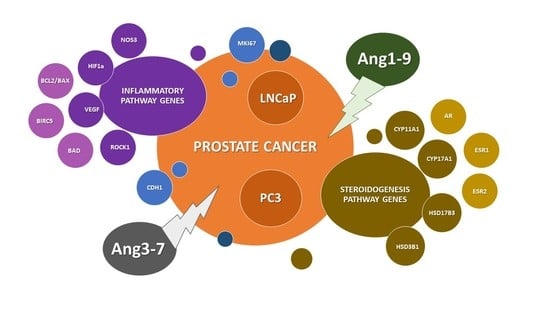
The Impact of Ang-(1-9) and Ang-(3-7) on the Biological Properties of Prostate Cancer Cells by Modulation of Inflammatory and Steroidogenesis Pathway Genes
Abstract
:1. Introduction
2. Results
2.1. Influence of Ang-(1-9) and Ang-(3-7) on Metabolic Activity of Prostate Cancer Cells
2.2. Influence of Ang-(1-9) and Ang-(3-7) on Cell Proliferation of Prostate Cancer Lines
2.3. Influence of Ang-(1-9) and Ang-(3-7) on Anchorage-Independent Cell Growth Ability and Cell Mobility of Prostate Cancer Lines
2.4. Influence of Ang-(1-9) and Ang-(3-7) on mRNA Level of Angiotensin Receptors Gene
2.5. Influence of Ang-(1-9) and Ang-(3-7) on mRNA Level of Inflammatory Pathway Genes
2.6. Influence of Ang-(1-9) and Ang-(3-7) on mRNA Level of Steroidogenesis Pathway Genes
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Reagents
4.2. Metabolic Activity Assay
4.3. The Cell Cycle Assay
4.4. Migration Assay
4.5. Soft Agar Anchorage-Independent Assay
4.6. RT-qPCR
4.7. Western Blot
4.8. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Ang-(1-9) | Angiotensin 1-9 | |
| Ang-(3-7) | Angiotensin 3-7 | |
| RAS | Renin–angiotensin system | |
| BCL2 | B-cell lymphoma 2 | |
| T | Testosterone | |
| ACE1 | Angiotensin Converting Enzyme-1 | |
| ACE2 | Angiotensin Converting Enzyme-2 | |
| NF-kB | Nuclear Factor kappa B | |
| HIF1a | Hypoxia-inducible factor 1-alpha | |
| ROCK1 | Rho Associated Coiled-Coil Containing Protein Kinase 1 | |
| VEGF | Vascular Endothelial Growth Factor | |
| MAS | Proto-oncogene Mas | |
| AR | Androgen eceptor | |
| ESR1 | Estrogen Receptor 1 | |
| ESR2 | Estrogen Receptor 2 | |
| I1 | Inhibitor AT1; losartan | |
| I2 | Inhibitor AT2; PD123319 | |
| I3 | Inhibitor AT1-7/MAS; A779 | |
| I4 | Inhibitor AT4/IRAP; HIF142 | |
| DHT | Dihydrotestosteron | |
| BIRC5 | Baculoviral IAP Repeat Containing 5; Survivin | |
| BAX | Bcl-2 Associated X-protein | |
| MKI67 | Marker Of Proliferation Ki-67 | |
| AT1 | Angiotensin Receptor Type 1 | |
| AT2 | Angiotensin Receptor Type 2 | |
| AT4/IRAP | Angiotensin Receptor Type 4/Insulin-Regulated Aminopeptidase Enzyme | |
| E2 | Estradiol-17β |
References
- Domińska, K.; Lachowicz-Ochedalska, A. The involvement of the renin–angiotensin system (RAS) in cancerogenesis. Postepy Biochem. 2008, 54, 294–300. [Google Scholar]
- Chow, L.; Rezmann, L.; Catt, K.J.; Louis, W.J.; Frauman, A.G.; Nahmias, C.; Louis, S.N.S. Role of the renin–angiotensin system in prostate cancer. Mol. Cell Endocrinol. 2009, 30, 2219–2229. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, P.E.; Arter, A.L.; Deng, G.; Tallant, E.A. Angiotensin-(1-7): A peptide hormone with anti-cancer activity. Curr. Med. Chem. 2014, 21, 2417–2423. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Fan, J.; Wu, F.; Huang, Q.; Guo, M.; Lv, Z.; Han, J.; Duan, L.; Hu, G.; Chen, L.; et al. The ACE2/Angiotensin-(1–7)/Mas Receptor Axis: Pleiotropic Roles. Cancer Front. Physiol. 2017, 8, 276. [Google Scholar] [CrossRef] [PubMed]
- Ławnicka, H.; Potocka, A.M.; Juzala, A.; Fournie-Zaluski, M.C.; Pawlikowski, M. Angiotensin II and its fragments (angiotensins III and IV) decrease the growth of DU-145 prostate cancer in vitro. Med. Sci. Monit. 2004, 10, 410–413. [Google Scholar]
