Antiarthritic Effects of a Root Extract from Harpagophytum procumbens DC: Novel Insights into the Molecular Mechanisms and Possible Bioactive Phytochemicals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Harpagophytum Procumbens Extract
2.2. Phytochemical Analysis
2.2.1. Determination of Total Polyphenols, Tannins, and Flavonoids
2.2.2. Solid-Phase Microextraction (SPME)
2.2.3. Gas Chromatography–Mass Spectrometry (GC–MS)
2.3. Human Tissue
2.4. Immunohistochemistry
2.5. Human Primary Cell Isolation
2.6. Immunofluorescence
2.7. Densitometric Analysis
2.8. Cell Treatment
2.9. Cell Viability
2.10. RNA Extraction and Reverse Transcription
2.11. Quantitative Real-Time PCR
2.12. FAAH Inhibition
2.13. Statistical Analysis
3. Results
3.1. Phytochemical Characterization
3.2. Expression of Cannabinoid Receptors in OA Synovial Membrane
3.3. Effects of Harpagophytum Extracts on Synoviocyte Cell Viability
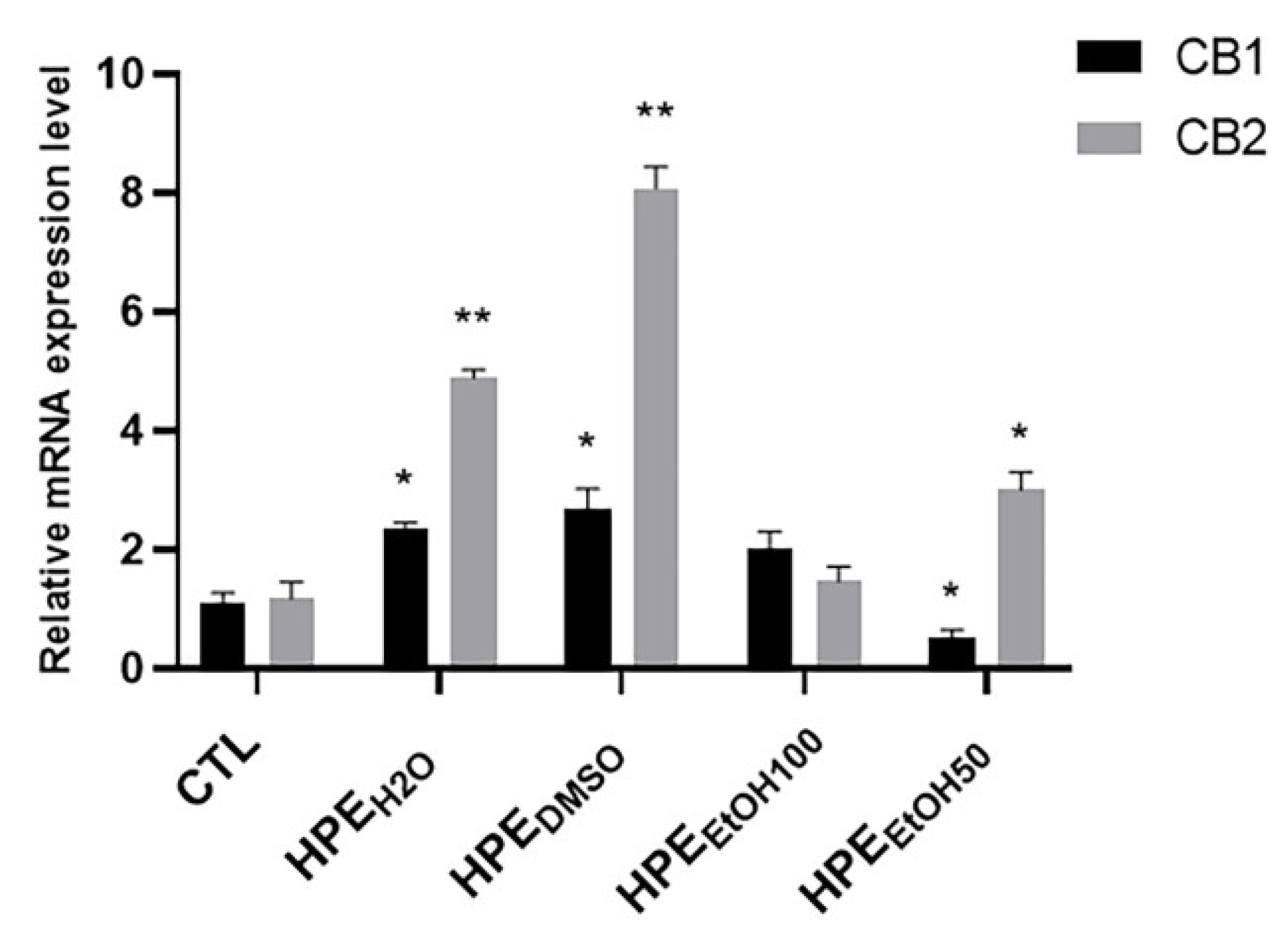
3.4. Mechanism of Action of Harpagophytum Extracts on FLSs
3.5. Inhibition of Fatty Acid Anandamide Hydrolase
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Robinson, W.H.; Lepus, C.M.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Goldring, S.R.; Goldring, M.B. Changes in the osteochondral unit during osteoarthritis: Structure, function and cartilage bone crosstalk. Nat. Rev. Rheumatol. 2016, 12, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Turcotte, C.; Blanchet, M.R.; Laviolette, M.; Flamand, N. The CB2 receptor and its role as a regulator of inflammation. Cell. Mol. Life Sci. 2016, 73, 4449–4470. [Google Scholar] [CrossRef] [Green Version]
- Sophocleous, A.; Börjesson, A.E.; Salter, D.M.; Ralston, S.H. The type 2 cannabinoid receptor regulates susceptibility to osteoarthritis in mice. Osteoarthr. Cartil. 2015, 23, 1586–1594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiou, L.C.; Hu, S.S.J.; Ho, Y.C. Targeting the cannabinoid system for pain relief? Acta Anaesthesiol. Taiwanica 2013, 51, 161–170. [Google Scholar] [CrossRef]
- Zoratti, C.; Kipmen-Korgun, D.; Osibow, K.; Malli, R.; Graier, W.F. Anandamide initiates Ca2+ signaling via CB2 receptor linked to phospholipase C in calf pulmonary endothelial cells. Br. J. Pharmacol. 2003, 140, 1351–1362. [Google Scholar] [CrossRef] [Green Version]
- Ferrara, A.L.; Piscitelli, F.; Petraroli, A.; Parente, R.; Galdiero, M.R.; Varricchi, G.; Marone, G.; Triggiani, M.; Di Marzo, V.; Loffredo, S. Altered metabolism of phospholipases, diacylglycerols, endocannabinoids, and N-Acylethanolamines in patients with mastocytosis. J. Immunol. Res. 2019. [Google Scholar] [CrossRef] [Green Version]
- Nagao, M.; Tanabe, N.; Manaka, S.; Naito, M.; Sekino, J.; Takayama, T.; Kawato, T.; Torigoe, G.; Kato, S.; Tsukune, N.; et al. LIPUS suppressed LPS-induced IL-1α through the inhibition of NF-κB nuclear translocation via AT1-PLCβ pathway in MC3T3-E1 cells. J. Cell. Physiol. 2017, 232, 3337–3346. [Google Scholar] [CrossRef]
