Chemopreventive Effect of Dietary Anthocyanins against Gastrointestinal Cancers: A Review of Recent Advances and Perspectives
Abstract
:1. Introduction
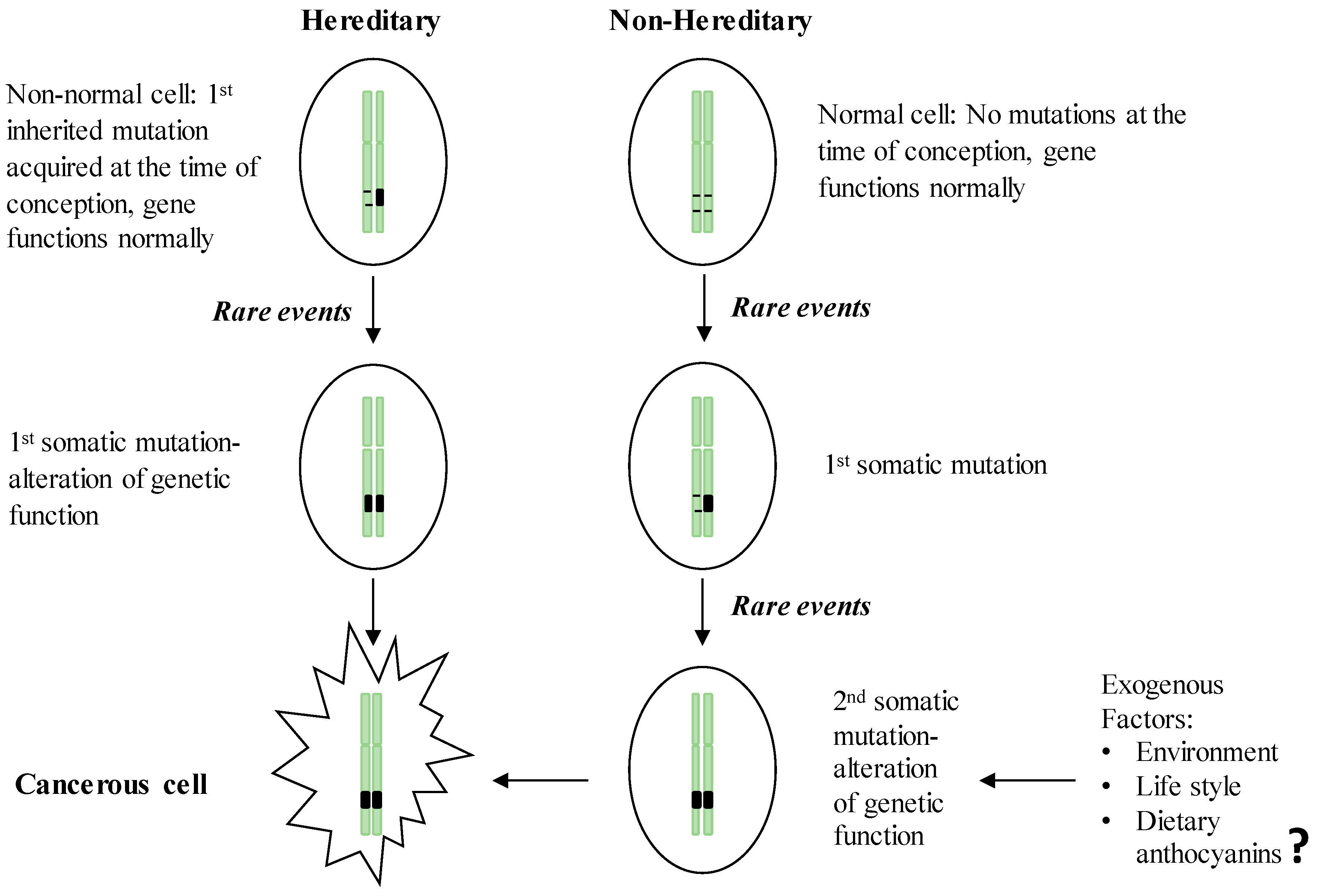
2. GI Carcinogenesis
2.1. Hereditary GI Cancers
2.2. Non-Hereditary GI Cancers
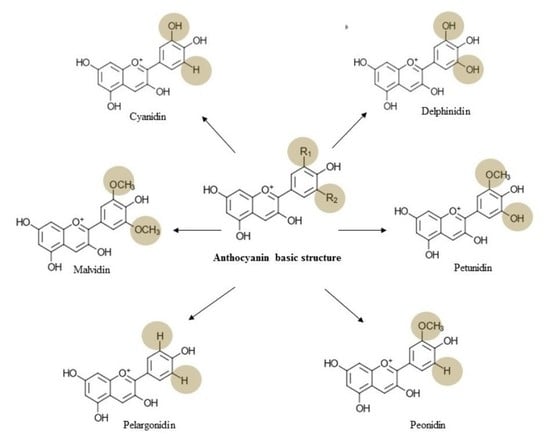
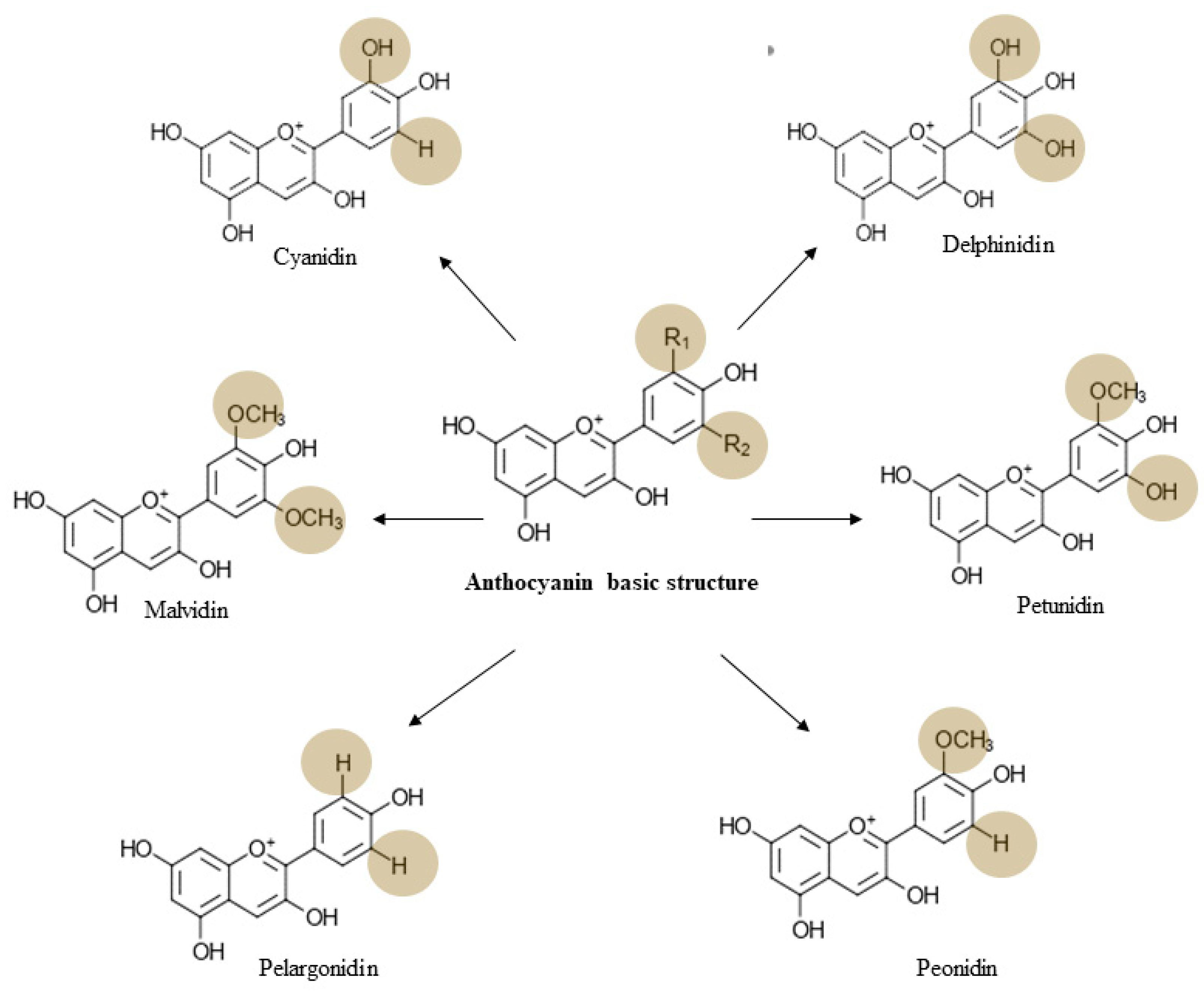
3. Chemistry, Dietary Sources, Bioavailability, and Toxicology of Anthocyanin
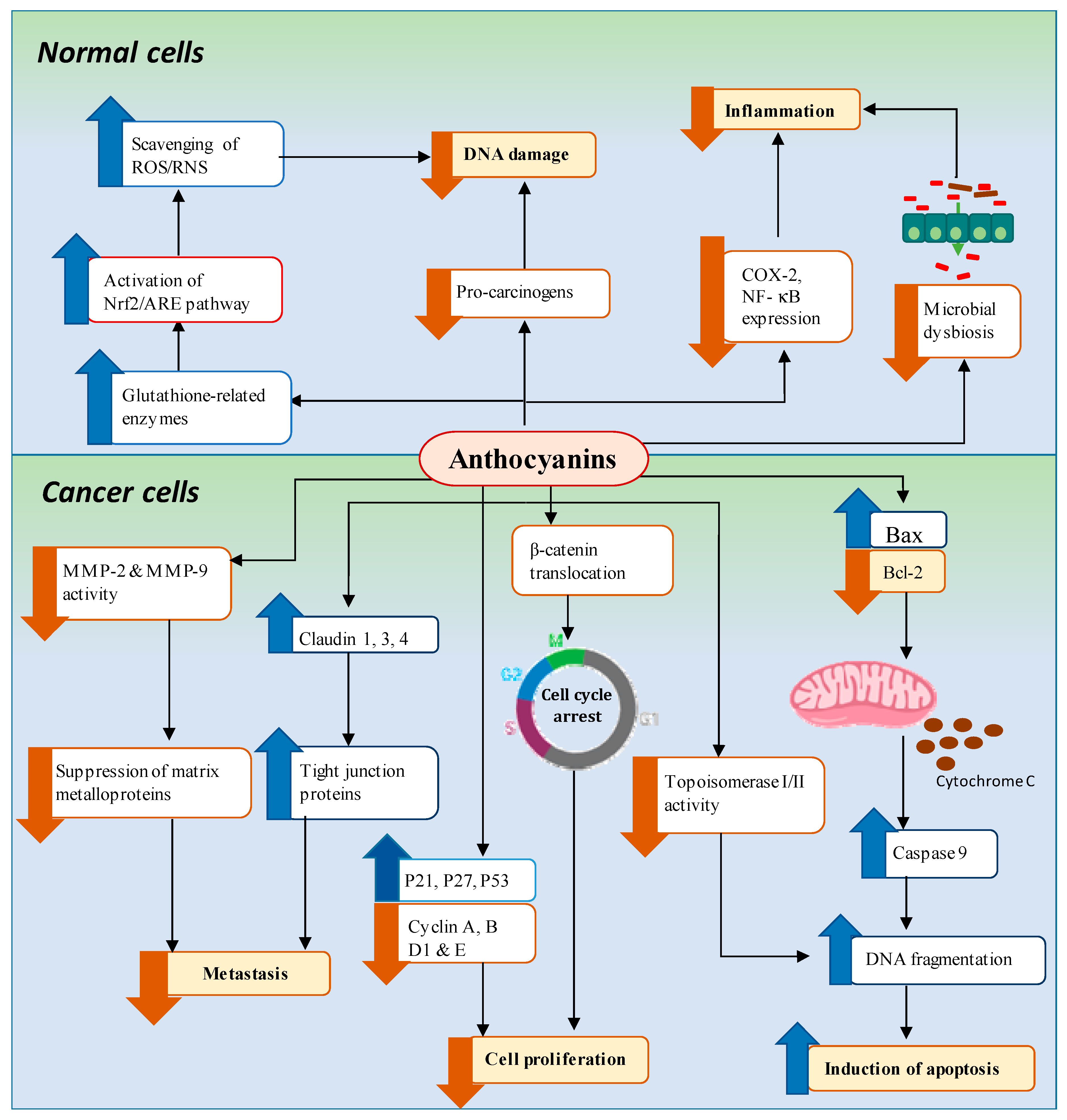
4. Mechanisms of Anthocyanin-Mediated Chemoprevention of GI Cancers
4.1. Downregulation of Pro-Inflammation and Oxidative Stress Associated with DNA Damage
4.1.1. Pro-Inflammation
4.1.2. Oxidative Stress Associated with DNA Damage
4.2. Inhibition of Cancer Cell Proliferation/Induction of Cell Cycle Arrest
4.3. Induction of Apoptosis
4.4. Regulation of Microbial Dysbiosis
5. Anti-GI Cancer Effect of Common Dietary Anthocyanins
5.1. Oral Cancer
5.2. Esophageal Cancer
5.3. Gastric Cancer
5.4. Liver Cancer
5.5. Colorectal Cancer
6. Epidemiological Studies
7. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACF | Aberrant crypt foci |
| AOM | Azoxymethane |
| ARE | Antioxidant response element |
| Bax | B-cell lymphoma-2-like protein 4 |
| Bcl-2 | B-cell lymphoma-2 |
| C3G | Cyanidin-3-O-glucoside |
| CDKs | Cyclin-dependent kinases |
| COX | Cyclooxygenase |
| CRC | Colorectal cancer |
| DNA | Deoxyribose nucleic acid |
| DSS | Dextran sulfate sodium |
| ESCC | Esophageal squamous cell carcinoma |
| FAP | Familial adenomatous polyposis |
| GI | Gastrointestinal |
| GSH | Glutathione-s-transferase |
| HCC | Hepatocellular carcinoma |
| IFN-γ | Interferon-gamma |
| IIR | Intestinal ischemia-reperfusion |
| IL | Interleukin |
| iNOS | Inducible nitric oxide synthase |
| MAPK | Mitogen-activated protein kinase |
| MMP | Matrix metalloproteinase |
| NF-κB | Nuclear factor-kappa B |
| NO | Nitrogen oxide |
| Nrf-2 | Nuclear factor-E2-related factor-2 |
| OIN | Oral intraepithelial neoplasia |
| PCA | protocatechuic acid |
| RNI | Reactive nitrogen intermediates |
| RNS | Reactive nitrogen species |
| ROS | Reactive oxygen species |
| SCC | Squamous cell carcinoma |
| TJ | Tight junction |
| TNF -α | Tumor necrosis factor-alpha |
| ΔΨm | Mitochondrial membrane potential |
References
- George, V.C.; Dellaire, G.; Rupasinghe, H.P.V. Plant flavonoids in cancer chemoprevention: Role in genome stability. J. Nutr. Biochem. 2017, 45, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Technical Report: WHO Report on Cancer: Setting Priorities, Investing Wisely and Providing Care for All; World Health Organization: Geneva, Switzerland, 2020.
- Arnold, M.; Abnet, C.C.; Neale, R.E.; Vignat, J.; Edward, L.; Mcglynn, K.A.; Bray, F. Global burden of 5 major types of gastrointestinal cancer. Gastroenterology 2020, 159, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Court, C.M.; Ankeny, J.S.; Sho, S.; Tomlinson, J.S. Circulating tumor cells in gastrointestinal cancer: Current practices and future directions. Cancer Treat. Res. 2016, 168, 345–376. [Google Scholar] [PubMed]
- Vedeld, H.M.; Goel, A.; Lind, G.E. Epigenetic biomarkers in gastrointestinal cancers: The current state and clinical perspectives. Semin. Cancer Biol. 2018, 51, 36–49. [Google Scholar] [CrossRef]