- Teranishi, J.; Ishiguro, H.; Hoshino, K.; Noguchi, K.; Kubota, Y.; Uemura, H. Evaluation of role of angiotensin III and aminopeptidases in prostate cancer cells. The Prostate 2008, 68, 1666–1673. [Google Scholar] [CrossRef] [PubMed]
- Domińska, K.; Piastowska-Ciesielska, A.W.; Lachowicz-Ochędalska, A.; Ochędalski, T. Similarities and differences between effects of angiotensin III and angiotensin II on human prostate cancer cell migration and proliferation. Peptides 2012, 37, 200–206. [Google Scholar] [CrossRef]
- Domińska, K.; Piastowska-Ciesielska, A.W.; Pluciennik, E.; Lachowicz-Ochedalska, A.; Ochedalski, T. A comparison of the effects of Angiotensin IV on androgen-dependent and androgen- independent prostate cancer cell lines J. Renin–angiotensin-Aldosterone Syst. JRAAS 2013, 14, 74–81. [Google Scholar]
- Domińska, K.; Kowalska, K.; Habrowska-Górczyńska, D.E.; Urbanek, K.A.; Ochędalski, T.; Piastowska-Ciesielska, A.W. The Opposite Effects of Angiotensin 1-9 and Angiotensin 3-7 in Prostate Epithelial Cells. Biochem. Biophys. Res. Commun. 2019, 519, 868–873. [Google Scholar] [CrossRef]
- Bosland, M.C. The Role of Estrogens in Prostate Carcinogenesis: A Rationale for Chemoprevention. Rev. Urol. 2005, 7, S4–S10. [Google Scholar]
- Nelles, J.L.; Hu, W.Y.; Prins, G.S. Estrogen action and prostate cancer. Expert Rev. Endocrinol. Metab. 2011, 6, 437–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowalska, K.; Piastowska-Ciesielska, A.W. Oestrogens and oestrogen receptors in prostate cancer. SpringerPlus 2016, 5, 522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domińska, K.; Okła, P.; Kowalska, K.; Habrowska-Górczyńska, D.; Urbanek, K.A.; Ochędalski, T.; Piastowska-Ciesielska, A.W. Angiotensin 1–7 modulates molecular and cellular processes central to the pathogenesis of prostate cancer. Sci. Rep. 2018, 8, 15772. [Google Scholar] [CrossRef] [Green Version]
- Domińska, K. The importance of the local renin–angiotensin system in the pathology of prostate. Folia Med. Lodz. 2009, 36, 73–86. [Google Scholar]
- Domińska, K.; Okła, P.; Kowalska, K.; Habrowska-Górczyńska, D.; Urbanek, K.A.; Ochędalski, T.; Piastowska-Ciesielska, A.W. Influence and mechanism of Angiotensin 1–7 on biological properties of normal prostate epithelial cells. Biochem. Biophys. Res. Commun. 2018, 502, 152–159. [Google Scholar] [CrossRef]
- Handa, R.K. Angiotensin-(1-7) can interact with the rat proximal tubule AT4 receptor system. Am. J. Physiol. 1999, 277, F75–F83. [Google Scholar] [CrossRef]
- O’Donnell, J.L.; Joyce, M.R.; Shannon, A.M.; Harmey, J.; Geraghty, J.; Bouchier-Hayes, D. Oncological Implications of Hypoxia Inducible factor-1alpha (HIF-1alpha) Expression. Cancer Treat. Rev. 2006, 32, 407–416. [Google Scholar] [CrossRef]
- Gian, J.; Bai, H.; Gao, Z.; Dong, Y.; Pei, J.; Ma, M.; Han, B. Downregulation of HIF-1α inhibits the proliferation and invasion of non-small cell lung cancer NCI-H157 cells. Oncol. Lett. 2016, 11, 1738–1744. [Google Scholar] [CrossRef] [Green Version]
- Masoud, G.N.; Li, W. HIF-1α pathway: Role, regulation and intervention for cancer therapy. Acta. Pharm. Sin. B 2015, 5, 378–389. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.; Li, X.; Wang, J.; Wang, Y.; Dong, H.; Li, J. Genetic variants in RhoA and ROCK1 genes are associated with the development, progression and prognosis of prostate cancer. Oncotarget 2017, 8, 19298–19309. [Google Scholar] [CrossRef] [Green Version]
