Candida albicans Virulence Factors and Pathogenicity for Endodontic Infections
Abstract
:1. Candida Infection in Endodontics
2. Virulence Factors and the Pathogenetic Mechanism in Pulp and Periapical Lesions
2.1. Recognition of C. albicans by Immune Cells
2.2. Recognition of C. albicans by Non-Immune Cells
2.3. Myeloid Cells Affected by C. albicans Infection
3. Treatment Options for Endodontic C. albicans Infection
3.1. Intraradicular Eradication
3.1.1. Mechanical Instrumentation
3.1.2. Chemical Irrigants
Sodium Hypochlorite
Chlorhexidine Digluconate
Alexidine Digluconate
Ethylene Diamine Tetraacetic Acid
3.1.3. Intracanal Medicaments
Calcium Hydroxide
Antimicrobial Peptides
Antifungal Agents
3.1.4. Root Canal Obturation
3.2. Extraradicular Eradication
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yu, C.; Abbott, P.V. An overview of the dental pulp: Its functions and responses to injury. Aust. Dent. J. 2007, 52, S4–S16. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.F., Jr.; Sen, B.H. Fungi in endodontic infections. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2004, 97, 632–641. [Google Scholar] [CrossRef]
- Mergoni, G.; Percudani, D.; Lodi, G.; Bertani, P.; Manfredi, M. Prevalence of Candida species in endodontic infections: Systematic review and meta-analysis. J. Endod. 2018, 44, 1616–1625.e1619. [Google Scholar] [CrossRef] [PubMed]
- Douglas, L.J. Candida biofilms and their role in infection. Trends Microbiol. 2003, 11, 30–36. [Google Scholar] [CrossRef]
- Gulati, M.; Nobile, C.J. Candida albicans biofilms: Development, regulation, and molecular mechanisms. Microbes Infect. 2016, 18, 310–321. [Google Scholar] [CrossRef] [Green Version]
- Chandra, J.; Kuhn, D.M.; Mukherjee, P.K.; Hoyer, L.L.; McCormick, T.; Ghannoum, M.A. Biofilm formation by the fungal pathogen Candida albicans: Development, architecture, and drug resistance. J. Bacteriol. 2001, 183, 5385–5394. [Google Scholar] [CrossRef] [Green Version]
- Sudbery, P.E. Growth of Candida albicans hyphae. Nat. Rev. Microbiol. 2011, 9, 737–748. [Google Scholar] [CrossRef]
- Jacobsen, I.D.; Wilson, D.; Wachtler, B.; Brunke, S.; Naglik, J.R.; Hube, B. Candida albicans dimorphism as a therapeutic target. Expert Rev. Anti-Infect. 2012, 10, 85–93. [Google Scholar] [CrossRef] [Green Version]
- Dalle, F.; Wachtler, B.; L’Ollivier, C.; Holland, G.; Bannert, N.; Wilson, D.; Labruere, C.; Bonnin, A.; Hube, B. Cellular interactions of Candida albicans with human oral epithelial cells and enterocytes. Cell. Microbiol. 2010, 12, 248–271. [Google Scholar] [CrossRef]
- Drago, L.; Mombelli, B.; De Vecchi, E.; Bonaccorso, C.; Fassina, M.C.; Gismondo, M.R. Candida albicans cellular internalization: A new pathogenic factor? Int. J. Antimicrob. Agents 2000, 16, 545–547. [Google Scholar] [CrossRef]
- Phan, Q.T.; Myers, C.L.; Fu, Y.; Sheppard, D.C.; Yeaman, M.R.; Welch, W.H.; Ibrahim, A.S.; Edwards, J.E., Jr.; Filler, S.G. Als3 is a Candida albicans invasin that binds to cadherins and induces endocytosis by host cells. PLoS Biol. 2007, 5, e64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoyer, L.L. The ALS gene family of Candida albicans. Trends Microbiol. 2001, 9, 176–180. [Google Scholar] [CrossRef]
- Liu, Y.; Filler, S.G. Candida albicans Als3, a multifunctional adhesin and invasin. Eukaryot. Cell. 2011, 10, 168–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nobile, C.J.; Andes, D.R.; Nett, J.E.; Smith, F.J.; Yue, F.; Phan, Q.T.; Edwards, J.E.; Filler, S.G.; Mitchell, A.P. Critical role of Bcr1-dependent adhesins in C. albicans biofilm formation in vitro and in vivo. PLoS Pathog. 2006, 2, e63. [Google Scholar] [CrossRef] [PubMed]
- Sheth, C.C.; Hall, R.; Lewis, L.; Brown, A.J.; Odds, F.C.; Erwig, L.P.; Gow, N.A. Glycosylation status of the C. albicans cell wall affects the efficiency of neutrophil phagocytosis and killing but not cytokine signaling. Med. Mycol. 2011, 49, 513–524. [Google Scholar] [CrossRef] [Green Version]
- Netea, M.G.; Brown, G.D.; Kullberg, B.J.; Gow, N.A. An integrated model of the recognition of Candida albicans by the innate immune system. Nat. Rev. Microbiol. 2008, 6, 67–78. [Google Scholar] [CrossRef]