- Dunn, S.L.; Wilkinson, J.M.; Crawford, A.; Bunning, R.A.D.; Le Maitre, C.L. Expression of Cannabinoid Receptors in Human Osteoarthritic Cartilage: Implications for Future Therapies. Cannabis Cannabinoid Res. 2016, 1, 3–15. [Google Scholar] [CrossRef] [Green Version]
- Richardson, D.; Pearson, R.G.; Kurian, N.; Latif, M.L.; Garle, M.J.; Barrett, D.A.; Kendall, D.A.; Scammell, B.E.; Reeve, A.J.; Chapman, V. Characterisation of the cannabinoid receptor system in synovial tissue and fluid in patients with osteoarthritis and rheumatoid arthritis. Arthritis Res. Ther. 2008. [Google Scholar] [CrossRef] [Green Version]
- McAlindon, T.E.; Bannuru, R.R. Latest advances in the management of knee OA. Nat. Rev. Rheumatol. 2018, 14, 73–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scotto d’Abusco, A.; Politi, L.; Giordano, C.; Scandurra, R. A peptidyl-glucosamine derivative affects IKKα kinase activity in human chondrocytes. Arthritis Res. Ther. 2010, 12. [Google Scholar] [CrossRef] [Green Version]
- D’Adamo, S.; Cetrullo, S.; Panichi, V.; Mariani, E.; Flamigni, F.; Borzì, R.M. Nutraceutical Activity in Osteoarthritis Biology: A Focus on the Nutrigenomic Role. Cells 2020, 9, 1232. [Google Scholar] [CrossRef] [PubMed]
- Stoppoloni, D.; Politi, L.; Leopizzi, M.; Gaetani, S.; Guazzo, R.; Basciani, S.; Moreschini, O.; De Santi, M.; Scandurra, R.; Scotto d’Abusco, A. Effect of glucosamine and its peptidyl-derivative on the production of extracellular matrix components by human primary chondrocytes. Osteoarthr. Cartil. 2015, 23, 103–113. [Google Scholar] [CrossRef] [Green Version]
- Committee on Herbal Medicinal Products (HMPC). Community Herbal Monograph on Harpagophytum Procumbens Dc. and/or Harpagophytum Zeyheri Decne, Radix; European Medicines Agency: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Dragos, D.; Gilca, M.; Gaman, L.; Vlad, A.; Iosif, L.; Stoian, I.; Lupescu, O. Phytomedicine in joint disorders. Nutrients 2017, 9, 70. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.W.; Kim, J.G.; Han, D.; Kim, Y.T. Analgesic effect of harpagophytum procumbens on postoperative and neuropathic pain in rats. Molecules 2014, 19, 1060–1068. [Google Scholar] [CrossRef]
- Gagnier, J.J.; Chrubasik, S.; Manheimer, E. Harpgophytum procumbens for osteoarthritis and low back pain: A systematic review. BMC Complement. Altern. Med. 2004, 4, 13. [Google Scholar] [CrossRef] [Green Version]
- Fiebich, B.L.; Fiebich, B.L.; Heinrich, M.; Hiller, K.O.; Kammerer, N. Inhibition of TNF-α synthesis in LPS-stimulated primary human monocytes by Harpagophytum extract SteiHap 69. Phytomedicine 2001, 8, 28–30. [Google Scholar] [CrossRef]
- Akhtar, N.; Haqqi, T.M. Current nutraceuticals in the management of osteoarthritis: A review. Ther. Adv. Musculoskelet. Dis. 2012, 4, 181–207. [Google Scholar] [CrossRef]