- Kroiss, A.; Vincent, S.; Decaussin-Petrucci, M.; Meugnier, E.; Viallet, J.; Ruffion, A.; Chalmel, F.; Samarut, J.; Allioli, N. Androgen-regulated microRNA-135a decreases prostate cancer cell migration and invasion through downregulating ROCK1 and ROCK2. Oncogene 2015, 34, 2846–2855. [Google Scholar] [CrossRef]
- Steurer, S.; Hager, B.; Büscheck, F.; Höflmayer, D.; Tsourlakis, M.C.; Minner, S.; Clauditz, T.S.; Hube-Magg, C.; Luebke, A.M.; Simon, R.; et al. Up regulation of Rho-associated coiled-coil containing kinase1 (ROCK1) is associated with genetic instability and poor prognosis in prostate cancer. Aging (Albany NY) 2019, 11, 7859–7879. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, L.J.; Duncan, K.; Yadav, N.; Regan, K.M.; Verone, A.R.; Lohse, C.M.; Pop, E.A.; Attwood, K.; Wilding, G.; Mohler, J.L.; et al. RhoA as a mediator of clinically relevant androgen action in prostate cancer cells. Mol. Endocrinol. 2012, 26, 716–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, H.; Zhou, L.; Khelfat, L.; Qiu, G.; Wang, Y.; Mao, K.; Chen, W. Rho-Associated Protein Kinase (ROCK) Promotes Proliferation and Migration of PC-3 and DU145 Prostate Cancer Cells by Targeting LIM Kinase 1 (LIMK1) and Matrix Metalloproteinase-2 (MMP-2). Med. Sci. Monit. 2019, 25, 3090–3099. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Breslin, J.W.; Zhu, J.; Yuan, S.Y.; Wu, M.H. Rho and ROCK Signaling in VEGF-induced Microvascular Endothelial Hyperpermeability. Microcirculation 2006, 13, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Bryan, B.A.; Dennstedt, B.; Mitchell, D.C.; Walshe, T.E.; Noma, K.; Loureiro, R.; Saint-Geniez, M.; Campaigniac, J.P.; Liao, J.K.; D’Amore, P.A. RhoA/ROCK signaling is essential for multiple aspects of VEGF-mediated angiogenesis. FASEB J. 2010, 24, 3186–3195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, J.R.; Lho, Y.; Connelly, L.; Wang, Y.; Yu, X.; Jean, L.S.; Case, T.C.; Ellwood-Yen, K.; Sawyers, C.L.; Bhowmick, N.A.; et al. The NF-κB Pathway Controls Progression of Prostate Cancer to Androgen Independent Growth. Cancer Res. 2008, 68, 6762–6769. [Google Scholar] [CrossRef] [Green Version]
- McCall, P.; Bennett, L.; Ahmad, I.; MacKenzie, L.M.; Forbes, I.W.G.; Leung, H.Y.; Sansom, O.J.; Orange, C.; Seywright, M.; Underwood, M.A.; et al. NFκB signalling is upregulated in a subset of castrate-resistant prostate cancer patients and correlates with disease progression. Br. J. Cancer 2012, 107, 1554–1563. [Google Scholar] [CrossRef] [Green Version]
- Verzella, D.; Fischietti, M.; Capece, D.; Vecchiotti, D.; Vecchio, F.D.; Cicciarelli, G.; Mastroiaco, V.; Tessitore, A.; Alesse, E.; Zazzeroni, F. Targeting the NF-κB Pathway in Prostate Cancer: A Promising Therapeutic Approach? Curr. Drug Targets 2016, 17, 311–320. [Google Scholar] [CrossRef]
- Staal, J.; Beyaert, R. Inflammation and NF-κB Signaling in Prostate Cancer: Mechanisms and Clinical Implications. Cells 2018, 7, 122. [Google Scholar] [CrossRef] [Green Version]