- Patel, T.N.; Roy, S.; Ravi, R. Gastric cancer and related epigenetic alterations. Ecancermedicalscience 2017, 11, 714. [Google Scholar] [CrossRef]
- Al-Ishaq, R.K.; Overy, A.J.; Büsselberg, D. Phytochemicals and gastrointestinal cancer: Cellular mechanisms and effects to change cancer progression. Biomolecules 2020, 10, 105. [Google Scholar] [CrossRef] [Green Version]
- Wei, D.; Kanai, M.; Huang, S.; Xie, K. Emerging role of KLF4 in human gastrointestinal cancer. Carcinogenesis 2006, 27, 23–31. [Google Scholar] [CrossRef] [Green Version]
- Ida, S.; Watanabe, M.; Baba, H. Chronic inflammation and gastrointestinal cancer. J. Cancer Metastasis Treat. 2015, 1, 138. [Google Scholar]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, 359–386. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, T.; Chen, G.Y. Flavonoids and colorectal cancer prevention. Antioxidants 2018, 7, 187. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-García, C.; Sánchez-Quesada, C.; Gaforio, J.J.; Gaforio, J.J. Dietary flavonoids as cancer chemopreventive agents: An updated review of human studies. Antioxidants 2019, 8, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozłowska, A.; Szostak-Węgierek, D. Flavonoids–Food Sources, Health Benefits, and Mechanisms Involved. In Bioactive Molecules in Food; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–27. [Google Scholar]
- Yao, L.H.; Jiang, Y.M.; Shi, J.; Tomás-Barberán, F.A.; Datta, N.; Singanusong, R.; Chen, S.S. Flavonoids in food and their health benefits. Plant Foods Hum. Nutr. 2004, 59, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Cruz, S.; Chaparro-Hernández, S.; Ruiz, K.L.H.; Cira-Chávez, L.A.; Estrada-Alvarado, M.I.; Ortega, L.E.G.; Ornelas-Paz, J.J.; Mata, M.A.L. Flavonoids: Important Biocompounds in Food. In Flavonoids—From Biosynthesis to Human Health; InTech: London, UK, 2017. [Google Scholar]
- Rupasinghe, H.P.V.; Arumuggam, N. Health Benefits of Anthocyanins. In Food Chemistry, Function and Analysis; Royal Society of Chemistry: London, UK, 2019; pp. 123–158. [Google Scholar]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, J. Classification of fruits based on anthocyanin types and relevance to their health effects. Nutrition 2015, 31, 1301–1306. [Google Scholar] [CrossRef]
- Benetou, V.; Lagiou, A.; Lagiou, P. Chemoprevention of cancer: Current evidence and future prospects. F1000Research 2015, 4, 916. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Wang, P.; Luo, Y.; Zhao, M.; Chen, F. Health benefits of anthocyanins and molecular mechanisms: Update from recent decade. Crit. Rev. Food Sci. Nutr. 2017, 57, 1729–1741. [Google Scholar] [CrossRef]
- De Sousa Moraes, L.F.; Sun, X.F.; Peluzio, M.d.C.G.; Zhu, M.J. Anthocyanins/anthocyanidins and colorectal cancer: What is behind the scenes? Crit. Rev. Food Sci. Nutr. 2017, 1–13. [Google Scholar]
- Forester, S.C.; Choy, Y.Y.; Waterhouse, A.L.; Oteiza, P.I. The anthocyanin metabolites gallic acid, 3-O-methylgallic acid, and 2,4,6-trihydroxybenzaldehyde decrease human colon cancer cell viability by regulating pro-oncogenic signals. Mol. Carcinog. 2014, 53, 432–439. [Google Scholar] [CrossRef]
- Global Cancer Facts & Figures 4th Edition; American Cancer Society: Atlanta, GA, USA, 2018.