- Wegener, T.; Lüpke, N.P. Treatment of Patients with Arthrosis of Hip or Knee with an Aqueous Extract of Devil’s Claw (Harpagophytum procumbens DC.). Phyther. Res. 2003, 17, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Chantre, P.; Cappelaere, A.; Leblan, D.; Guedon, D.; Vandermander, J.; Fournie, B. Efficacy and tolerance of Harpagophytum procumbens versus diacerhein in treatment of osteoarthritis. Phytomedicine 2000, 7, 177–183. [Google Scholar] [CrossRef]
- Haseeb, A.; Ansari, M.Y.; Haqqi, T.M. Harpagoside suppresses IL-6 expression in primary human osteoarthritis chondrocytes. J. Orthop. Res. 2017, 35, 311–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Ascola, A.; Irrera, N.; Ettari, R.; Bitto, A.; Pallio, G.; Mannino, F.; Atteritano, M.; Campo, G.M.; Minutoli, L.; Arcoraci, V.; et al. Exploiting curcumin synergy with natural products using quantitative analysis of dose-effect relationships in an experimental in vitro model of osteoarthritis. Front. Pharmacol. 2019, 10, 1347. [Google Scholar] [CrossRef] [Green Version]
- Irrera, N.; D’ascola, A.; Pallio, G.; Bitto, A.; Mazzon, E.; Mannino, F.; Squadrito, V.; Arcoraci, V.; Minutoli, L.; Campo, G.M.; et al. β-Caryophyllene Mitigates Collagen Antibody Induced Arthritis (CAIA) in Mice Through a Cross-Talk between CB2 and PPAR-γ Receptors. Biomolecules 2019, 9, 326. [Google Scholar] [CrossRef] [Green Version]
- Ferland, C.E.; Beaudry, F.; Vachon, P. Antinociceptive effects of eugenol evaluated in a monoiodoacetate-induced osteoarthritis rat model. Phyther. Res. 2012, 26, 1278–1285. [Google Scholar] [CrossRef]
- Di Sotto, A.; Di Giacomo, S.; Amatore, D.; Locatelli, M.; Vitalone, A.; Toniolo, C.; Rotino, G.L.; Lo Scalzo, R.; Palamara, A.T.; Marcocci, M.E.; et al. A polyphenol rich extract from solanum melongena L. DR2 peel exhibits antioxidant properties and anti-herpes simplex virus type 1 activity In Vitro. Molecules 2018, 23, 2066. [Google Scholar] [CrossRef] [Green Version]
- Krenn, V.; Morawietz, L.; Häupl, T.; Neidel, J.; Petersen, I.; König, A. Grading of chronic synovitis—A histopathological grading system for molecular and diagnostic pathology. Pathol. Res. Pract. 2002, 198, 317–325. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Li, F.; Tang, Y.; Song, B.; Yu, M.; Li, Q.; Zhang, C.; Hou, J.; Yang, R. Nomenclature clarification: Synovial fibroblasts and synovial mesenchymal stem cells. Stem Cell Res. Ther. 2019, 10, 260. [Google Scholar] [CrossRef] [Green Version]