- Mishra, J.S.; Hankins, G.D.; Kumar, S. Testosterone downregulates angiotensin II type-2 receptor via androgen receptor-mediated ERK1/2 MAP kinase pathway in rat aorta. J. Renin Angiotensin Aldosterone Syst. 2016, 17, 1470320316674875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domińska, K.; Kowalski, A.; Ochędalski, T.; Rębas, E. Effects of Testosterone and 17β estradiol on angiotensin induced Changes in Tyrosine Kinase Activity in the androgen independent Human Prostate Cancer Cell Line, DU145. Int. J. Mol. Med. 2017, 40, 1573–1581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domińska, K.; Ochędalski, T.; Kowalska, K.; Matysiak-Burzyńska, Z.E.; Płuciennik, E.; Piastowska-Ciesielska, A.W. Interaction Between Angiotensin II and Relaxin 2 in the Progress of Growth and Spread of Prostate Cancer Cells. Int. J. Oncol. 2016, 48, 2619–2628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capper, C.P.; Rae, J.M.; Auchus, R.J. The metabolism, analysis, and targeting of steroid hormones in breast and prostate cancer. Horm. Cancer. 2016, 7, 149–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armandari, I.; Hamid, A.R.; Verhaegh, G.; Schalken, J. Intratumoral steroidogenesis in castration-resistant prostate cancer: A target for therapy. Prostate Int. 2014, 2, 105–113. [Google Scholar] [CrossRef] [Green Version]
- Payne, A.H.; Hales, D.B. Overview of Steroidogenic Enzymes in the Pathway From Cholesterol to Active Steroid Hormones. Endocr. Rev. 2004, 25, 947–970. [Google Scholar] [CrossRef]
- Kumazawa, T.; Tsuchiya, N.; Wang, L.; Sato, K.; Kamoto, T.; Ogawa, O.; Nakamura, A.; Kato, T.; Habuchi, T. Microsatellite polymorphism of steroid hormone synthesis gene CYP11A1 is associated with advanced prostate cancer. Int. J. Cancer 2004, 110, 140–144. [Google Scholar] [CrossRef]
- Stanford, J.L.; Noonan, E.A.; Iwasaki, L.; Kolb, S.; Chadwick, R.B.; Feng, Z.; Ostrander, E.A. A Polymorphism in the CYP17 Gene and Risk of Prostate Cancer. Cancer Epidemiol. Biomarkers Prev. 2002, 11, 243–247. [Google Scholar]
- Sivoňová, M.K.; Jurečeková, J.; Tatarková, Z.; Kaplán, P.; Lichardusová, L.; Hatok, J. The role of CYP17A1 in prostate cancer development: Structure, function, mechanism of action, genetic variations and its inhibition. Gen. Physiol. Biophys. 2017, 36, 487–499. [Google Scholar] [CrossRef] [Green Version]
- Gomez, L.; Kovac, J.R.; Lamb, D.J. CYP17A1 Inhibitors in Castration-Resistant Prostate Cancer. Steroids 2015, 95, 80–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Margiotti, K.; Kim, E.; Pearce, C.L.; Spera, E.; Novelli, G.; Reichardt, J.K.V. Association of the G289S Single Nucleotide Polymorphism in the HSD17B3 Gene With Prostate Cancer in Italian Men. Prostate 2002, 53, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Hettel, D.; Sharifi, N. HSD3B1 Status as a Biomarker of Androgen Deprivation Resistance and Implications for Prostate Cancer. Nat. Rev. Urol. 2018, 15, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Wilson, E.M. More evidence intratumoral DHT synthesis drives castration-resistant prostate cancer. Asian J. Androl. 2014, 16, 99–101. [Google Scholar] [CrossRef]
- Tan, T.; Yu, R.M.K.; Wu, R.S.S.; Kong, R.Y.C. Overexpression and Knockdown of Hypoxia-Inducible Factor 1 Disrupt the Expression of Steroidogenic Enzyme Genes and Early Embryonic Development in Zebrafish. Gene Regul. Syst. Biol. 2017, 11, 1177625017713193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanamura, T.; Niwa, T.; Gohno, T.; Kurosumi, M.; Takei, H.; Yamaguchi, Y.; Ito, K.; Hayashi, S. Possible Role of the Aromatase-Independent Steroid Metabolism Pathways in Hormone Responsive Primary Breast Cancers. Breast Cancer Res. Treat. 2014, 143, 69–80. [Google Scholar] [CrossRef]