- Katona, B.W.; Lynch, J.P. Mechanisms of Gastrointestinal Malignancies. In Physiology of the Gastrointestinal Tract: Sixth Edition; Elsevier Inc: Cambridge, MN, USA, 2018; Volume 2, pp. 1615–1642. [Google Scholar]
- Thirumurthi, S.; Vilar, E.; Lynch, P.J. Hereditary Gastrointestinal Cancers. In Textbook of Gastrointestinal Oncology; Springer: Cham, Switzerland, 2019; pp. 595–611. [Google Scholar]
- Rahner, N.; Steinke, V. Hereditary cancer syndromes. Dtsch. Arztebl. 2008, 105, 706–714. [Google Scholar] [CrossRef]
- Coleman, W.B. Neoplasia. In Molecular Pathology: The Molecular Basis of Human Disease; Elsevier Inc.: Cambridge, MN, USA, 2018; pp. 71–97. [Google Scholar]
- Knudson, A.G. Mutation and cancer: Statistical study of retinoblastoma. Proc. Natl. Acad. Sci. USA 1971, 68, 820–823. [Google Scholar] [CrossRef] [Green Version]
- Van Nistelrooij, A.M.J.; Dinjens, W.N.M.; Wagner, A.; Spaander, M.C.W.; van Lanschot, J.J.B.; Wijnhoven, B.P.L. Hereditary factors in esophageal adenocarcinoma. Gastrointest. Tumors 2014, 1, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Ellis, A.; Risk, J.M.; Maruthappu, T.; Kelsell, D.P. Tylosis with oesophageal cancer: Diagnosis, management and molecular mechanisms. Orphanet. J. Rare Dis. 2015, 10, 126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colvin, H.; Yamamoto, K.; Wada, N.; Mori, M. Hereditary gastric cancer syndromes. Surg. Oncol. Clin. N. Am. 2015, 24, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, H.; Kobayashi, S.; Gotoh, K.; Noda, T.; Doki, Y. Characteristics of early-onset pancreatic cancer and its association with familial pancreatic cancer and hereditary pancreatic cancer syndromes. Ann. Gastroenterol. Surg. 2020, 4, 229–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wells, K.; Wise, P.E. Hereditary colorectal cancer syndromes. Surg. Clin. N. Am. 2017, 97, 605–625. [Google Scholar] [CrossRef] [PubMed]
- Kanth, P.; Grimmett, J.; Champine, M.; Burt, R.; Samadder, J.N. Hereditary colorectal polyposis and cancer syndromes: A primer on diagnosis and management. Am. J. Gastroenterol. 2017, 112, 1509–1525. [Google Scholar] [CrossRef]
- Shenoy, S. Genetic risks and familial associations of small bowel carcinoma. World J. Gastrointest. Oncol. 2016, 8, 509. [Google Scholar] [CrossRef]
- Vanier, M.T. Niemann-Pick diseases. In Handbook of Clinical Neurology; Elsevier B.V.: Berlin/Heidelberg, Germany, 2013; Volume 113, pp. 1717–1721. [Google Scholar]
- Yang Chou, J.; Mansfield, B.C. Molecular genetics of type 1 glycogen storage diseases. Trends Endocrinol. Metab. 1999, 10, 104–113. [Google Scholar] [CrossRef]
- Villanueva, A.; Newell, P.; Hoshida, Y. Inherited hepatocellular carcinoma. Best Pract. Res. Clin. Gastroenterol. 2010, 24, 725–734. [Google Scholar] [CrossRef]
- Chang, I.J.; Hahn, S.H. The genetics of Wilson disease. In Handbook of Clinical Neurology; Elsevier B.V.: Berlin/Heidelberg, Germany, 2017; Volume 142, pp. 19–34. [Google Scholar]
- MacIas, I.; Laín, A.; Bernardo-Seisdedos, G.; Gil, D.; Gonzalez, E.; Falcon-Perez, J.M.; Millet, O. Hereditary tyrosinemia type I-associated mutations in fumarylacetoacetate hydrolase reduce the enzyme stability and increase its aggregation rate. J. Biol. Chem. 2019, 294, 13051–13060. [Google Scholar] [CrossRef] [Green Version]
- Gidi, A.D.G.; González-Chávez, M.A.; Villegas-Tovar, E.; Visag-Castillo, V.; Pantoja-Millan, J.P.; Vélez-Pérez, F.M.; Cano-García, F.; Contreras, A.G. An unusual type of biliar cyst: A case report. Ann. Hepatol. 2016, 15, 788–794. [Google Scholar] [PubMed]
- Moss, A.; Nalankilli, K. The association between diet and colorectal cancer risk: Moving beyond generalizations. Gastroenterology 2017, 152, 1821–1823. [Google Scholar] [CrossRef] [Green Version]
- Mehta, R.S.; Song, M.; Nishihara, R.; Drew, D.A.; Wu, K.; Qian, Z.R.; Fung, T.T.; Hamada, T.; Masugi, Y.; da Silva, A.; et al. Dietary patterns and risk of colorectal cancer: Analysis by tumor location and molecular subtypes. Gastroenterology 2017, 152, 1944–1953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaddy, J.A.; Radin, J.N.; Loh, J.T.; Zhang, F.; Kay Washington, M.; Peek, R.M.; Scott Algood, H.M.; Cover, T.L. Hiharriscagh dietary salt intake exacerbates Helicobacter pylori-induced gastric carcinogenesis. Infect. Immun. 2013, 81, 2258–2267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Elia, L.; Galletti, F.; Strazzullo, P. Dietary salt intake and risk of gastric cancer. Cancer Treat. Res. 2014, 159, 83–95. [Google Scholar]
- Ulrich, C.M.; Himbert, C.; Holowatyj, A.N.; Hursting, S.D. Energy balance and gastrointestinal cancer: Risk, interventions, outcomes and mechanisms. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 683–698. [Google Scholar] [CrossRef]
- Gunter, M.J.; Riboli, E. Obesity and gastrointestinal cancers—Where do we go from here? Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 651–652. [Google Scholar] [CrossRef]
- Seitz, H.K.; Stickel, F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nat. Rev. Cancer 2007, 7, 599–612. [Google Scholar] [CrossRef]
- Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; El Ghissassi, F.; Bouvard, V.; Altieri, A.; Cogliano, V. Carcinogenicity of alcoholic beverages. Lancet Oncol. 2007, 8, 292–293. [Google Scholar] [CrossRef]
- Sierra, J.C.; Piazuelo, M.B.; Luis, P.B.; Barry, D.P.; Allaman, M.M.; Asim, M.; Sebrell, T.A.; Finley, J.L.; Rose, K.L.; Hill, S.; et al. Spermine oxidase mediates Helicobacter pylori-induced gastric inflammation, DNA damage, and carcinogenic signaling. Oncogene 2020, 1–10. [Google Scholar] [CrossRef]
- Huang, F.L.; Yu, S.J. Esophageal cancer: Risk factors, genetic association, and treatment. Asian J. Surg. 2018, 41, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Giovannucci, E.; Fuchs, C.S.; Michaud, D.S. Passive smoking and pancreatic cancer in women: A prospective cohort study. Cancer Epidemiol. Biomarkers Prev. 2009, 18, 2292–2296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Praud, D.; Rota, M.; Pelucchi, C.; Bertuccio, P.; Rosso, T.; Galeone, C.; Zhang, Z.F.; Matsuo, K.; Ito, H.; Hu, J.; et al. Cigarette smoking and gastric cancer in the Stomach Cancer Pooling (StoP) Project. Eur. J. Cancer Prev. 2018, 27, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Moy, K.A.; Fan, Y.; Wang, R.; Gao, Y.T.; Yu, M.C.; Yuan, J.M. Alcohol and tobacco use in relation to gastric cancer: A prospective study of men in Shanghai, China. Cancer Epidemiol. Biomark. Prev. 2010, 19, 2287–2297. [Google Scholar] [CrossRef] [Green Version]
- Sjödahl, K.; Jansson, C.; Bergdahl, I.A.; Adami, J.; Boffetta, P.; Lagergren, J. Airborne exposures and risk of gastric cancer: A prospective cohort study. Int. J. Cancer. 2007, 120, 2013–2018. [Google Scholar] [CrossRef]
- De Oliveira, G.A.; Cheng, R.Y.S.; Ridnour, L.A.; Basudhar, D.; Somasundaram, V.; McVicar, D.W.; Monteiro, H.P.; Wink, D.A. Inducible nitric oxide synthase in the carcinogenesis of gastrointestinal cancers. Antioxid. Redox Signal. 2017, 26, 1059–1077. [Google Scholar] [CrossRef]
- Adam, M.A.A.; Tabana, Y.M.; Musa, K.B.; Sandai, D.A. Effects of different mycotoxins on humans, cell genome and their involvement in cancer (Review). Oncol. Rep. 2017, 37, 1321–1336. [Google Scholar] [CrossRef] [Green Version]
- Marchese, S.; Polo, A.; Ariano, A.; Velotto, S.; Costantini, S.; Severino, L. Aflatoxin B1 and M1: Biological properties and their involvement in cancer development. Toxins 2018, 10, 214. [Google Scholar] [CrossRef] [Green Version]
- Myburg, R.B.; Dutton, M.F.; Chuturgoon, A.A. Cytotoxicity of fumonisin B1, diethylnitrosamine, and catechol on the SNO esophageal cancer cell line. Environ. Health Perspect. 2002, 110, 813–815. [Google Scholar] [CrossRef]
- Mahmoodi, M.; Alizadeh, A.M.; Sohanaki, H.; Rezaei, N.; Amini-Najafi, F.; Khosravi, A.R.; Hosseini, S.K.; Safari, Z.; Hydarnasab, D.; Khori, V. Impact of fumonisin B1 on the production of inflammatory cytokines by gastric and colon cell lines. Iran. J. Allergy Asthma Immunol. 2012, 11, 165–173. [Google Scholar]