- Scanzello, C.R. Role of low-grade inflammation in osteoarthritis. Curr. Opin. Rheumatol. 2017, 29, 79–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Honvo, G.; Reginster, J.Y.; Rabenda, V.; Geerinck, A.; Mkinsi, O.; Charles, A.; Rizzoli, R.; Cooper, C.; Avouac, B.; Bruyère, O. Safety of Symptomatic Slow-Acting Drugs for Osteoarthritis: Outcomes of a Systematic Review and Meta-Analysis. Drugs Aging 2019, 36, 65–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mncwangi, N.; Chen, W.; Vermaak, I.; Viljoen, A.M.; Gericke, N. Devil’s Claw–A review of the ethnobotany, phytochemistry and biological activity of Harpagophytum procumbens. J. Ethnopharmacol. 2012, 143, 755–771. [Google Scholar] [CrossRef]
- Zhang, L.; Feng, L.; Jia, Q.; Xu, J.; Wang, R.; Wang, Z.; Wu, Y.; Li, Y. Effects of β-glucosidase hydrolyzed products of harpagide and harpagoside on cyclooxygenase-2 (COX-2) in vitro. Bioorg. Med. Chem. 2011, 19, 4882–4886. [Google Scholar] [CrossRef] [PubMed]
- Pichersky, E.; Gershenzon, J. The formation and function of plant volatiles: Perfumes for pollinator attraction and defense. Curr. Opin. Plant Biol. 2002, 5, 237–243. [Google Scholar] [CrossRef]
- Wenke, K.; Kai, M.; Piechulla, B. Belowground volatiles facilitate interactions between plant roots and soil organisms. Planta 2010, 231, 499–506. [Google Scholar] [CrossRef]
- Gfeller, V.; Huber, M.; Förster, C.; Huang, W.; Köllner, T.G.; Erb, M. Root volatiles in plant–plant interactions I: High root sesquiterpene release is associated with increased germination and growth of plant neighbours. Plant Cell Environ. 2019, 42, 1950–1963. [Google Scholar] [CrossRef] [Green Version]
- Kriukova, A.; Vladimirova, I. The GC-MS determination of chemical constituents from harpagophytum procumbens dc roots. Technol. Transf. Innov. Solut. Med. 2017, 52–54. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, E.S.; Passos, G.F.; Medeiros, R.; da Cunha, F.M.; Ferreira, J.; Campos, M.M.; Pianowski, L.F.; Calixto, J.B. Anti-inflammatory effects of compounds alpha-humulene and (-)-trans-caryophyllene isolated from the essential oil of Cordia verbenacea. Eur. J. Pharmacol. 2007, 569, 228–236. [Google Scholar] [CrossRef]
- Barboza, J.N.; da Silva Maia Bezerra Filho, C.; Silva, R.O.; Medeiros, J.V.R.; de Sousa, D.P. An overview on the anti-inflammatory potential and antioxidant profile of eugenol. Oxid. Med. Cell. Longev. 2018, 2018, 3957262. [Google Scholar] [CrossRef]
- Gertsch, J.; Leonti, M.; Raduner, S.; Racz, I.; Chen, J.Z.; Xie, X.Q.; Altmann, K.H.; Karsak, M.; Zimmer, A. Beta-caryophyllene is a dietary cannabinoid. Proc. Natl. Acad. Sci. USA 2008, 105, 9099–9104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chicca, A.; Caprioglio, D.; Minassi, A.; Petrucci, V.; Appendino, G.; Taglialatela-Scafati, O.; Gertsch, J. Functionalization of β-caryophyllene generates novel polypharmacology in the endocannabinoid system. ACS Chem. Biol. 2014, 107, 1499–1507. [Google Scholar] [CrossRef] [PubMed]
- Ames-Sibin, A.P.; Barizão, C.L.; Castro-Ghizoni, C.V.; Silva, F.M.S.; Sá-Nakanishi, A.B.; Bracht, L.; Bersani-Amado, C.A.; Marçal-Natali, M.R.; Bracht, A.; Comar, J.F. β-Caryophyllene, the major constituent of copaiba oil, reduces systemic inflammation and oxidative stress in arthritic rats. J. Cell. Biochem. 2018, 119, 10262–10277. [Google Scholar] [CrossRef] [PubMed]