- Ishizaki, F.; Nishiyama, T.; Kawasaki, T.; Miyashiro, Y.; Hara, N.; Takizawa, I.; Naito, M.; Takahashi, K. Androgen Deprivation Promotes Intratumoral Synthesis of Dihydrotestosterone From Androgen Metabolites in Prostate Cancer. Sci. Rep. 2013, 3, 1528. [Google Scholar] [CrossRef] [Green Version]
- Alimirah, F.; Chen, J.; Basrawala, Z.; Xin, H.; Choubey, D. DU-145 and PC-3 Human Prostate Cancer Cell Lines Express Androgen Receptor: Implications for the Androgen Receptor Functions and Regulation. FEBS Lett. 2006, 580, 2294–3000. [Google Scholar] [CrossRef] [Green Version]
- Song, C.; Kim, Y.; Min, G.E.; Ahn, H. Dihydrotestosterone Enhances Castration-Resistant Prostate Cancer Cell Proliferation Through STAT5 Activation via Glucocorticoid Receptor Pathway. Prostate 2014, 74, 1240–1248. [Google Scholar] [CrossRef]
- Domińska, K.; Kowalska, K.; Matysiak, Z.E.; Płuciennik, E.; Ochędalski, T.; Piastowska-Ciesielska, A.W. Regulation of mRNA gene expression of members of the NF-κB transcription factor gene family by angiotensin II and relaxin 2 in normal and cancer prostate cell lines. Mol. Med. Rep. 2017, 15, 4352–4359. [Google Scholar] [CrossRef] [Green Version]
- Kowalska, K.; Habrowska-Górczyńska, D.E.; Domińska, K.; Piastowska-Ciesielska, A.W. The dose-dependent effect of zearalenone on mitochondrial metabolism, plasma membrane permeabilization and cell cycle in human prostate cancer cell lines. Chemosphere 2017, 180, 455–466. [Google Scholar] [CrossRef]









© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domińska, K.; Kowalska, K.; Urbanek, K.A.; Habrowska-Górczyńska, D.E.; Ochędalski, T.; Piastowska Ciesielska, A.W. The Impact of Ang-(1-9) and Ang-(3-7) on the Biological Properties of Prostate Cancer Cells by Modulation of Inflammatory and Steroidogenesis Pathway Genes. Int. J. Mol. Sci. 2020, 21, 6227. https://doi.org/10.3390/ijms21176227
Domińska K, Kowalska K, Urbanek KA, Habrowska-Górczyńska DE, Ochędalski T, Piastowska Ciesielska AW. The Impact of Ang-(1-9) and Ang-(3-7) on the Biological Properties of Prostate Cancer Cells by Modulation of Inflammatory and Steroidogenesis Pathway Genes. International Journal of Molecular Sciences. 2020; 21(17):6227. https://doi.org/10.3390/ijms21176227
Chicago/Turabian StyleDomińska, Kamila, Karolina Kowalska, Kinga Anna Urbanek, Dominika Ewa Habrowska-Górczyńska, Tomasz Ochędalski, and Agnieszka Wanda Piastowska Ciesielska. 2020. "The Impact of Ang-(1-9) and Ang-(3-7) on the Biological Properties of Prostate Cancer Cells by Modulation of Inflammatory and Steroidogenesis Pathway Genes" International Journal of Molecular Sciences 21, no. 17: 6227. https://doi.org/10.3390/ijms21176227
APA StyleDomińska, K., Kowalska, K., Urbanek, K. A., Habrowska-Górczyńska, D. E., Ochędalski, T., & Piastowska Ciesielska, A. W. (2020). The Impact of Ang-(1-9) and Ang-(3-7) on the Biological Properties of Prostate Cancer Cells by Modulation of Inflammatory and Steroidogenesis Pathway Genes. International Journal of Molecular Sciences, 21(17), 6227. https://doi.org/10.3390/ijms21176227