- Alfarouk, K.O.; Bashir, A.H.H.; Aljarbou, A.N.; Ramadan, A.M.; Muddathir, A.K.; AlHoufie, S.T.S.; Hifny, A.; Elhassan, G.O.; Ibrahim, M.E.; Alqahtani, S.S.; et al. The possible role of Helicobacter pylori gastric cancer and its management. Front. Oncol. 2019, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Axelrad, J.E.; Lichtiger, S.; Yajnik, V. Inflammatory bowel disease and cancer: The role of inflammation, immunosuppression, and cancer treatment. World J. Gastroenterol. 2016, 22, 4794–4801. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Baby, D.; Rajguru, J.; Patil, P.; Thakkannavar, S.; Pujari, V. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, F.U.; Chhipa, A.S.; Sagar, N.; Pathak, C. Oxidative Stress and Inflammation Can Fuel Cancer. In Role of Oxidative Stress in Pathophysiology of Diseases; Springer: Singapore, 2020; pp. 229–258. [Google Scholar]
- Yu, J.H.; Kim, H. Oxidative stress and cytokines in the pathogenesis of pancreatic cancer. J. Cancer Prev. 2014, 19, 97–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Federico, A.; Morgillo, F.; Tuccillo, C.; Ciardiello, F.; Loguercio, C. Chronic inflammation and oxidative stress in human carcinogenesis. Int. J. Cancer 2007, 121, 2381–2386. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, J.; Lysaght, J.; Donohoe, C.L.; Reynolds, J.V. Obesity and gastrointestinal cancer: The interrelationship of adipose and tumour microenvironments. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 699–714. [Google Scholar] [CrossRef] [PubMed]
- Stickel, F.; Schuppan, D.; Hahn, E.G.; Seitz, H.K. Cocarcinogenic effects of alcohol in hepatocarcinogenesis. Gut 2002, 51, 132–139. [Google Scholar] [CrossRef] [Green Version]
- Schroeder, B.O.; Birchenough, G.M.H.; Ståhlman, M.; Arike, L.; Johansson, M.E.V.; Hansson, G.C.; Bäckhed, F. Bifidobacteria or fiber protects against diet-induced microbiota-mediated colonic mucus deterioration. Cell Host Microbe 2018, 23, 27–40. [Google Scholar] [CrossRef] [Green Version]
- Weng, M.T.; Chiu, Y.T.; Wei, P.Y.; Chiang, C.W.; Fang, H.L.; Wei, S.C. Microbiota and gastrointestinal cancer. J. Formos. Med. Assoc. 2019, 118, 32–41. [Google Scholar] [CrossRef]
- Amararathna, M.; Johnston, M.R.; Rupasinghe, H.P.V. Plant polyphenols as chemopreventive agents for lung cancer. Int. J. Mol. Sci. 2016, 17, 1352. [Google Scholar] [CrossRef] [Green Version]
- Sayed, M.; ElHamid Mahmou, A.A. Cancer chemoprevention by dietary polyphenols. In Carcinogenesis; InTech: London, UK, 2013. [Google Scholar]
- Fernandes, I.; de Freitas, V.; Mateus, N. Anthocyanins and human health: How gastric absorption may influence acute human physiology. Nutr. Aging 2014, 2, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Engwa, G.A. Free radicals and the role of plant phytochemicals as antioxidants against oxidative stress-related diseases. In Phytochemicals—Source of Antioxidants and Role in Disease Prevention; InTech: London, UK, 2018. [Google Scholar]
- Yang, C.S.; Landau, J.M.; Huang, M.T.; Newmark, H.L. Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu. Rev. Nutr. 2001, 21, 381–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Yang, D.Y.; Yang, L.Q.; Zhao, W.Z.; Cai, L.Y.; Shi, H.P. Anthocyanin consumption and risk of colorectal cancer: A meta-analysis of observational studies. J. Am. Coll. Nutr. 2019, 38, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Shin, A.; Oh, J.H.; Kim, J. Colors of vegetables and fruits and the risks of colorectal cancer. World J. Gastroenterol. 2017, 23, 2527–2538. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zeng, X.T.; Liu, T.Z.; Zhang, C.; Yang, Z.H.; Li, S.; Chen, X.Y. Fruits and vegetables intake and risk of bladder cancer: A PRISMA-compliant systematic review and dose-response meta-analysis of prospective cohort studies. Medicine 2015, 94, 759. [Google Scholar] [CrossRef]
- Larsson, S.C.; Bergkvist, L.; Wolk, A. Fruit and vegetable consumption and incidence of gastric cancer: A prospective study. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1998–2001. [Google Scholar] [CrossRef] [Green Version]
- Clifford, M.N. Anthocyanins—Nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 80, 1063–1072. [Google Scholar] [CrossRef]
- He, J.; Giusti, M.M. Anthocyanins: Natural colorants with health-promoting properties. Annu. Rev. Food Sci. Technol. 2010, 1, 163–187. [Google Scholar] [CrossRef]
- Rupasinghe, H.P.V.; Arumuggam, N.; Amararathna, M.; De Silva, A.B.K.H. The potential health benefits of haskap (Lonicera caerulea L.): Role of cyanidin-3-O-glucoside. J. Funct. Foods. 2018, 44, 24–39. [Google Scholar] [CrossRef]
- Fang, J. Bioavailability of anthocyanins. Drug Metab. Rev. 2014, 46, 508–520. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, D.; Wu, Y.; Wang, D.; Wei, Y.; Wu, J.; Ji, B. Stability and absorption of anthocyanins from blueberries subjected to a simulated digestion process. Int. J. Food Sci. Nutr. 2014, 65, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Huang, S.; Cai, S.; Cao, J.; Han, P. Digestion property and synergistic effect on biological activity of purple rice (Oryza sativa L.) anthocyanins subjected to a simulated gastrointestinal digestion in vitro. Food Res. Int. 2015, 78, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Czank, C.; Cassidy, A.; Zhang, Q.; Morrison, D.J.; Preston, T.; Kroon, P.A.; Botting, N.P.; Kay, C.D. Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: A 13C-tracer study. Am. J. Clin. Nutr. 2013, 97, 995–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mueller, D.; Jung, K.; Winter, M.; Rogoll, D.; Melcher, R.; Richling, E. Human intervention study to investigate the intestinal accessibility and bioavailability of anthocyanins from bilberries. Food Chem. 2017, 231, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Felgines, C.; Talavéra, S.; Texier, O.; Besson, C.; Fogliano, V.; Lamaison, J.L.; la Fauci, L.; Galvano, G.; Rémésy, C.; Galvano, F. Absorption and metabolism of red orange juice anthocyanins in rats. Br. J. Nutr. 2006, 95, 898–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talavéra, S.; Felgines, C.; Texier, O.; Besson, C.; Lamaison, J.L.; Rémésy, C. Anthocyanins are efficiently absorbed from the stomach in anesthetized rats. J. Nutr. 2003, 133, 4178–4182. [Google Scholar] [CrossRef]
- Passamonti, S.; Vrhovsek, U.; Vanzo, A.; Mattivi, F. The stomach as a site for anthocyanins absorption from food. FEBS Lett. 2003, 544, 210–213. [Google Scholar] [CrossRef] [Green Version]
- Novotny, J.A.; Clevidence, B.A.; Kurilich, A.C. Anthocyanin kinetics are dependent on anthocyanin structure. Br. J. Nutr. 2012, 107, 504–509. [Google Scholar] [CrossRef] [Green Version]
- Felgines, C.; Texier, O.; Besson, C.; Vitaglione, P.; Lamaison, J.L.; Fogliano, V.; Scalbert, A.; Vanella, L.; Galvano, F. Influence of glucose on cyanidin 3-glucoside absorption in rats. Mol. Nutr. Food Res. 2008, 52, 959–964. [Google Scholar] [CrossRef]
- Verine Talavé, S.; Felgines, C.; Texier, O.; Besson, C.; Manach, C.; Lamaison, J.L.; Ré, C.; Sy, M. Anthocyanins are efficiently absorbed from the small intestine in rats. Nutr. Metab. 2004, 134, 2275–2279. [Google Scholar]
- Valdez, J.C.; Bolling, B.W. View of anthocyanins and intestinal barrier function: A review. J. Food Bioact. 2019, 5, 18–30. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, I.; Faria, A.; de Freitas, V.; Calhau, C.; Mateus, N. Multiple-approach studies to assess anthocyanin bioavailability. Phytochem. Rev. 2015, 14, 899–919. [Google Scholar] [CrossRef]
- Hidalgo, M.; Oruna-Concha, M.J.; Kolida, S.; Walton, G.E.; Kallithraka, S.; Spencer, J.P.E.; Gibson, G.R.; De Pascual-Teresa, S. Metabolism of anthocyanins by human gut microflora and their influence on gut bacterial growth. J. Agric. Food Chem. 2012, 60, 3882–3890. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, Q.; Zhao, T.; Zhang, Z.; Mao, G.; Feng, W.; Wu, X.; Yang, L. Biotransformation and metabolism of three mulberry anthocyanin monomers by rat gut microflora. Food Chem. 2017, 237, 887–894. [Google Scholar] [CrossRef]
- Hanske, L.; Engst, W.; Loh, G.; Sczesny, S.; Blaut, M.; Braune, A. Contribution of gut bacteria to the metabolism of cyanidin 3-glucoside in human microbiota-associated rats. Br. J. Nutr. 2013, 109, 1433–1441. [Google Scholar] [CrossRef]
- Tsuda, T. Anthocyanins as functional food factors—Chemistry, nutrition and health promotion. Food Sci. Technol. Res. 2012, 18, 315–324. [Google Scholar] [CrossRef] [Green Version]