- Gantner, B.N.; Simmons, R.M.; Underhill, D.M. Dectin-1 mediates macrophage recognition of Candida albicans yeast but not filaments. EMBO J. 2005, 24, 1277–1286. [Google Scholar] [CrossRef] [Green Version]
- Bates, S.; Hughes, H.B.; Munro, C.A.; Thomas, W.P.; MacCallum, D.M.; Bertram, G.; Atrih, A.; Ferguson, M.A.; Brown, A.J.; Odds, F.C.; et al. Outer chain N-glycans are required for cell wall integrity and virulence of Candida albicans. J. Biol. Chem. 2006, 281, 90–98. [Google Scholar] [CrossRef] [Green Version]
- Chaffin, W.L.; Lopez-Ribot, J.L.; Casanova, M.; Gozalbo, D.; Martinez, J.P. Cell wall and secreted proteins of Candida albicans: Identification, function, and expression. Microbiol. Mol. Biol. Rev. 1998, 62, 130–180. [Google Scholar] [CrossRef] [Green Version]
- Machova, E.; Fiacanova, L.; Cizova, A.; Korcova, J. Mannoproteins from yeast and hyphal form of Candida albicans considerably differ in mannan and protein content. Carbohydr. Res. 2015, 408, 12–17. [Google Scholar] [CrossRef]
- Sakko, M.; Tjaderhane, L.; Rautemaa-Richardson, R. Microbiology of Root Canal Infections. Prim. Dent. J. 2016, 5, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.A.; Garlet, G.P.; Fukada, S.Y.; Silva, J.S.; Cunha, F.Q. Chemokines in oral inflammatory diseases: Apical periodontitis and periodontal disease. J. Dent. Res. 2007, 86, 306–319. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.N. Pathogenesis of apical periodontitis and the causes of endodontic failures. Crit. Rev. Oral Biol. Med. 2004, 15, 348–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, P.N. Apical periodontitis: A dynamic encounter between root canal infection and host response. Periodontol. 2000 1997, 13, 121–148. [Google Scholar] [CrossRef]
- Rodini, C.O.; Lara, V.S. Study of the expression of CD68+ macrophages and CD8+ T cells in human granulomas and periapical cysts. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2001, 92, 221–227. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Rodini, C.; Batista, A.C.; Lara, V.S. Comparative immunohistochemical study of the presence of mast cells in apical granulomas and periapical cysts: Possible role of mast cells in the course of human periapical lesions. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2004, 97, 59–63. [Google Scholar] [CrossRef]
- Tong, Y.; Tang, J. Candida albicans infection and intestinal immunity. Microbiol. Res. 2017, 198, 27–35. [Google Scholar] [CrossRef]
- Jouault, T.; Ibata-Ombetta, S.; Takeuchi, O.; Trinel, P.A.; Sacchetti, P.; Lefebvre, P.; Akira, S.; Poulain, D. Candida albicans phospholipomannan is sensed through toll-like receptors. J. Infect. Dis. 2003, 188, 165–172. [Google Scholar] [CrossRef] [Green Version]
- Netea, M.G.; Gow, N.A.; Munro, C.A.; Bates, S.; Collins, C.; Ferwerda, G.; Hobson, R.P.; Bertram, G.; Hughes, H.B.; Jansen, T.; et al. Immune sensing of Candida albicans requires cooperative recognition of mannans and glucans by lectin and Toll-like receptors. J. Clin. Investig. 2006, 116, 1642–1650. [Google Scholar] [CrossRef]
- van de Veerdonk, F.L.; Marijnissen, R.J.; Kullberg, B.J.; Koenen, H.J.; Cheng, S.C.; Joosten, I.; van den Berg, W.B.; Williams, D.L.; van der Meer, J.W.; Joosten, L.A.; et al. The macrophage mannose receptor induces IL-17 in response to Candida albicans. Cell Host Microbe 2009, 5, 329–340. [Google Scholar] [CrossRef] [Green Version]
- Wells, C.A.; Salvage-Jones, J.A.; Li, X.; Hitchens, K.; Butcher, S.; Murray, R.Z.; Beckhouse, A.G.; Lo, Y.L.; Manzanero, S.; Cobbold, C.; et al. The macrophage-inducible C-type lectin, mincle, is an essential component of the innate immune response to Candida albicans. J. Immunol. 2008, 180, 7404–7413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Wang, F.; Yang, J.; Zhao, D.; Wang, H.; Shao, F.; Wang, W.; Sun, R.; Ling, M.; Zhai, J.; et al. Mannan-binding lectin inhibits Candida albicans-induced cellular responses in PMA-activated THP-1 cells through Toll-like receptor 2 and Toll-like receptor 4. PLoS ONE 2013, 8, e83517. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.; Davies, L.C.; Liao, C.T.; da Fonseca, D.M.; Griffiths, J.S.; Andrews, R.; Jones, A.V.; Clement, M.; Brown, G.D.; Humphreys, I.R.; et al. The protective effect of inflammatory monocytes during systemic C. albicans infection is dependent on collaboration between C-type lectin-like receptors. PLoS Pathog. 2019, 15, e1007850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wellington, M.; Koselny, K.; Sutterwala, F.S.; Krysan, D.J. Candida albicans triggers NLRP3-mediated pyroptosis in macrophages. Eukaryot. Cell 2014, 13, 329–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joly, S.; Ma, N.; Sadler, J.J.; Soll, D.R.; Cassel, S.L.; Sutterwala, F.S. Cutting edge: Candida albicans hyphae formation triggers activation of the Nlrp3 inflammasome. J. Immunol. 2009, 183, 3578–3581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogiers, O.; Frising, U.C.; Kucharikova, S.; Jabra-Rizk, M.A.; van Loo, G.; Van Dijck, P.; Wullaert, A. Candidalysin crucially contributes to Nlrp3 inflammasome activation by Candida albicans hyphae. mBio 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Kasper, L.; Konig, A.; Koenig, P.A.; Gresnigt, M.S.; Westman, J.; Drummond, R.A.; Lionakis, M.S.; Gross, O.; Ruland, J.; Naglik, J.R.; et al. The fungal peptide toxin Candidalysin activates the NLRP3 inflammasome and causes cytolysis in mononuclear phagocytes. Nat. Commun. 2018, 9, 4260. [Google Scholar] [CrossRef]
- Pietrella, D.; Pandey, N.; Gabrielli, E.; Pericolini, E.; Perito, S.; Kasper, L.; Bistoni, F.; Cassone, A.; Hube, B.; Vecchiarelli, A. Secreted aspartic proteases of Candida albicans activate the NLRP3 inflammasome. Eur. J. Immunol. 2013, 43, 679–692. [Google Scholar] [CrossRef]
- Ganesan, S.; Rathinam, V.A.K.; Bossaller, L.; Army, K.; Kaiser, W.J.; Mocarski, E.S.; Dillon, C.P.; Green, D.R.; Mayadas, T.N.; Levitz, S.M.; et al. Caspase-8 modulates dectin-1 and complement receptor 3-driven IL-1beta production in response to beta-glucans and the fungal pathogen, Candida albicans. J. Immunol. 2014, 193, 2519–2530. [Google Scholar] [CrossRef] [Green Version]
- Joly, S.; Eisenbarth, S.C.; Olivier, A.K.; Williams, A.; Kaplan, D.H.; Cassel, S.L.; Flavell, R.A.; Sutterwala, F.S. Cutting edge: Nlrp10 is essential for protective antifungal adaptive immunity against Candida albicans. J. Immunol. 2012, 189, 4713–4717. [Google Scholar] [CrossRef] [Green Version]
- Wagener, J.; MacCallum, D.M.; Brown, G.D.; Gow, N.A. Candida albicans chitin increases arginase-1 activity in human macrophages, with an Impact on macrophage antimicrobial functions. mBio 2017, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- d’Ostiani, C.F.; Del Sero, G.; Bacci, A.; Montagnoli, C.; Spreca, A.; Mencacci, A.; Ricciardi-Castagnoli, P.; Romani, L. Dendritic cells discriminate between yeasts and hyphae of the fungus Candida albicans. Implications for initiation of T helper cell immunity in vitro and in vivo. J. Exp. Med. 2000, 191, 1661–1674. [Google Scholar] [CrossRef]
- Munoz-Duarte, A.R.; Castrejon-Jimenez, N.S.; Baltierra-Uribe, S.L.; Perez-Rangel, S.J.; Carapia-Minero, N.; Castaneda-Sanchez, J.I.; Luna-Herrera, J.; Lopez-Santiago, R.; Rodriguez-Tovar, A.V.; Garcia-Perez, B.E. Candida glabrata survives and replicates in human osteoblasts. Pathog. Dis. 2016, 74, ftw030. [Google Scholar] [CrossRef] [Green Version]
- Romani, L.; Bistoni, F.; Puccetti, P. Fungi, dendritic cells and receptors: A host perspective of fungal virulence. Trends Microbiol. 2002, 10, 508–514. [Google Scholar] [CrossRef]
- De Zuani, M.; Paolicelli, G.; Zelante, T.; Renga, G.; Romani, L.; Arzese, A.; Pucillo, C.E.M.; Frossi, B. Mast cells respond to Candida albicans infections and modulate macrophages phagocytosis of the fungus. Front. Immunol. 2018, 9, 2829. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.H.; Richardson, J.P.; Zhou, C.; Coleman, B.M.; Moyes, D.L.; Ho, J.; Huppler, A.R.; Ramani, K.; McGeachy, M.J.; Mufazalov, I.A.; et al. Oral epithelial cells orchestrate innate type 17 responses to Candida albicans through the virulence factor candidalysin. Sci. Immunol. 2017, 2, eaam8834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swidergall, M.; Solis, N.V.; Lionakis, M.S.; Filler, S.G. EphA2 is an epithelial cell pattern recognition receptor for fungal beta-glucans. Nat. Microbiol. 2018, 3, 53–61. [Google Scholar] [CrossRef] [Green Version]