- El-Sheikh, S.M.A.; Abd El-Alim, A.E.A.F.; Galal, A.A.A.; El-Sayed, R.G.; El-naseery, N.I. Anti-arthritic effect of β-caryophyllene and its ameliorative role on methotrexate and/or leflunomide-induced side effects in arthritic rats. Life Sci. 2019, 233, 116750. [Google Scholar] [CrossRef]
- Scanzello, C.R.; Goldring, S.R. The role of synovitis in osteoarthritis pathogenesis. Bone 2012, 51, 249–250. [Google Scholar] [CrossRef] [Green Version]
- Fukuda, S.; Kohsaka, H.; Takayasu, A.; Yokoyama, W.; Miyabe, C.; Miyabe, Y.; Harigai, M.; Miyasaka, N.; Nanki, T. Cannabinoid receptor 2 as a potential therapeutic target in rheumatoid arthritis. BMC Musculoskelet. Disord. 2014, 15, 275. [Google Scholar] [CrossRef] [Green Version]
- Marcu, K.B.; Otero, M.; Olivotto, E.; Maria Borzi, R.; Goldring, M.B. NF-κB Signaling: Multiple Angles to Target OA. Curr. Drug Targets 2010, 11, 599–613. [Google Scholar] [CrossRef]
- Scotto d’Abusco, A.; Corsi, A.; Grillo, M.G.; Cicione, C.; Calamia, V.; Panzini, G.; Sansone, A.; Giordano, C.; Politi, L.; Scandurra, R. Effects of intra-articular administration of glucosamine and a peptidyl-glucosamine derivative in a rabbit model of experimental osteoarthritis: A pilot study. Rheumatol. Int. 2008, 28, 437–443. [Google Scholar] [CrossRef]
- Veronesi, F.; Giavaresi, G.; Maglio, M.; Scotto d’Abusco, A.; Politi, L.; Scandurra, R.; Olivotto, E.; Grigolo, B.; Borzì, R.M.; Fini, M. Chondroprotective activity of N-acetyl phenylalanine glucosamine derivative on knee joint structure and inflammation in a murine model of osteoarthritis. Osteoarthr. Cartil. 2017, 25, 589–599. [Google Scholar] [CrossRef] [Green Version]
- Suh, P.G.; Park, J.I.; Manzoli, L.; Cocco, L.; Peak, J.C.; Katan, M.; Fukami, K.; Kataoka, T.; Yun, S.; Sung, H.R. Multiple roles of phosphoinositide-specific phospholipase C isozymes. J. Biochem. Mol. Biol. 2008, 41, 415–434. [Google Scholar] [CrossRef]
- Zini, N.; Lisignoli, G.; Solimando, L.; Bavelloni, A.; Grassi, F.; Guidotti, L.; Trimarchi, C.; Facchini, A.; Maraldi, N.M. IL1-β and TNF-α induce changes in the nuclear polyphosphoinositide signalling system in osteoblasts similar to that occurring in patients with rheumatoid arthritis: An immunochemical and immunocytochemical study. Histochem. Cell Biol. 2003, 120, 243–250. [Google Scholar] [CrossRef] [PubMed]




| Gene | Primer Sequences |
|---|---|
| CB1 NM_016083 | Forward: 5′-TTCCTTCTTGTGAAGGCACTG-3′ Reverse: 5′-TCTTGACCGTGCTCTTGATGC-3′ |
| CB2 NM_001841 | Forward: 5′-ATGCTGTGCCTCATCAACTC-3′ Reverse: 5′-CTCACACACTTCTTCCAGTG-3′ |
| FAAH NM_001441 | Forward: 5′-CAGCTTTCCTCAGCAACATG-3′ Reverse: 5′-CAATCACGGTTTTGCGGTAC-3′ |
| 18S NM_003286 | Forward: 5′-CGCCGCTAGAGGTGAAATTC-3′ Reverse: 5′-CATTCTTGGCAAATGCTTTCG-3′ |