- Amararathna, M.; Hoskin, D.W.; Rupasinghe, H.P.V. Anthocyanin-rich haskap (Lonicera caerulea L.) berry extracts reduce nitrosamine-induced DNA damage in human normal lung epithelial cells in vitro. Food Chem. Toxicol. 2020, 141, 111404. [Google Scholar] [CrossRef]
- Samuels, T.L.; Pearson, A.C.S.; Wells, C.W.; Stoner, G.D.; Johnston, N. Curcumin and anthocyanin inhibit pepsin-mediated cell damage and carcinogenic changes in airway epithelial cells. Ann. Otol. Rhinol. Laryngol. 2013, 122, 632–641. [Google Scholar] [CrossRef]
- Guttenplan, J.B.; Chen, K.M.; Sun, Y.W.; Lajara, B.; Shalaby, N.A.E.; Kosinska, W.; Benitez, G.; Gowda, K.; Amin, S.; Stoner, G.; et al. Effects of black raspberry extract and berry compounds on repair of DNA damage and mutagenesis induced by chemical and physical agents in human oral leukoplakia and rat oral fibroblasts. Chem. Res. Toxicol. 2017, 30, 2159–2164. [Google Scholar] [CrossRef]
- Karlsen, A.; Retterstøl, L.; Laake, P.; Paur, I.; Kjølsrud-Bøhn, S.; Sandvik, L.; Blomhoff, R. Anthocyanins inhibit nuclear factor-kB activation in monocytes and reduce plasma concentrations of pro-inflammatory mediators in healthy adults. J. Nutr. Nutr. Dis. 2007, 137, 1951–1954. [Google Scholar]
- Li, S.; Wu, B.; Fu, W.; Reddivari, L. The anti-inflammatory effects of dietary anthocyanins against ulcerative colitis. Int. J. Mol. Sci. 2019, 20, 2588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cremonini, E.; Daveri, E.; Mastaloudis, A.; Adamo, A.M.; Mills, D.; Kalanetra, K.; Hester, S.N.; Wood, S.M.; Fraga, C.G.; Oteiza, P.I. Anthocyanins protect the gastrointestinal tract from high fat diet-induced alterations in redox signaling, barrier integrity and dysbiosis. Redox Biol. 2019, 26, 101269. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.; Freitas, V.; Almeida, L.; Laranjinha, J. Red wine extract preserves tight junctions in intestinal epithelial cells under inflammatory conditions: Implications for intestinal inflammation. Food Funct. 2019, 10, 1364–1374. [Google Scholar] [CrossRef]
- Sun, X.; Du, M.; Navarre, D.A.; Zhu, M.-J. Purple Potato Extract Promotes Intestinal Epithelial Differentiation and Barrier Function by Activating AMP-Activated Protein Kinase. Mol. Nutr. Food Res. 2018, 62, 1700536. [Google Scholar] [CrossRef]
- Ferrari, D.; Speciale, A.; Cristani, M.; Fratantonio, D.; Molonia, M.S.; Ranaldi, G.; Saija, A.; Cimino, F. Cyanidin-3-O-glucoside inhibits NF-kB signalling in intestinal epithelial cells exposed to TNF-α and exerts protective effects via Nrf2 pathway activation. Toxicol. Lett. 2016, 264, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Yan, Y.; Wan, P.; Chen, D.; Ding, Y.; Ran, L.; Mi, J.; Lu, L.; Zhang, Z.; Li, X.; et al. Gut microbiota modulation and anti-inflammatory properties of anthocyanins from the fruits of Lycium ruthenicum Murray in dextran sodium sulfate-induced colitis in mice. Free Radic. Biol. Med. 2019, 136, 96–108. [Google Scholar] [CrossRef]
- Li, J.; Wu, T.; Li, N.; Wang, X.; Chen, G.; Lyu, X. Bilberry anthocyanin extract promotes intestinal barrier function and inhibits digestive enzyme activity by regulating the gut microbiota in aging rats. Food Funct. 2019, 10, 333–343. [Google Scholar] [CrossRef]
- Rupasinghe, H.P.V.; Boehm, M.; Sekhon-Loodu, S.; Parmar, I.; Bors, B.; Jamieson, A. Anti-inflammatory activity of haskap cultivars is polyphenols-dependent. Biomolecules 2015, 5, 1079–1098. [Google Scholar] [CrossRef]
- Phan, M.A.T.; Bucknall, M.P.; Arcot, J. Interferences of anthocyanins with the uptake of lycopene in Caco-2 cells, and their interactive effects on anti-oxidation and anti-inflammation in vitro and ex vivo. Food Chem. 2019, 276, 402–409. [Google Scholar] [CrossRef]
- Lee, S.G.; Brownmiller, C.R.; Lee, S.O.; Kang, H.W. Anti-inflammatory and antioxidant effects of anthocyanins of Trifolium pratense (red clover) in lipopolysaccharide-stimulated RAW-267.4 macrophages. Nutrients 2020, 12, 1089. [Google Scholar] [CrossRef] [Green Version]
- Limtrakul, P.; Yodkeeree, S.; Pitchakarn, P.; Punfa, W. Suppression of inflammatory responses by black rice extract in RAW 264.7 macrophage cells via downregulation of NF-kB and AP-1 signaling pathways. Asian Pac. J. Cancer Prev. 2015, 16, 4277–4283. [Google Scholar] [CrossRef] [Green Version]
- Domitrovic, R. The molecular basis for the pharmacological activity of anthocyans. Curr. Med. Chem. 2011, 18, 4454–4469. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Yan, Y.; Wan, P.; Dong, W.; Huang, K.; Ran, L.; Mi, J.; Lu, L.; Zeng, X.; Cao, Y. Effects of long-term intake of anthocyanins from Lycium ruthenicum Murray on the organism health and gut microbiota in vivo. Food Res. Int. 2020, 130, 108952. [Google Scholar] [CrossRef] [PubMed]
- Venancio, V.P.; Cipriano, P.A.; Kim, H.; Antunes, L.M.G.; Talcott, S.T.; Mertens-Talcott, S.U. Cocoplum (Chrysobalanus icaco L.) anthocyanins exert anti-inflammatory activity in human colon cancer and non-malignant colon cells. Food Funct. 2017, 8, 307–314. [Google Scholar] [CrossRef]
- Li, L.; Wang, L.; Wu, Z.; Yao, L.; Wu, Y.; Huang, L.; Liu, K.; Zhou, X.; Gou, D. Anthocyanin-rich fractions from red raspberries attenuate inflammation in both RAW264.7 macrophages and a mouse model of colitis. Sci. Rep. 2014, 4, 6234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, L.; Zhang, Y.; Liu, G.; Hao, S.; Wang, C.; Wang, Y. Black rice anthocyanin-rich extract and rosmarinic acid, alone and in combination, protect against DSS-induced colitis in mice. Food Funct. 2018, 9, 2796–2808. [Google Scholar] [CrossRef]
- Ghattamaneni, N.K.; Sharma, A.; Panchal, S.K.; Brown, L. Pelargonidin 3-glucoside-enriched strawberry attenuates symptoms of DSS-induced inflammatory bowel disease and diet-induced metabolic syndrome in rats. Eur. J. Nutr. 2019, 1–14. [Google Scholar] [CrossRef]
- Sies, H. Oxidative Stress: Eustress and Distress in Redox Homeostasis. In Stress: Physiology, Biochemistry, and Pathology; Academic Press: Cambridge, MA, USA, 2019; pp. 153–163. [Google Scholar]
- Ahmed, O.M. Relationships between oxidative stress, cancer development and therapeutic interventions. J. Cancer Sci. Res. 2018, 1, 1. [Google Scholar] [CrossRef]
- Céspedes-Acuña, C.L.; Xiao, J.; Wei, Z.J.; Chen, L.; Bastias, J.M.; Avila, J.G.; Alarcon-Enos, J.; Werner-Navarrete, E.; Kubo, I. Antioxidant and anti-inflammatory effects of extracts from Maqui berry Aristotelia chilensis in human colon cancer cells. J. Berry Res. 2018, 8, 275–296. [Google Scholar] [CrossRef]
- Shih, P.H.; Yeh, C.T.; Yen, G.C. Anthocyanins induce the activation of phase II enzymes through the antioxidant response element pathway against oxidative stress-induced apoptosis. J. Agric. Food Chem. 2007, 55, 9427–9435. [Google Scholar] [CrossRef]
- Yi, L.; Chen, C.Y.; Jin, X.; Mi, M.T.; Yu, B.; Chang, H.; Ling, W.H.; Zhang, T. Structural requirements of anthocyanins in relation to inhibition of endothelial injury induced by oxidized low-density lipoprotein and correlation with radical scavenging activity. FEBS Lett. 2010, 584, 583–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juadjur, A.; Mohn, C.; Schantz, M.; Baum, M.; Winterhalter, P.; Richling, E. Fractionation of an anthocyanin-rich bilberry extract and in vitro antioxidative activity testing. Food Chem. 2015, 167, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Olejnik, A.; Rychlik, J.; Kidoń, M.; Czapski, J.; Kowalska, K.; Juzwa, W.; Olkowicz, M.; Dembczyński, R.; Moyer, M.P. Antioxidant effects of gastrointestinal digested purple carrot extract on the human cells of colonic mucosa. Food Chem. 2016, 190, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Claudio, S.R.; Gollucke, A.P.B.; Yamamura, H.; Morais, D.R.; Bataglion, G.A.; Eberlin, M.N.; Peres, R.C.; Oshima, C.T.F.; Ribeiro, D.A. Purple carrot extract protects against cadmium intoxication in multiple organs of rats: Genotoxicity, oxidative stress and tissue morphology analyses. J. Trace Elem. Med. Biol. 2016, 33, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Su, H.; Xu, Y.; Bao, T.; Zheng, X. Protective effect of wild raspberry (Rubus hirsutus Thunb.) extract against acrylamide-induced oxidative damage is potentiated after simulated gastrointestinal digestion. Food Chem. 2016, 196, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Jakesevic, M.; Aaby, K.; Borge, G.I.A.; Jeppsson, B.; Ahrné, S.; Molin, G. Antioxidative protection of dietary bilberry, chokeberry and Lactobacillus plantarum HEAL19 in mice subjected to intestinal oxidative stress by ischemia-reperfusion. BMC Complement. Altern. Med. 2011, 11, 8. [Google Scholar] [CrossRef] [Green Version]