- Ishida, Y.; Ohta, K.; Naruse, T.; Kato, H.; Fukui, A.; Shigeishi, H.; Nishi, H.; Tobiume, K.; Takechi, M. Candida albicans beta-glucan-containing particles increase HO-1 expression in oral keratinocytes via a reactive oxygen species/p38 mitogen-activated protein kinase/Nrf2 pathway. Infect. Immunol. 2018, 86, e00575–e00617. [Google Scholar] [CrossRef] [Green Version]
- Taille, C.; El-Benna, J.; Lanone, S.; Dang, M.C.; Ogier-Denis, E.; Aubier, M.; Boczkowski, J. Induction of heme oxygenase-1 inhibits NAD(P)H oxidase activity by down-regulating cytochrome b558 expression via the reduction of heme availability. J. Biol. Chem. 2004, 279, 28681–28688. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Liang, G.; Wang, Q.; She, X.; Shi, D.; Shen, Y.; Su, X.; Wang, X.; Wang, W.; Li, D.; et al. Different host immunological response to C. albicans by human oral and vaginal epithelial cells. Mycopathologia 2019, 184, 1–12. [Google Scholar] [CrossRef]
- Tamai, R.; Kiyoura, Y. Candida albicans and Candida parapsilosis rapidly up-regulate galectin-3 secretion by human gingival epithelial cells. Mycopathologia 2014, 177, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Villar, C.C.; Zhao, X.R. Candida albicans induces early apoptosis followed by secondary necrosis in oral epithelial cells. Mol. Oral Microbiol. 2010, 25, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Dongari-Bagtzoglou, A.; Villar, C.C.; Kashleva, H. Candida albicans-infected oral epithelial cells augment the anti-fungal activity of human neutrophils in vitro. Med. Mycol. 2005, 43, 545–549. [Google Scholar] [CrossRef] [Green Version]
- Ohta, K.; Nishi, H.; Fukui, A.; Shigeishi, H.; Takechi, M.; Kamata, N. CX3CL1 expression induced by Candida albicans in oral fibroblasts. FEMS Immunol. Med. Microbiol. 2010, 60, 179–185. [Google Scholar] [CrossRef]
- Alsalleeh, F.; Young, A.; Algarawi, Z.; Petro, T. C. Albicans biofilm formation is restricted by periodontal ligament cells and induces differential cytokines response compared to planktonic C. Albicans. J. Dent. Appl. 2014, 1, 139–144. [Google Scholar]
- Yu, Q.; Li, J.; Zhang, Y.; Wang, Y.; Liu, L.; Li, M. Inhibition of gold nanoparticles (AuNPs) on pathogenic biofilm formation and invasion to host cells. Sci. Rep. 2016, 6, 26667. [Google Scholar] [CrossRef] [PubMed]
- Angata, T. Siglec-15: A potential regulator of osteoporosis, cancer, and infectious diseases. J. Biomed. Sci. 2020, 27, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maher, C.O.; Dunne, K.; Comerford, R.; O’Dea, S.; Loy, A.; Woo, J.; Rogers, T.R.; Mulcahy, F.; Dunne, P.J.; Doherty, D.G. Candida albicans stimulates IL-23 release by human dendritic cells and downstream IL-17 secretion by Vdelta1 T cells. J. Immunol. 2015, 194, 5953–5960. [Google Scholar] [CrossRef] [Green Version]
- Nur, S.; Sparber, F.; Lemberg, C.; Guiducci, E.; Schweizer, T.A.; Zwicky, P.; Becher, B.; LeibundGut-Landmann, S. IL-23 supports host defense against systemic Candida albicans infection by ensuring myeloid cell survival. PLoS Pathog. 2019, 15, e1008115. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Zhao, Y.; Jiang, Y.; Qin, T.; Chen, J.; Chu, X.; Yi, Q.; Gao, S.; Wang, S. Dectin-1 signaling inhibits osteoclastogenesis via IL-33-induced inhibition of NFATc1. Oncotarget 2017, 8, 53366–53374. [Google Scholar] [CrossRef] [Green Version]