| Compounds | HPEDMSO | HPEEtOH100 | HPEEtOH50 | HPEH2O |
|---|---|---|---|---|
| µg/mg of Dry Extract (Mean ± SE) | ||||
| Polyphenols (TAE) | 99.6 ± 0.05 ***§ | 69.6 ± 0.05 | 97.9 ± 0.01 ***§ | 84.2 ± 0.04 ** |
| Tannins (TAE) | 14.2 ± 0.02 ** | 9.2 ± 0.04 | 9.2 ± 0.02 | 17.9 ± 0.03 *** |
| Flavonoids (QE) | 113.0± 5.1 ***§§ | 71.3 ± 6.6 §§ | 75.1 ± 6.1 §§ | 37.6 ± 4.3 |
| Flavonols (QE) | 43.8 ± 2.4 ***§§ | 27.6 ± 3.2 | 30.1 ± 2.0 § | 25.1 ± 2.0 |
| No. 1 | Compound 2 | LRI 3 | LRI 4 | MS 5 | HPEDMSO (%) | HPEEtOH100 (%) | HPEEtOH50 (%) | HPEH2O (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | β-pinene | 978 | 974 | + | - | - | 5.1 | - |
| 2 | thymol | 1308 | 1310 | + | 0.8 | - | - | - |
| 3 | eugenol | 1339 | 1344 | + | 6.0 | 14.6 | 51.6 | - |
| 4 | α-copaene | 1381 | 1387 | + | - | 6.3 | 3.7 | - |
| 5 | β-caryophyllene | 1412 | 1416 | + | 77.4 | 77.1 | 38.6 | - |
| 6 | isoeugenol | 1432 | 1439 | + | - | - | 1.0 | - |
| 7 | α-humulene | 1450 | 1454 | + | 10.0 | 0.8 | - | - |
| 8 | δ-cadinene | 1528 | 1530+ | + | 5.8 | 1.3 | - | - |
| Total (%) | 100.0 | 100.0 | 100.0 |
| Harpagophytum procumbens Root Extract | IC50 (CL) μg/mL |
|---|---|
| HPEDMSO | 94.7 (23.8–97.5) |
| HPEEtOH100 | 65.5 (19.2–87.9) * |
| HPEEtOH50 | 73.8 (24.5–94.3) |
| HPEH2O | - |
| JZL 195 | 0.03 (0.01–0.06) * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mariano, A.; Di Sotto, A.; Leopizzi, M.; Garzoli, S.; Di Maio, V.; Gullì, M.; Dalla Vedova, P.; Ammendola, S.; Scotto d’Abusco, A. Antiarthritic Effects of a Root Extract from Harpagophytum procumbens DC: Novel Insights into the Molecular Mechanisms and Possible Bioactive Phytochemicals. Nutrients 2020, 12, 2545. https://doi.org/10.3390/nu12092545
Mariano A, Di Sotto A, Leopizzi M, Garzoli S, Di Maio V, Gullì M, Dalla Vedova P, Ammendola S, Scotto d’Abusco A. Antiarthritic Effects of a Root Extract from Harpagophytum procumbens DC: Novel Insights into the Molecular Mechanisms and Possible Bioactive Phytochemicals. Nutrients. 2020; 12(9):2545. https://doi.org/10.3390/nu12092545
Chicago/Turabian StyleMariano, Alessia, Antonella Di Sotto, Martina Leopizzi, Stefania Garzoli, Valeria Di Maio, Marco Gullì, Pietro Dalla Vedova, Sergio Ammendola, and Anna Scotto d’Abusco. 2020. "Antiarthritic Effects of a Root Extract from Harpagophytum procumbens DC: Novel Insights into the Molecular Mechanisms and Possible Bioactive Phytochemicals" Nutrients 12, no. 9: 2545. https://doi.org/10.3390/nu12092545
APA StyleMariano, A., Di Sotto, A., Leopizzi, M., Garzoli, S., Di Maio, V., Gullì, M., Dalla Vedova, P., Ammendola, S., & Scotto d’Abusco, A. (2020). Antiarthritic Effects of a Root Extract from Harpagophytum procumbens DC: Novel Insights into the Molecular Mechanisms and Possible Bioactive Phytochemicals. Nutrients, 12(9), 2545. https://doi.org/10.3390/nu12092545