- Jakesevic, M.; Xu, J.; Aaby, K.; Jeppsson, B.; Ahrné, S.; Molin, G. Effects of bilberry (Vaccinium myrtillus) in combination with lactic acid bacteria on intestinal oxidative stress induced by ischemia-reperfusion in mouse. J. Agric. Food Chem. 2013, 61, 3468–3478. [Google Scholar] [CrossRef]
- Janšáková, K.; Bábíčková, J.; Filová, B.; Lengyelová, E.; Havrlentová, M.; Kraic, J.; Celec, P.; Tóthová, L. Anthocyanin-rich diet in chemically induced colitis in mice. Folia Biol. (Praha) 2015, 61, 104–109. [Google Scholar]
- Thoppil, R.J.; Bhatia, D.; Barnes, K.F.; Haznagy-Radnai, E.; Hohmann, J.; Darvesh, A.S.; Bishayee, A. Black currant anthocyanins abrogate oxidative stress through Nrf2-mediated antioxidant mechanisms in a rat model of hepatocellular carcinoma. Curr. Cancer Drug Targets 2012, 12, 1244–1257. [Google Scholar]
- Visconti, R.; Della Monica, R.; Grieco, D. Cell cycle checkpoint in cancer: A therapeutically targetable double-edged sword. J. Exp. Clin. Cancer Res. 2016, 35, 153. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Kim, W.; Kang, H.G.; Kim, W.J.; Lee, S.C.; Kim, S.J. Geranium thunbergii extract-induced G1 phase cell cycle arrest and apoptosis in gastric cancer cells. Animal Cells Syst. 2020, 24, 26–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Hu, X.; Zuo, X.; Wang, M. Chemopreventive effects of some popular phytochemicals on human colon cancer: A review. Food Funct. 2018, 9, 4548–4568. [Google Scholar] [CrossRef] [PubMed]
- Tavakolian, S.; Goudarzi, H.; Faghihloo, E. Cyclin-dependent kinases and CDK inhibitors in virus-associated cancers. Infect. Agent. Cancer 2020, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.H.; Stoeber, K. The cell cycle and cancer. J. Pathol. 2012, 226, 352–364. [Google Scholar] [CrossRef]
- Seeram, N.P. Berry fruits for cancer prevention: Current status and future prospects. J. Agric. Food Chem. 2008, 56, 630–635. [Google Scholar] [CrossRef]
- Kristo, A.S.; Klimis-Zacas, D.; Sikalidis, A.K. Protective role of dietary berries in cancer. Antioxidants 2016, 5, 37. [Google Scholar] [CrossRef] [Green Version]
- Lin, B.W.; Gong, C.C.; Song, H.F.; Cui, Y.Y. Effects of anthocyanins on the prevention and treatment of cancer. Br. J. Pharmacol. 2017, 174, 1226–1243. [Google Scholar] [CrossRef] [Green Version]
- Malik, M.; Zhao, C.; Schoene, N.; Guisti, M.M.; Moyer, M.P.; Magnuson, B.A. Anthocyanin-rich extract from Aronia meloncarpa E. induces a cell cycle block in colon cancer but not normal colonic cells. Nutr. Cancer 2003, 46, 186–196. [Google Scholar] [CrossRef]
- Qi, C.; Li, S.; Jia, Y.; Wang, L. Blueberry anthocyanins induce G2/M cell cycle arrest and apoptosis of oral cancer KB cells through down-regulation methylation of p53. Yi Chuan 2014, 36, 566–573. [Google Scholar]
- Hsu, C.-P.P.; Shih, Y.-T.T.; Lin, B.-R.R.; Chiu, C.-F.F.; Lin, C.-C.C. Inhibitory effect and mechanisms of an anthocyanins- and anthocyanidins-rich extract from purple-shoot tea on colorectal carcinoma cell proliferation. J. Agric. Food Chem. 2012, 60, 3686–3692. [Google Scholar] [CrossRef]
- Yun, J.-M.M.; Afaq, F.; Khan, N.; Mukhtar, H. Delphinidin, an anthocyanidin in pigmented fruits and vegetables, induces apoptosis and cell cycle arrest in human colon cancer HCT116 cells. Mol. Carcinog. 2009, 48, 260–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, C.; Giusti, M.M.; Malik, M.; Moyer, M.P.; Magnuson, B.A. Effects of commercial anthocyanin-rich on colonic cancer and nontumorigenic colonic cell growth. J. Agric. Food Chem. 2004, 52, 6122–6128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Vareed, S.K.; Nair, M.G. Human tumor cell growth inhibition by nontoxic anthocyanidins, the pigments in fruits and vegetables. Life Sci. 2005, 76, 1465–1472. [Google Scholar] [CrossRef]
- Chen, P.N.; Chu, S.C.; Chiou, H.L.; Chiang, C.L.; Yang, S.F.; Hsieh, Y.S. Cyanidin 3-glucoside and peonidin 3-glucoside inhibit tumor cell growth and induce apoptosis in vitro and suppress tumor growth in vivo. Nutr. Cancer 2005, 53, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Blanco, A.; Blanco, G. Chapter 32—Apoptosis; Academic Press: Cambridge, MA, USA, 2017; pp. 791–796. [Google Scholar]
- Jin, Z.; El-Deiry, W.S. Overview of cell death signaling pathways. Cancer Biol. Ther. 2005, 4, 147–171. [Google Scholar] [CrossRef] [Green Version]
- Fernald, K.; Kurokawa, M. Evading apoptosis in cancer. Trends Cell Biol. 2013, 23, 620–633. [Google Scholar] [CrossRef] [Green Version]
- Baba, A.B.; Nivetha, R.; Chattopadhyay, I.; Nagini, S. Blueberry and malvidin inhibit cell cycle progression and induce mitochondrial-mediated apoptosis by abrogating the JAK/STAT-3 signalling pathway. Food Chem. Toxicol. 2017, 109, 534–543. [Google Scholar] [CrossRef]
- Mazewski, C.; Liang, K.; Gonzalez de Mejia, E. Comparison of the effect of chemical composition of anthocyanin-rich plant extracts on colon cancer cell proliferation and their potential mechanism of action using in vitro, in silico, and biochemical assays. Food Chem. 2018, 242, 378–388. [Google Scholar] [CrossRef]
- Shin, D.Y.; Ryu, C.H.; Lee, W.S.; Kim, D.C.; Kim, S.H.; Hah, Y.-S.S.; Lee, S.J.; Shin, S.C.; Kang, H.S.; Choi, Y.H. Induction of apoptosis and inhibition of invasion in human hepatoma cells by anthocyanins from meoru. Ann. N. Y. Acad. Sci. 2009, 1171, 137–148. [Google Scholar] [CrossRef]
- Jo, W.S.; Jeong, M.-H.; Jin, Y.-H.; Jang, J.Y.; Nam, B.H.; Son, S.H.; Choi, S.S.; Yoo, Y.H.; Kang, C.D.; Lee, J.D.; et al. Loss of mitochondrial membrane potential and caspase activation enhance apoptosis in irradiated K562 cells treated with herbimycin A. Int. J. Radiat. Biol. 2005, 81, 531–543. [Google Scholar] [CrossRef]
- Lazze, M.C.; Savio, M.; Pizzala, R.; Cazzalini, O.; Perucca, P.; Scovassi, A.I.; Stivala, L.A.; Bianchi, L.; Lazz, M.C.; Savio, M.; et al. Anthocyanins induce cell cycle perturbations and apoptosis in different human cell lines. Carcinogenesis 2004, 25, 1427–1433. [Google Scholar] [CrossRef] [PubMed]
- Shih, P.H.; Yeh, C.T.; Yen, G.C. Effects of anthocyanidin on the inhibition of proliferation and induction of apoptosis in human gastric adenocarcinoma cells. Food Chem. Toxicol. 2005, 43, 1557–1566. [Google Scholar] [CrossRef]
- Wu, Q.K.; Koponen, J.M.; Mykkänen, H.M.; Törrönen, A.R. Berry phenolic extracts modulate the expression of p21WAF1 and Bax but Not Bcl-2 in HT-29 colon cancer cells. J. Agric. Food Chem. 2007, 55, 1156–1163. [Google Scholar] [CrossRef] [PubMed]
- Sordet, O.; Khan, Q.A.; Kohn, K.W.; Pommier, Y. Apoptosis induced by topoisomerase inhibitors. Curr. Med. Chem. Anti-Cancer Agents 2003, 3, 271–290. [Google Scholar] [CrossRef] [PubMed]
- Webb, M.R.; Min, K.; Ebeler, S.E. Anthocyanin interactions with DNA: Interactions, topoisomerase I inhibition and oxidative reactions. J. Food Biochem. 2008, 32, 576–596. [Google Scholar] [CrossRef] [PubMed]
- Habermeyer, M.; Fritz, J.; Barthelmes, H.U.; Christensen, M.O.; Larsen, M.K.; Boege, F.; Marko, D. Anthocyanidins modulate the activity of human DNA topoisomerases I and II and affect cellular DNA integrity. Chem. Res. Toxicol. 2005, 18, 1395–1404. [Google Scholar] [CrossRef]
- Esselen, M.; Barth, S.W.; Winkler, S.; Baechler, S.; Briviba, K.; Watzl, B.; Skrbek, S.; Marko, D. Anthocyanins suppress the cleavable complex formation by irinotecan and diminish its DNA-strand-breaking activity in the colon of Wistar rats. Carcinogenesis 2013, 34, 835–840. [Google Scholar] [CrossRef] [Green Version]
- Esselen, M.; Fritz, J.; Hutter, M.; Teller, N.; Baechler, S.; Boettler, U.; Marczylo, T.H.; Gescher, A.J.; Marko, D. Anthocyanin-rich extracts suppress the DNA-damaging effects of topoisomerase poisons in human colon cancer cells. Mol. Nutr. Food Res. 2011, 55, 143–153. [Google Scholar] [CrossRef]