- Maruyama, K.; Takayama, Y.; Kondo, T.; Ishibashi, K.I.; Sahoo, B.R.; Kanemaru, H.; Kumagai, Y.; Martino, M.M.; Tanaka, H.; Ohno, N.; et al. Nociceptors boost the resolution of fungal osteoinflammation via the TRP channel-CGRP-Jdp2 axis. Cell Rep. 2017, 19, 2730–2742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amanai, T.; Nakamura, Y.; Aoki, S.; Mataga, I. Micro-CT analysis of experimental Candida osteoarthritis in rats. Mycopathologia 2008, 166, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Noda, S.; Masuhara, T.; Ito-Kuwa, S.; Nakamura, K.; Aoki, S. Radiographic features of experimental Candida arthritis in rats. Mycopathologia 1993, 121, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Doughty-Shenton, D.; Joseph, J.D.; Zhang, J.; Pagliarini, D.J.; Kim, Y.; Lu, D.; Dixon, J.E.; Casey, P.J. Pharmacological targeting of the mitochondrial phosphatase PTPMT1. J. Pharm. Exp. 2010, 333, 584–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnhold, J.; Wiegel, D.; Richter, O.; Hammerschmidt, S.; Arnold, K.; Krumbiegel, M. Modification of low density lipoproteins by sodium hypochlorite. Biomed. Biochim. Acta 1991, 50, 967–973. [Google Scholar] [PubMed]
- Webb, B.C.; Willcox, M.D.; Thomas, C.J.; Harty, D.W.; Knox, K.W. The effect of sodium hypochlorite on potential pathogenic traits of Candida albicans and other Candida species. Oral Microbiol. Immunol. 1995, 10, 334–341. [Google Scholar] [CrossRef]
- Parsons, G.J.; Patterson, S.S.; Miller, C.H.; Katz, S.; Kafrawy, A.H.; Newton, C.W. Uptake and release of chlorhexidine by bovine pulp and dentin specimens and their subsequent acquisition of anti-bacterial properties. Oral Surg. Oral Med. Oral Pathol. 1980, 49, 455–459. [Google Scholar] [CrossRef]
- Bobichon, H.; Bouchet, P. Action of chlorhexidine on budding Candida albicans: Scanning and transmission electron microscopic study. Mycopathologia 1987, 100, 27–35. [Google Scholar] [CrossRef]
- Yoo, Y.J.; Kwon, I.; Oh, S.R.; Perinpanayagam, H.; Lim, S.M.; Ahn, K.B.; Lee, Y.; Han, S.H.; Chang, S.W.; Baek, S.H.; et al. Antifungal effects of synthetic human beta-defensin-3-C15 peptide on Candida albicans-infected root dentin. J. Endod. 2017, 43, 1857–1861. [Google Scholar] [CrossRef]
- Krithikadatta, J.; Indira, R.; Dorothykalyani, A.L. Disinfection of dentinal tubules with 2% chlorhexidine, 2% metronidazole, bioactive glass when compared with calcium hydroxide as intracanal medicaments. J. Endod. 2007, 33, 1473–1476. [Google Scholar] [CrossRef]
- Ercan, E.; Dalli, M.; Dulgergil, C.T. In vitro assessment of the effectiveness of chlorhexidine gel and calcium hydroxide paste with chlorhexidine against Enterococcus faecalis and Candida albicans. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 102, e27–e31. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, Z.; Abbott, P.V. The properties and applications of chlorhexidine in endodontics. Int. Endod. J. 2009, 42, 288–302. [Google Scholar] [CrossRef] [PubMed]
- Okino, L.A.; Siqueira, E.L.; Santos, M.; Bombana, A.C.; Figueiredo, J.A. Dissolution of pulp tissue by aqueous solution of chlorhexidine digluconate and chlorhexidine digluconate gel. Int. Endod. J. 2004, 37, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Mamouei, Z.; Alqarihi, A.; Singh, S.; Xu, S.Y.; Mansour, M.K.; Ibrahim, A.S.; Uppuluri, P. Alexidine dihydrochloride has broad-spectrum activities against diverse fungal pathogens. mSphere 2018, 3, e00539–e00618. [Google Scholar] [CrossRef] [Green Version]
- Barrios, R.; Ferrer-Luque, C.M.; Arias-Moliz, M.T.; Ruiz-Linares, M.; Bravo, M.; Baca, P. Antimicrobial substantivity of alexidine and chlorhexidine in dentin. J. Endod. 2013, 39, 1413–1415. [Google Scholar] [CrossRef]
- Kim, H.S.; Zhu, Q.; Baek, S.H.; Jung, I.Y.; Son, W.J.; Chang, S.W.; Lee, W.; Gu, Y.; Lee, Y.; Hong, S.T.; et al. Chemical interaction of alexidine and sodium hypochlorite. J. Endod. 2012, 38, 112–116. [Google Scholar] [CrossRef]
- Orstavik, D.; Haapasalo, M. Disinfection by endodontic irrigants and dressings of experimentally infected dentinal tubules. Endod. Dent. Traumatol. 1990, 6, 142–149. [Google Scholar] [CrossRef]
- Sen, B.H.; Akdeniz, B.G.; Denizci, A.A. The effect of ethylenediamine-tetraacetic acid on Candida albicans. Oral Surg. Oral Med. Oral Pathol. 2000, 90, 651–655. [Google Scholar] [CrossRef]
- Pugh, D.; Cawson, R.A. Calcium, sequestering agents and nystatin-interactions on cell wall morphology and fungistasis of Candida albicans. Sabouraudia 1980, 18, 157–159. [Google Scholar] [CrossRef]
- Bedell, G.W.; Soll, D.R. Effects of low concentrations of zinc on the growth and dimorphism of Candida albicans: Evidence for zinc-resistant and -sensitive pathways for mycelium formation. Infect. Immunol. 1979, 26, 348–354. [Google Scholar] [CrossRef] [Green Version]
