | 0.23 ± 0.03 bc |
| Glycine | 2.04±0.04 b | 1.93 ± 0.03 c | 2.13 ± 0.01 a | 1.99 ± 0.01 b | 2.14 ± 0.01 a | 2.14 ± 0.02 a |
| Glutamic acid | 4.29 ± 0.01 bc | 4.14 ± 0.02 c | 4.82 ± 0.02 a | 4.38 ± 0.02 c | 4.74 ± 0.02 b | 4.79 ± 0.02 a |
| Proline | 1.92 ± 0.03 b | 1.82 ± 0.02 c | 2.20 ± 0.10 a | 2.04 ± 0.02 b | 2.18 ± 0.01 a | 2.19 ± 0.01 a |
| Serine | 1.74 ± 0.04 b | 1.67 ± 0.02 b | 1.90 ± 0.10 a | 1.77 ± 0.02 c | 1.89 ± 0.01 b | 1.93 ± 0.01 a |
| Tyrosine | 1.48 ± 0.02 a | 1.38 ± 0.02 c | 1.46 ± 0.02 ab | 1.42 ± 0.02 c | 1.61 ± 0.01 a | 1.55 ± 0.01 b |
| Ammonia | 0.49 ± 0.01 ab | 0.47 ± 0.02 b | 0.52 ± 0.02 a | 0.52 ± 0.02 a | 0.48 ± 0.02 b | 0.54 ± 0.02 a |
| No. | Compound | Formula | Retention Time | Measured Mass (m/z) | Fragments 1 | Fragments 2 | Fragments 3 | Fragments 4 | Fragments 5 | |
|---|---|---|---|---|---|---|---|---|---|---|
| [M + H]+ | [M − H]- | |||||||||
| 1 | γ-Aminobutyric acid | C4H9NO2 | 1.25 | 104.07116 | 87.0446 | 86.0607 | 69.0342 | 58.0658 | ||
| 2 | Quinic acid | C7H12O6 | 1.27 | 191.05557 | 173.0447 | 171.0289 | 127.0388 | 93.0331 | 85.0280 | |
| 3 | Betaine (Trimethylglycine) | C5H11NO2 | 1.28 | 118.08681 | 59.0737 | 58.0659 | ||||
| 4 | Malic acid | C4H6O5 | 1.33 | 133.01370 | 115.0024 | 89.0230 | 87.0075 | 72.9916 | 71.0123 | |
| 5 | Nicotinic acid (Niacin) | C6H5NO2 | 1.51 | 124.03986 | 96.0450 | 80.0501 | 78.0347 | |||
| 6 | Citric acid | C6H8O7 | 1.73 | 191.01918 | 173.0082 | 129.0182 | 111.0075 | 87.0073 | 85.0280 | |
| 7 | Neochlorogenic acid (5-O-Caffeoylquinic acid) | C16H18O9 | 10.14 | 353.08726 | 191.0557 | 179.0344 | 173.0448 | 135.0441 | ||
| 8 | Salicylic acid-2-O-glucoside | C13H16O8 | 13.56 | 299.07670 | 137.0234 | 113.0229 | 93.0331 | 85.0280 | 71.0123 | |
| 9 | Chlorogenic acid (3-O-Caffeoylquinic acid) | C16H18O9 | 14.83 | 353.08726 | 191.0556 | 179.0344 | 173.0443 | 161.0234 | 135.0441 | |
| 10 | Cryptochlorogenic acid (4-O-Caffeoylquinic acid) | C16H18O9 | 16.11 | 353.08726 | 191.0555 | 179.0344 | 173.0447 | 161.0232 | 135.0441 | |
| 11 | 4-O-(4-Coumaroyl) quinic acid | C16H18O8 | 16.14 | 337.09235 | 191.0555 | 173.0447 | 163.0390 | 119.0489 | 93.0331 | |
| 12 | Vanillin (4-Hydroxy-3-methoxybenzaldehyde) | C8H8O3 | 16.22 | 153.05517 | 125.0600 | 111.0445 | 110.0366 | 93.0341 | 65.0393 | |
| 13 | 5-O-(4-Coumaroyl)quinic acid | C16H18O8 | 17.38 | 337.09235 | 191.0556 | 173.0447 | 163.0391 | 119.0490 | 93.0332 | |
| 14 | Indole-3-acetic acid | C10H9NO2 | 17.98 | 174.05551 | 146.0601 | 144.0440 | 130.0651 | 128.0492 | ||
| 15 | 4-O-(4-Coumaroyl)quinic acid cis isomer | C16H18O8 | 18.04 | 337.09235 | 191.0556 | 173.0447 | 163.0391 | 119.0489 | 93.0331 | |
| 16 | Isoscopoletin (6-Hydroxy-7-methoxycoumarin) | C10H8O4 | 18.33 | 193.05009 | 178.0264 | 165.0550 | 149.0598 | 137.0600 | 133.0287 | |
| 17 | 5-O-Feruloylquinic acid | C17H20O9 | 18.42 | 367.10291 | 193.0503 | 191.0556 | 173.0447 | 134.0362 | 93.0331 | |