- Esselen, M.; Boettler, U.; Teller, N.; Bächler, S.; Hutter, M.; Rüfer, C.E.; Skrbek, S.; Marko, D. Anthocyanin-rich blackberry extract suppresses the DNA-damaging properties of topoisomerase i and II poisons in colon carcinoma cells. J. Agric. Food Chem. 2011, 59, 6966–6973. [Google Scholar] [CrossRef]
- Wroblewski, L.E.; Peek, R.M.; Coburn, L.A. The role of the microbiome in gastrointestinal cancer. Gastroenterol. Clin. N. Am. 2016, 45, 543–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, P.; Lam, V.; Salzman, N.; Huang, Y.W.; Yu, J.; Zhang, J.; Wang, L.S. Black raspberries and their anthocyanin and fiber fractions alter the composition and diversity of gut microbiota in F-344 rats. Nutr. Cancer 2017, 69, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, T.T.Y.Y.; Tang, Q.; Xue, C.; Li, R.W.; Wu, V.C.H.H. Malvidin 3-glucoside modulated gut microbial dysbiosis and global metabolome disrupted in a murine colitis model induced by dextran sulfate sodium. Mol. Nutr. Food Res. 2019, 63, 1900455. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Lin, J.; Gao, N.; Sun, X.; Meng, X.; Liu, R.; Liu, Y.; Wang, W.; Wang, Y.; Li, B. Blueberry malvidin-3-galactoside modulated gut microbial dysbiosis and microbial TCA cycle KEGG pathway disrupted in a liver cancer model induced by HepG2 cells. Food Sci. Hum. Wellness 2020, in press. [Google Scholar] [CrossRef]
- Khairnar, M.R.; Wadgave, U.; Jadhav, H.; Naik, R. Anticancer activity of chlorhexidine and cranberry extract: An in-vitro study. J. Exp. Ther. Oncol. 2018, 12, 201–205. [Google Scholar]
- Fan, M.J.; Wang, I.C.; Hsiao, Y.T.; Lin, H.Y.; Tang, N.Y.; Hung, T.C.; Quan, C.; Lien, J.C.; Chung, J.G. Anthocyanins from black rice (Oryza sativa L.) demonstrate antimetastatic properties by reducing MMPs and NF-B expressions in human oral cancer CAL 27 cells. Nutr. Cancer 2015, 67, 327–338. [Google Scholar] [CrossRef]
- Yue, E.; Tuguzbaeva, G.; Chen, X.; Qin, Y.; Li, A.; Sun, X.; Dong, C.; Liu, Y.; Yu, Y.; Zahra, S.M.; et al. Anthocyanin is involved in the activation of pyroptosis in oral squamous cell carcinoma. Phytomedicine 2019, 56, 286–294. [Google Scholar] [CrossRef]
- De Moura, C.F.G.; Soares, G.R.; Ribeiro, F.A.P.; Silva, M.J.D.; Vilegas, W.; Santamarina, A.B.; Pisani, L.P.; Estadella, D.; Ribeiro, D.A. Evaluation of the chemopreventive activity of grape skin extract using medium-term oral carcinogenesis assay induced by 4-nitroquinoline 1-oxide. Anticancer Res. 2019, 39, 177–182. [Google Scholar] [CrossRef]
- Casto, B.C.; Knobloch, T.J.; Galioto, R.L.; Yu, Z.; Accurso, B.T.; Warner, B.M. Chemoprevention of oral cancer by lyophilized strawberries. Anticancer Res. 2013, 33, 4757–4766. [Google Scholar]
- Medda, R.; Lyros, O.; Schmidt, J.L.; Jovanovic, N.; Nie, L.; Link, B.J.; Otterson, M.F.; Stoner, G.D.; Shaker, R.; Rafiee, P. Anti-inflammatory and anti angiogenic effect of black raspberry extract on human esophageal and intestinal microvascular endothelial cells. Microvasc. Res. 2015, 97, 167–180. [Google Scholar] [CrossRef] [Green Version]
- Zikri, N.N.; Riedl, K.M.; Wang, L.S.; Lechner, J.; Schwartz, S.J.; Stoner, G.D. Black raspberry components inhibit proliferation, induce apoptosis, and modulate gene expression in rat esophageal epithelial cells. Nutr. Cancer 2009, 61, 816–826. [Google Scholar] [CrossRef] [PubMed]
- Aiyer, H.S.; Li, Y.; Losso, J.N.; Gao, C.; Schiffman, S.C.; Slone, S.P.; Martin, R.C.G. Effect of freeze-dried berries on the development of reflux-induced esophageal adenocarcinoma. Nutr. Cancer 2011, 63, 1256–1262. [Google Scholar] [CrossRef] [PubMed]
- Seguin, C.M.; Wang, L.S.; Stoner, G.D. Chemopreventive effects of berries and berry components in the rodent esophagus. In Berries and Cancer Prevention; Springer: New York, NY, USA, 2011; pp. 143–161. [Google Scholar]
- Wang, L.S.; Hecht, S.S.; Carmella, S.G.; Yu, N.; Larue, B.; Henry, C.; McIntyre, C.; Rocha, C.; Lechner, J.F.; Stoner, G.D. Anthocyanins in black raspberries prevent esophageal tumors in rats. Cancer Prev. Res. 2009, 2, 84–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoner, G.D.; Wang, L.S.; Seguin, C.; Rocha, C.; Stoner, K.; Chiu, S.; Kinghorn, A.D. Multiple berry types prevent N-nitrosomethylbenzylamine-induced esophageal cancer in rats. Pharm. Res. 2010, 27, 1138–1145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peiffer, D.S.; Wang, L.S.; Zimmerman, N.P.; Ransom, B.W.S.; Carmella, S.G.; Kuo, C.T.; Chen, J.H.; Oshima, K.; Huang, Y.W.; Hecht, S.S.; et al. Dietary consumption of black raspberries or their anthocyanin constituents alters innate immune cell trafficking in esophageal cancer. Cancer Immunol. Res. 2016, 4, 72–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.M.; Kim, K.M.; Park, E.H.; Seo, J.H.; Song, J.Y.; Shin, S.C.; Kang, H.L.; Lee, W.K.; Cho, M.J.; Rhee, K.H.; et al. Anthocyanins from black soybean inhibit Helicobacter pylori-induced inflammation in human gastric epithelial AGS cells. Microbiol. Immunol. 2013, 57, 366–373. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, J.; Tian, J.; Si, X.; Jiao, X.; Zhang, W.; Gong, E.; Li, B. Blueberry malvidin-3-galactoside suppresses hepatocellular carcinoma by regulating apoptosis, proliferation, and metastasis pathways in vivo and in vitro. J. Agric. Food Chem. 2019, 67, 625–636. [Google Scholar] [CrossRef]
- Shin, D.Y.; Lee, W.S.; Kim, S.H.; Kim, M.J.; Yun, J.W.; Lu, J.N.; Lee, S.J.; Tsoy, I.; Kim, H.J.; Ryu, C.H.; et al. Anti-invasive activity of anthocyanins isolated from Vitis coignetiae in human hepatocarcinoma cells. J. Med. Food 2009, 12, 967–972. [Google Scholar] [CrossRef]
- Savio, M.; Stivala, L.A.; Prosperi, E. Anthocyanins protect against DNA damage induced by tert-butyl-hydroperoxide in rat smooth muscle and hepatoma cells. Mutat. Res. 2003, 535, 103–115. [Google Scholar]
- Longo, L.; Platini, F.; Scardino, A.; Alabiso, O.; Vasapollo, G.; Tessitore, L. Autophagy inhibition enhances anthocyanin-induced apoptosis in hepatocellular carcinoma. Mol. Cancer Ther. 2008, 7, 2476–2485. [Google Scholar] [CrossRef] [Green Version]
- Yeh, C.T.; Yen, G.C. Induction of apoptosis by the anthocyanidins through regulation of Bcl-2 gene and activation of c-Jun N-terminal kinase cascade in hepatoma cells. J. Agric. Food Chem. 2005, 53, 1740–1749. [Google Scholar] [CrossRef] [PubMed]
- Bishayee, A.; Háznagy-Radnai, E.; Mbimba, T.; Sipos, P.; Morazzoni, P.; Darvesh, A.S.; Bhatia, D.; Hohmann, J. Anthocyanin-rich black currant extract suppresses the growth of human hepatocellular carcinoma cells. Nat. Prod. Commun. 2010, 5, 1613–1618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anwar, S.; Fratantonio, D.; Ferrari, D.; Saija, A.; Cimino, F.; Speciale, A. Berry anthocyanins reduce proliferation of human colorectal carcinoma cells by inducing caspase-3 activation and p21 upregulation. Mol. Med. Rep. 2016, 14, 1397–1403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, W.; Fischer, J.; Krewer, G.; Akoh, C.C. Phenolic compounds from blueberries can inhibit colon cancer cell proliferation and induce apoptosis. J. Agric. Food Chem. 2005, 53, 7320–7329. [Google Scholar] [CrossRef]
- Harris, G.K.; Gupta, A.; Nines, R.G.; Kresty, L.A.; Habib, S.G.; Frankel, W.L.; LaPerle, K.; Gallaher, D.D.; Schwartz, S.J.; Stoner, G.D. Effects of lyophilized black raspberries on azoxymethane-induced colon cancer and 8-hydroxy-2′-deoxyguanosine levels in the fischer 344 rat. Nutr. Cancer 2001, 40, 125–133. [Google Scholar] [CrossRef]
- Asadi, K.; Ferguson, L.R.; Philpott, M.; Karunasinghe, N. Cancer-preventive properties of an anthocyanin-enriched sweet potato in the APC Min mouse model. J. Cancer Prev. 2017, 22, 135–146. [Google Scholar] [CrossRef] [Green Version]
- Pan, P.; Skaer, C.W.; Wang, H.-T.; Stirdivant, S.M.; Young, M.R.; Oshima, K.; Stoner, G.D.; Lechner, J.F.; Huang, Y.-W.; Wang, L.-S. Black raspberries suppress colonic adenoma development in Apc Min/+ mice: Relation to metabolite profiles. Carcinogenesis 2015, 36, 1245–1253. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.; Xu, J.; Kim, J.; Chen, T.Y.Y.; Su, X.; Standard, J.; Carey, E.; Griffin, J.; Herndon, B.; Katz, B.; et al. Role of anthocyanin-enriched purple-fleshed sweet potato p40 in colorectal cancer prevention. Mol. Nutr. Food Res. 2013, 57, 1908–1917. [Google Scholar] [CrossRef] [Green Version]