- Holmes, A.R.; Cannon, R.D.; Shepherd, M.G. Effect of calcium ion uptake on Candida albicans morphology. FEMS Microbiol. Lett. 1991, 61, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Sohnle, P.G.; Hahn, B.L.; Karmarkar, R. Effect of metals on Candida albicans growth in the presence of chemical chelators and human abscess fluid. J. Lab. Clin. Med. 2001, 137, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.A.M. Candida (Monilia) Albicans—Effect of amino acids, glucose, pH, chlortetracycline (aureomycin), dibasic sodium and calcium phosphates, and anaerobic and aerobic conditions on Its growth. AMA Arch. Derm. Syph. 1954, 70, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Waltimo, T.M.; Siren, E.K.; Orstavik, D.; Haapasalo, M.P. Susceptibility of oral Candida species to calcium hydroxide in vitro. Int. Endod. J. 1999, 32, 94–98. [Google Scholar] [CrossRef]
- Denison, S.H. pH regulation of gene expression in fungi. Fungal Genet. Biol. 2000, 29, 61–71. [Google Scholar] [CrossRef]
- Ramon, A.M.; Porta, A.; Fonzi, W.A. Effect of environmental pH on morphological development of Candida albicans is mediated via the PacC-related transcription factor encoded by PRR2. J. Bacteriol. 1999, 181, 7524–7530. [Google Scholar] [CrossRef] [Green Version]
- Wilson, R.B.; Davis, D.; Mitchell, A.P. Rapid hypothesis testing with Candida albicans through gene disruption with short homology regions. J. Bacteriol. 1999, 181, 1868–1874. [Google Scholar] [CrossRef] [Green Version]
- Sen, B.H.; Safavi, K.E.; Spangberg, L.S. Antifungal effects of sodium hypochlorite and chlorhexidine in root canals. J. Endod. 1999, 25, 235–238. [Google Scholar] [CrossRef]
- Shai, Y. Molecular recognition between membrane-spanning polypeptides. Trends Biochem. Sci. 1995, 20, 460–464. [Google Scholar] [CrossRef]
- Debono, M.; Gordee, R.S. Antibiotics that inhibit fungal cell-wall development. Annu. Rev. Microbiol. 1994, 48, 471–497. [Google Scholar] [CrossRef]
- White, S.H.; Wimley, W.C.; Selsted, M.E. Structure, function, and membrane integration of defensins. Curr. Opin. Struct. Biol. 1995, 5, 521–527. [Google Scholar] [CrossRef]
- Schneider, J.J.; Unholzer, A.; Schaller, M.; Schafer-Korting, M.; Korting, H.C. Human defensins. J. Mol. Med. 2005, 83, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Hoover, D.M.; Wu, Z.; Tucker, K.; Lu, W.; Lubkowski, J. Antimicrobial characterization of human beta-defensin 3 derivatives. Antimicrob. Agents Chemother. 2003, 47, 2804–2809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnakumari, V.; Rangaraj, N.; Nagaraj, R. Antifungal activities of human beta-defensins HBD-1 to HBD-3 and their C-terminal analogs Phd1 to Phd3. Antimicrob Agents Chemother. 2009, 53, 256–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, S.M.; Ahn, K.B.; Kim, C.; Kum, J.W.; Perinpanayagam, H.; Gu, Y.; Yoo, Y.J.; Chang, S.W.; Han, S.H.; Shon, W.J.; et al. Antifungal effects of synthetic human beta-defensin 3-C15 peptide. Restor. Dent. Endod. 2016, 41, 91–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, Z.; Jiang, B.; Chandra, J.; Ghannoum, M.; Nelson, S.; Weinberg, A. Human beta-defensins: Differential activity against candidal species and regulation by Candida albicans. J. Dent. Res. 2005, 84, 445–450. [Google Scholar] [CrossRef]
- Lee, J.Y.; Suh, J.S.; Kim, J.M.; Kim, J.H.; Park, H.J.; Park, Y.J.; Chung, C.P. Identification of a cell-penetrating peptide domain from human beta-defensin 3 and characterization of its anti-inflammatory activity. Int. J. Nanomed. 2015, 10, 5423–5434. [Google Scholar] [CrossRef] [Green Version]
- Hancock, R.E. Peptide antibiotics. Lancet 1997, 349, 418–422. [Google Scholar] [CrossRef]
- Ota, C.; Unterkircher, C.; Fantinato, V.; Shimizu, M.T. Antifungal activity of propolis on different species of Candida. Mycoses 2001, 44, 375–378. [Google Scholar] [CrossRef]
- Chua, E.G.; Parolia, A.; Ahlawat, P.; Pau, A.; Amalraj, F.D. Antifungal effectiveness of various intracanal medicaments against Candida albicans: An ex-vivo study. BMC Oral Health 2014, 14, 53. [Google Scholar] [CrossRef] [Green Version]