| 18 | Riboflavin | C17H20N4O6 | 19.03 | 377.14611 | 359.1352 | 243.0879 | 200.0824 | 172.0872 | 69.0342 | |
| 19 | Scopoletin (7-Hydroxy-6-methoxycoumarin) | C10H8O4 | 19.08 | 193.05009 | 178.0263 | 165.0546 | 149.0597 | 137.0601 | 133.0287 | |
| 20 | Azelaamic acid (9-Amino-9-oxononanoic acid) | C9H17NO3 | 19.21 | 186.11302 | 125.0959 | 97.0647 | ||||
| 21 | 6-Methylcoumarin | C10H8O2 | 19.44 | 161.06026 | 133.0651 | 115.0547 | 105.0704 | 91.0547 | 79.0549 | |
| 22 | 5-O-(4-Coumaroyl)quinic acid cis isomer | C16H18O8 | 19.63 | 337.09235 | 191.0555 | 173.0446 | 163.0390 | 119.0491 | 93.0330 | |
| 23 | Indole-4-carbaldehyde | C9H7NO | 19.67 | 146.06059 | 118.0655 | 117.0574 | 91.0548 | |||
| 24 | Fraxidin or Isofraxidin | C11H10O5 | 19.72 | 221.04500 | 206.0219 | 190.9983 | 163.0030 | |||
| 25 | Loliolide | C11H16O3 | 20.05 | 197.11777 | 179.1069 | 161.0962 | 135.1171 | 133.1015 | 107.0860 | |
| 26 | 4-Hydroxy-3-methoxycinnamaldehyde (Coniferyl aldehyde) | C10H10O3 | 20.59 | 179.07082 | 161.0599 | 147.0442 | 133.0652 | 119.0495 | 55.0186 | |
| 27 | 7-Deoxyloganic acid isomer | C16H24O9 | 22.36 | 359.13421 | 197.0815 | 153.0909 | 135.0805 | 109.0643 | 89.0230 | |
| 28 | Di-O-caffeoylquinic acid isomer 1 | C25H24O12 | 22.61 | 515.11896 | 353.0884 | 191.0556 | 179.0342 | 173.0447 | 135.0441 | |
| 29 | Di-O-caffeoylquinic acid isomer 2 | C25H24O12 | 22.77 | 515.11896 | 353.0884 | 191.0556 | 179.0342 | 173.0446 | 135.0440 | |
| 30 | Salvianolic acid derivative isomer 1 | C27H22O12 | 22.80 | 537.10331 | 375.0705 | 201.0165 | 179.0343 | 161.0234 | 135.0440 | |
| 31 | Butein (2′,3,4,4′-Tetrahydroxychalcone) | C15H12O5 | 23.00 | 273.07630 | 255.0656 | 227.0699 | 209.0602 | 163.0391 | 137.0235 | |
| 32 | Quercetin-3-O-glucuronide | C21H18O13 | 23.26 | 477.06692 | 301.0359 | 178.9980 | 163.0028 | 151.0026 | 121.0281 | |
| 33 | Isoquercitrin (Hirsutrin, Quercetin-3-O-glucoside) | C21H20O12 | 23.47 | 463.08765 | 301.0358 | 300.0283 | 271.0253 | 255.0300 | ||
| 34 | Chrysoeriol-O-glucoside | C22H22O11 | 23.87 | 461.10839 | 299.0560 | 298.0484 | 270.0537 | 255.0292 | 227.0346 | |
| 35 | Salvianolic acid derivative isomer 2 | C27H22O12 | 24.60 | 537.10331 | 375.0705 | 201.0166 | 179.0343 | 161.0236 | 135.0440 | |
| 36 | Di-O-caffeoylquinic acid isomer 3 | C25H24O12 | 24.62 | 515.11896 | 353.0884 | 191.0557 | 179.0342 | 173.0447 | 135.0440 | |
| 37 | Azelaic acid | C9H16O4 | 25.05 | 187.09704 | 169.0863 | 143.1070 | 125.0959 | 123.0803 | ||
| 38 | Kaempferol-3-O-glucuronide | C21H18O12 | 25.18 | 461.07200 | 285.0410 | 229.0505 | 113.0231 | |||
| 39 | Apigenin-O-malonylglucoside | C24H22O13 | 25.21 | 517.09822 | 473.1116 | 269.0461 | 268.0376 | |||
| 40 | Astragalin (Kaempferol-3-O-glucoside) | C21H20O11 | 25.26 | 447.09274 | 285.0410 | 284.0331 | 255.0302 | 227.0350 | ||
| 41 | Isorhamnetin-3-O-glucoside | C22H22O12 | 25.48 | 477.10330 | 315.0524 | 314.0437 | 285.0406 | 271.0248 | 243.0292 | |