- Carbajal Mejia, J.B. Antimicrobial effects of calcium hydroxide, chlorhexidine, and propolis on Enterococcus faecalis and Candida albicans. J. Investig. Clin. Dent. 2014, 5, 194–200. [Google Scholar] [CrossRef] [PubMed]
- McHugh, C.P.; Zhang, P.; Michalek, S.; Eleazer, P.D. pH required to kill Enterococcus faecalis in vitro. J. Endod. 2004, 30, 218–219. [Google Scholar] [CrossRef] [PubMed]
- Al-Hezaimi, K.; Al-Hamdan, K.; Naghshbandi, J.; Oglesby, S.; Simon, J.H.S.; Rotstein, I. Effect of white-colored mineral trioxide aggregate in different concentrations on Candida albicans in vitro. J. Endod. 2005, 31, 684–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adl, A.; Sadat Shojaee, N.; Pourhatami, N. Evaluation of the dislodgement resistance of a new Pozzolan-based cement (EndoSeal MTA) compared to ProRoot MTA and Biodentine in the presence and absence of blood. Scanning 2019, 2019, 3863069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reyes-Carmona, J.F.; Felippe, M.S.; Felippe, W.T. The biomineralization ability of mineral trioxide aggregate and Portland cement on dentin enhances the push-out strength. J. Endod. 2010, 36, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.S.; Chang, S.W.; Oh, S.R.; Perinpanayagam, H.; Lim, S.M.; Yoo, Y.J.; Oh, Y.R.; Woo, S.B.; Han, S.H.; Zhu, Q.; et al. Bacterial entombment by intratubular mineralization following orthograde mineral trioxide aggregate obturation: A scanning electron microscopy study. Int. J. Oral Sci. 2014, 6, 227–232. [Google Scholar] [CrossRef] [Green Version]
- Nair, P.N. On the causes of persistent apical periodontitis: A review. Int. Endod. J. 2006, 39, 249–281. [Google Scholar] [CrossRef]
- Nair, P.N.; Sjogren, U.; Krey, G.; Kahnberg, K.E.; Sundqvist, G. Intraradicular bacteria and fungi in root-filled, asymptomatic human teeth with therapy-resistant periapical lesions: A long-term light and electron microscopic follow-up study. J. Endod. 1990, 16, 580–588. [Google Scholar] [CrossRef]
- Lomcali, G.; Sen, B.H.; Cankaya, H. Scanning electron microscopic observations of apical root surfaces of teeth with apical periodontitis. Endod. Dent. Traumatol. 1996, 12, 70–76. [Google Scholar] [CrossRef]
- Damm, D.D.; Neville, B.W.; Geissler, R.H., Jr.; White, D.K.; Drummond, J.F.; Ferretti, G.A. Dentinal candidiasis in cancer patients. Oral Surg. Oral Med. Oral Pathol. 1988, 65, 56–60. [Google Scholar] [CrossRef]
- Eidelman, D.; Neuman, I.; Kuttin, E.S.; Pinto, M.; Beemer, A.M. Dental sepsis due to Candida albicans causing urticaria–case report. Ann. Allergy 1978, 41, 179–181. [Google Scholar] [PubMed]

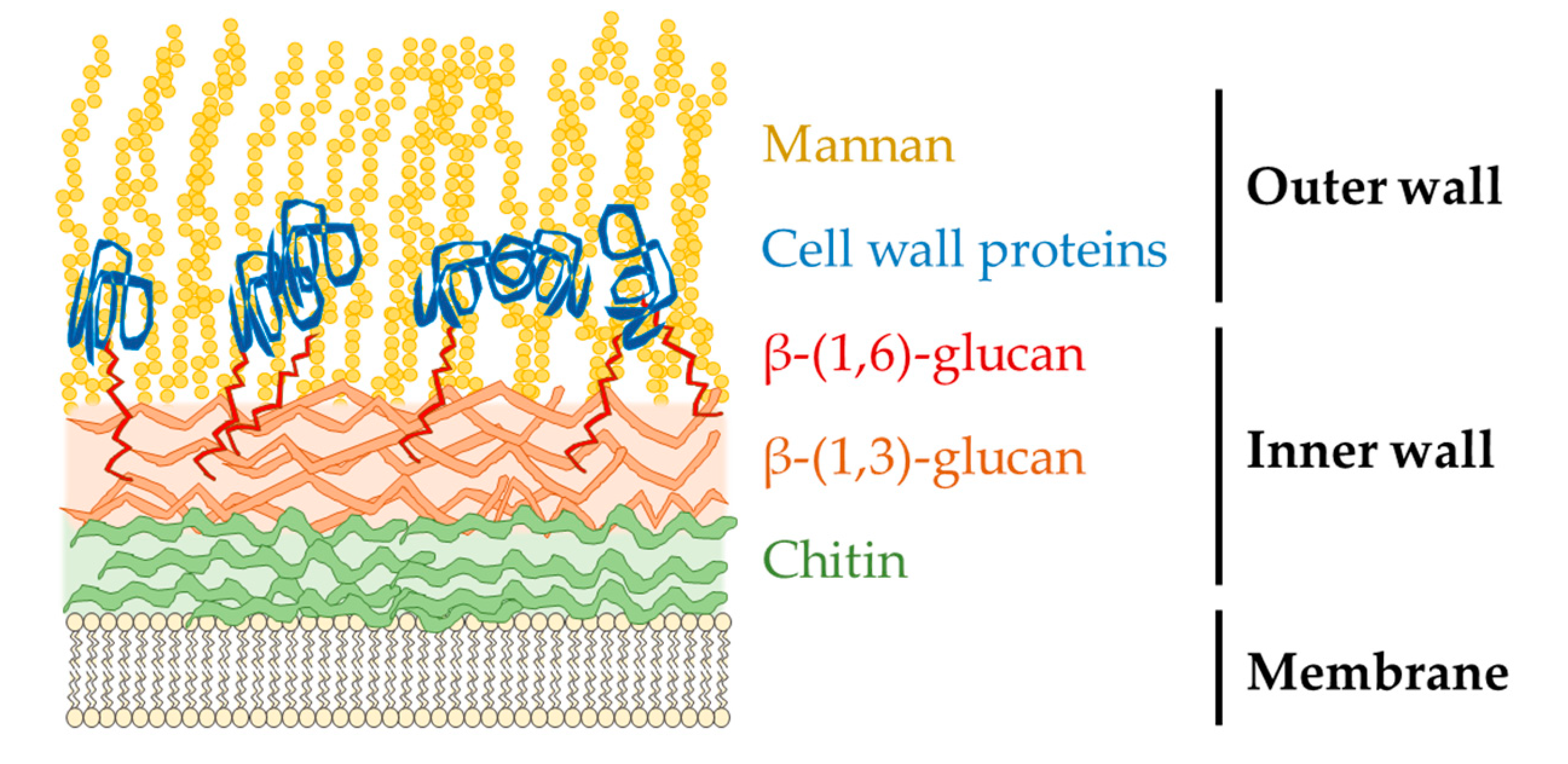
| Host Cell | Component of C. albicans | Receptor or Mechanisms | Increase | Decrease | Ref |
|---|---|---|---|---|---|
| macrophages | phospholipomannan | TLR2 | TNF-a | / | [28] |
| human mononuclear cell, murine macrophages | purified mannan | / | TNF, IL-6 | / | [29] |
| human mononuclear cells | O-linked mannosyl residues | TLR4 | / | / | [29] |
| human mononuclear cells | N-linked mannosyl residues | mannose receptor | / | / | [29] |
| human peripheral blood mononuclear cells | mannan | mannose receptor | IL-17 | / | [30] |
| human peripheral blood mononuclear cells | mannan, β-glucan | TLR2/denctin-1 | mannose receptor-induced IL-17 | / | [30] |
| human mononuclear cells | β-glucan | TLR2/denctin-1 | induced cytokine production | / | [29] |
| macrophages | β-glucan | dectin-1 | reactive oxygen, phagocytosis | / | [17] |
| macrophages | / | mincle | TNF-α | / | [31] |
| human macrophages | / | mannan-binding lectin | / | C. albicans-induced proinflammatory cytokines | [32] |
| monocyte | / | multiple CLR (Dectin-1, Dectin-2 and Mincle) | / | remove fungus | [33] |
| macrophage | transcription factor UPC2 | caspase-1, ASC, and NLRP3 Inflammasome | pyroptosis | / | [34] |
| macrophage, monocuclear phagocytes | hyphae, hyphae-derived toxin candidalysin | NLRP3 Inflammasome | pyroptosis and IL-1β secretion | / | [35,36,37] |
| monocyte, macrophage, dendritic cells | Secreted aspartic proteases 2 and 6 | NLRP3 inflammasome | induced IL-1β, TNF-α, and IL-6 production | / | [38] |
| dendritic cells | β-glucans | caspase-8 and NLRP3 inflammasome, Complement receptor 3 and Dectin-1 signaling | IL-1β secretion | / | [39] |
| macrophage and dendritic cell | / | NLRP10 | Th1 and Th17 responses | / | [40] |
| human macrophages | purified chitin or increased exposure of chitin | enhancement of host arginase activity | / | nitric oxide production | [41] |
| dendritic cells | morphology of C. albicans and type of receptor mediated the entry into cells | mannose receptor/CR3 | production of proinflammatory cytokines | / | [42] |
| mast cell | / | α-tubulin cytoskeleton rearrangement and accumulation LAMP1+ vesicles | production of TNF-α, IL-6, IL-13, and IL-4, phagocytosis of hyphae form of C. albicans, enhanced chemotaxis and movement of macrophages. | / | [45] |
| TCRαβ+ cells | hyphae, candidalysin | / | IL-17+ TCRαβ+ cell proliferation | / | [46] |
| Host Cell | Component of C. albicans | Receptor or Mechanisms | Increase | Decrease | Ref |
|---|---|---|---|---|---|
| oral epithelium | β-glucans -containing particles | reactive oxygen species (ROS)/p38 MAPK/Nrf2 | heme oxygenase-1 (HO-1) expression | / | [48] |
| oral epithelial cells | Als3p and Ssa1p gene | / | production of cytokine and chemokine | / | [50] |
| human gingival epithelial cell, fibroblasts | / | cytoskeletal changes, protease activity, or PI3K signaling | secretion of galectin-3 | / | [51] |
| oral epithelial cells | / | / | release of cytokines such as IL-1α or IL-1β and chemokines such as IL-8 or Macrophage Inflammatory Protein (MIP)-3 α | / | [50] |
| oral epithelial cells | / | / | caspase-dependent apoptosis | / | [52] |
| oral epithelial cells | / | neutrophils, IL-1 signaling | C. albicans hyphae damage | / | [53] |
| oral fibroblasts | / | / | fractalkine/CX3CL1 (CX3CL1) | / | [54] |
| keratinocyte | / | / | increased mRNA levels of several chemokines excepting CX3CL1 | / | [54] |
| periodontal ligament (PDL) cell | / | / | induced expression of IL-6 and TNF-α, or IL-1β, RANKL, IL-23 p19, and IL-17R | reduced IL-10 expression | [55] |
| dental pulp cells | Hyphal | / | cell damage, expression of CLEC7A, TLR2, and TLR4, IFN-α | / | [56] |
| Host | Component of C. albicans | Receptor or Mechanisms | Increase | Decrease | Ref |
|---|---|---|---|---|---|
| human myeloid cells, epithelial cells | / | / | Siglec-15 messenger RNA (mRNA) expression (associated with osteoclast differentiation on osteoclast precursor) | / | [57] |
| monocytes, macrophages, dendritic cells, neutrophils | / | / | IL-23 (associated with osteoclast differentiation in mouse macrophage) | / | [58,59] |
| osteoclast precursor | / | dectin-1 signaling (β-1-3-glucans of C. albicans are ligands of Dectin-1), IL-33 | RANKL-mediated osteoclast differentiation | / | [60] |
| mice | C. albicans-derived β-glucan | dectin-1, nociceptors | the production of calcitonin gene-related peptides | inhibit osteoporosis and osteomyelitis in response to β-glucan | [61] |
| rat | / | / | irregular new bone formation and resorptions, severe deformation of joint bones | / | [62,63] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, Y.-J.; Kim, A.R.; Perinpanayagam, H.; Han, S.H.; Kum, K.-Y. Candida albicans Virulence Factors and Pathogenicity for Endodontic Infections. Microorganisms 2020, 8, 1300. https://doi.org/10.3390/microorganisms8091300
Yoo Y-J, Kim AR, Perinpanayagam H, Han SH, Kum K-Y. Candida albicans Virulence Factors and Pathogenicity for Endodontic Infections. Microorganisms. 2020; 8(9):1300. https://doi.org/10.3390/microorganisms8091300
Chicago/Turabian StyleYoo, Yeon-Jee, A Reum Kim, Hiran Perinpanayagam, Seung Hyun Han, and Kee-Yeon Kum. 2020. "Candida albicans Virulence Factors and Pathogenicity for Endodontic Infections" Microorganisms 8, no. 9: 1300. https://doi.org/10.3390/microorganisms8091300
APA StyleYoo, Y. -J., Kim, A. R., Perinpanayagam, H., Han, S. H., & Kum, K. -Y. (2020). Candida albicans Virulence Factors and Pathogenicity for Endodontic Infections. Microorganisms, 8(9), 1300. https://doi.org/10.3390/microorganisms8091300