| 42 | Kukulkanin B (2′,4′,4-Trihydroxy-3′-methoxyxchalcone) | C16H14O5 | 25.50 | 287.09195 | 269.0810 | 241.0864 | 177.0548 | 145.0286 | 137.0235 | |
| 43 | Isorhamnetin-3-O-glucuronide | C22H20O13 | 25.70 | 491.08257 | 315.0517 | 300.0275 | 271.0249 | |||
| 44 | Dihydroactinidiolide | C11H16O2 | 27.16 | 181.12286 | 163.1119 | 145.1014 | 135.1171 | 121.1015 | 107.0860 | |
| 45 | Dimethoxy-tetrahydroxyflavone | C17H14O8 | 28.38 | 345.06105 | 330.0386 | 315.0153 | 287.0204 | 215.0347 | 178.9978 | |
| 46 | Dihydroxy-methoxyflavone | C16H12O5 | 29.89 | 283.06065 | 268.0381 | 267.0305 | 240.0427 | 239.0350 | 211.0396 | |
| 47 | Dimethoxy-trihydroxyflavone isomer 1 | C17H14O7 | 30.09 | 329.06613 | 314.0439 | 299.0197 | 283.0869 | 271.0247 | 255.0913 | |
| 48 | Trihydroxy-trimethoxyflavone | C18H16O8 | 30.36 | 359.07670 | 344.0541 | 329.0307 | 314.0075 | 301.0358 | 286.0129 | |
| 49 | Dimethoxy-trihydroxyflavone isomer 2 | C17H14O7 | 30.38 | 329.06613 | 314.0439 | 299.0201 | 283.0871 | 271.0252 | 253.0763 | |
| 50 | Liquiritigenin (4′,7-Dihydroxyflavanone) | C15H12O4 | 30.56 | 255.06574 | 153.0183 | 135.0077 | 119.0489 | 91.0175 | ||
| 51 | Hymenoxin (5,7,Dihydroxy-3′,4′,6,8-tetramethoxyflavone) | C19H18O8 | 32.11 | 375.10800 | 360.0840 | 345.0606 | 342.0736 | 330.0367 | 317.0659 | |
| 52 | Epiafzelechin trimethyl ether | C18H20O5 | 33.32 | 317.13890 | 167.0704 | 163.0755 | 155.0705 | 137.0598 | 121.0651 | |
| 53 | Nevadensin (5,7-Dihydroxy-4′,6,8-trimethoxyflavone) | C18H16O7 | 33.91 | 345.09743 | 330.0736 | 315.0501 | 312.0631 | 287.0554 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaszás, L.; Alshaal, T.; El-Ramady, H.; Kovács, Z.; Koroknai, J.; Elhawat, N.; Nagy, É.; Cziáky, Z.; Fári, M.; Domokos-Szabolcsy, É. Identification of Bioactive Phytochemicals in Leaf Protein Concentrate of Jerusalem Artichoke (Helianthus tuberosus L.). Plants 2020, 9, 889. https://doi.org/10.3390/plants9070889
Kaszás L, Alshaal T, El-Ramady H, Kovács Z, Koroknai J, Elhawat N, Nagy É, Cziáky Z, Fári M, Domokos-Szabolcsy É. Identification of Bioactive Phytochemicals in Leaf Protein Concentrate of Jerusalem Artichoke (Helianthus tuberosus L.). Plants. 2020; 9(7):889. https://doi.org/10.3390/plants9070889
Chicago/Turabian StyleKaszás, László, Tarek Alshaal, Hassan El-Ramady, Zoltán Kovács, Judit Koroknai, Nevien Elhawat, Éva Nagy, Zoltán Cziáky, Miklós Fári, and Éva Domokos-Szabolcsy. 2020. "Identification of Bioactive Phytochemicals in Leaf Protein Concentrate of Jerusalem Artichoke (Helianthus tuberosus L.)" Plants 9, no. 7: 889. https://doi.org/10.3390/plants9070889
APA StyleKaszás, L., Alshaal, T., El-Ramady, H., Kovács, Z., Koroknai, J., Elhawat, N., Nagy, É., Cziáky, Z., Fári, M., & Domokos-Szabolcsy, É. (2020). Identification of Bioactive Phytochemicals in Leaf Protein Concentrate of Jerusalem Artichoke (Helianthus tuberosus L.). Plants, 9(7), 889. https://doi.org/10.3390/plants9070889