- Madiwale, G.P.; Reddivari, L.; Holm, D.G.; Vanamala, J. Storage elevates phenolic content and antioxidant activity but suppresses antiproliferative and pro-apoptotic properties of colored-flesh potatoes against human colon cancer cell lines. J. Agric. Food Chem. 2011, 59, 8155–8166. [Google Scholar] [CrossRef]
- Lee, D.Y.; Yun, S.M.; Song, M.Y.; Jung, K.; Kim, E.H. Cyanidin chloride induces apoptosis by inhibiting NF-κB signaling through activation of Nrf2 in colorectal cancer cells. Antioxidants 2020, 9, 285. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.S.; Kuo, C.T.; Cho, S.J.; Seguin, C.; Siddiqui, J.; Stoner, K.; Yu, I.W.; Huang, T.H.M.; Tichelaar, J.; Yearsley, M.; et al. Black raspberry-derived anthocyanins demethylate tumor suppressor genes through the inhibition of DNMT1 and DNMT3B in colon cancer cells. Nutr. Cancer 2013, 65, 118–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, J.; Patel, J.D.; Mumper, R.J. Characterization of blackberry extract and its antiproliferative and anti-inflammatory properties. J. Med. Food 2007, 10, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Lala, G.; Malik, M.; Zhao, C.; He, J.; Kwon, Y.; Giusti, M.M.; Magnuson, B.A. Anthocyanin-rich extracts inhibit multiple biomarkers of colon cancer in rats. Nutr. Cancer 2006, 54, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Lippert, E.; Ruemmele, P.; Obermeier, F.; Goelder, S.; Kunst, C.; Rogler, G.; Dunger, N.; Messmann, H.; Hartmann, A.; Endlicher, E. Anthocyanins prevent colorectal cancer development in a mouse model. Digestion 2017, 95, 275–280. [Google Scholar] [CrossRef] [Green Version]
- Rivera, C. Essentials of oral cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 11884–11894. [Google Scholar]
- Ugalde, C.M.; Liu, Z.; Ren, C.; Chan, K.K.; Rodrigo, K.A.; Ling, Y.; Larsen, P.E.; Chacon, G.E.; Stoner, G.D.; Mumper, R.J.; et al. Distribution of anthocyanins delivered from a bioadhesive black raspberry gel following topical intraoral application in normal healthy volunteers. Pharm. Res. 2009, 26, 977–986. [Google Scholar] [CrossRef] [Green Version]
- Mallery, S.R.; Stoner, G.D.; Larsen, P.E.; Fields, H.W.; Rodrigo, K.A.; Schwartz, S.J.; Tian, Q.; Dai, J.; Mumper, R.J. Formulation and in-vitro and in-vivo evaluation of a mucoadhesive gel containing freeze dried black raspberries: Implications for oral cancer chemoprevention. Pharm. Res. 2007, 24, 728–737. [Google Scholar] [CrossRef]
- Cui, L.; Liu, X.; Tian, Y.; Xie, C.; Li, Q.; Cui, H.; Sun, C. Flavonoids, flavonoid subclasses, and esophageal cancer risk: A meta-analysis of epidemiologic studies. Nutrients 2016, 8, 350. [Google Scholar] [CrossRef]
- Zhang, Y. Epidemiology of esophageal cancer. World J. Gastroenterol. 2013, 19, 5598–5606. [Google Scholar] [CrossRef]
- Reen, R.K.; Nines, R.; Stoner, G.D. Modulation of N-nitrosomethylbenzylamine metabolism by black raspberries in the esophagus and liver of Fischer 344 rats. Nutr Cancer 2006, 54, 47–57. [Google Scholar] [CrossRef] [Green Version]
- Stoner, G.D.; Chen, T.; Kresty, L.A.; Aziz, R.M.; Reinemann, T.; Nines, R. Protection against esophageal cancer in rodents with lyophilized berries: Potential mechanisms. Nutr Cancer 2006, 54, 33–46. [Google Scholar] [CrossRef] [Green Version]
- Peiffer, D.S.; Zimmerman, N.P.; Wang, L.S.; Ransom, B.W.S.; Carmella, S.G.; Kuo, C.T.; Siddiqui, J.; Chen, J.H.; Oshima, K.; Huang, Y.W.; et al. Chemoprevention of esophageal cancer with black raspberries, their component anthocyanins, and a major anthocyanin metabolite, protocatechuic acid. Cancer Prev. Res. 2014, 7, 574–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Z.; Lu, C.; Li, C.; Jiao, Y.; Li, Y.; Zhang, G. Dracorhodin perchlorate induces apoptosis and G2/M cell cycle arrest in human esophageal squamous cell carcinoma through inhibition of the JAK2/STAT3 and AKT/FOXO3a pathways. Mol. Med. Rep. 2019, 20, 2091–2100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, N.; Riedl, K.M.; Schwartz, S.J.; Zhang, X.; Clinton, S.K.; Chen, T. Efficacy comparison of lyophilised black raspberries and combination of celecoxib and PBIT in prevention of carcinogen-induced oesophageal cancer in rats. J. Funct. Foods 2016, 27, 84–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, X.J.; Lin, J.C.; Tu, S.P. Etiology and prevention of gastric cancer. Gastrointest. Tumors 2016, 3, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.Y.; Wang, X.; Yuan, W.J.; Chen, Z.H. Intake of anthocyanins and gastric cancer risk: A comprehensive meta-analysis on cohort and case-control studies. J. Nutr. Sci. Vitaminol. 2019, 65, 72–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woo, H.D.; Lee, J.; Choi, I.J.; Kim, C.G.; Lee, J.Y.; Kwon, O.; Kim, J. Dietary flavonoids and gastric cancer risk in a Korean population. Nutrients 2014, 6, 4961–4973. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Lee, M.H.; Park, M.; Woo, H.J.; Kim, Y.S.; Tharmalingam, N.; Seo, W.D.; Kim, J.B. Regulatory effects of black rice extract on Helicobacter pylori infection-induced apoptosis. Mol. Nutr. Food Res. 2018, 62, 1700586. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, J.; Luo, J.; Wang, Q.; Liu, J.; Zeng, Q. Study on mulberry anthocyanins induced autophagy and apoptosis of human gastric cancer SGC-7901 cell autophagy. Zhong Yao Cai 2016, 39, 1134–1138. [Google Scholar]
- Lu, J.N.; Lee, W.S.; Nagappan, A.; Chang, S.-H.; Choi, Y.H.; Kim, H.J.; Kim, G.S.; Ryu, C.H.; Shin, S.C.; Jung, J.-M.; et al. Anthocyanins from the fruit of Vitis coignetiae Pulliat potentiate the cisplatin activity by inhibiting PI3K/Akt signaling pathways in human gastric cancer cells. J. Cancer Prev. 2015, 20, 50–56. [Google Scholar] [CrossRef] [Green Version]
- Savitha, G.; Vishnupriya, V.; Krishnamohan, S. Hepatocellular carcinoma—A review. J. Pharm. Sci. Res. 2017, 9, 1276–1280. [Google Scholar]
- Liu, C.Y.; Chen, K.F.; Chen, P.J. Treatment of liver cancer. Cold Spring Harb. Perspect. Med. 2015, 5, a021535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.; Wang, H.; Yi, J.; Yang, B.; Li, M.; He, D.; Yang, W.; Zhang, Y.; Ni, H. Anti-tumor properties of anthocyanins from Lonicera caerulea ‘Beilei’ fruit on human hepatocellular carcinoma: In vitro and in vivo study. Biomed. Pharmacother. 2018, 104, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Bishayee, A.; Thoppil, R.J.; Mandal, A.; Darvesh, A.S.; Ohanyan, V.; Meszaros, J.G.; Háznagy-Radnai, E.; Hohmann, J.; Bhatia, D. Black currant phytoconstituents exert chemoprevention of diethylnitrosamine-initiated hepatocarcinogenesis by suppression of the inflammatory response. Mol. Carcinog. 2013, 52, 304–317. [Google Scholar] [CrossRef]
- Suwannakul, N.; Punvittayagul, C.; Jarukamjorn, K.; Wongpoomchai, R. Purple rice bran extract attenuates the aflatoxin B1-induced initiation stage of hepatocarcinogenesis by alteration of xenobiotic metabolizing enzymes. Asian Pac. J. Cancer Prev. 2015, 16, 3371–3376. [Google Scholar] [CrossRef] [Green Version]
- Marley, A.R.; Nan, H. Epidemiology of colorectal cancer. Int. J. Mol. Epidemiol. Genet. 2016, 7, 105–114. [Google Scholar]
- Moghimi-Dehkordi, B. An overview of colorectal cancer survival rates and prognosis in Asia. World J. Gastrointest. Oncol. 2012, 4, 71. [Google Scholar] [CrossRef]
- Kuipers, E.J.; Grady, W.M.; Lieberman, D.; Seufferlein, T.; Sung, J.J.; Boelens, P.G.; Van De Velde, C.J.H.; Watanabe, T. Colorectal cancer. Nat. Rev. Dis. Prim. 2015, 1, 15065. [Google Scholar] [CrossRef] [Green Version]
- Medic, N.; Tramer, F.; Passamonti, S. Anthocyanins in colorectal cancer prevention. A systematic review of the literature in search of molecular oncotargets. Front. Pharmacol. 2019, 10, 675. [Google Scholar] [CrossRef]
- Ding, L.; Lan, Z.; Xiong, X.; Ao, H.; Feng, Y.; Gu, H.; Yu, M.; Cui, Q. The dual role of microRNAs in colorectal cancer progression. Int. J. Mol. Sci. 2018, 19, 2791. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Guo, J.; Mao, L.; Li, Q.; Guo, M.; Mu, T.; Zhang, Q.; Bi, X. Up-regulation of MIR-24-1-5p is involved in the chemoprevention of colorectal cancer by black raspberry anthocyanins. Br. J. Nutr. 2019, 122, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Yang, Z.; Zhou, H.; Yue, J.; Mu, T.; Zhang, Q.; Bi, X. Upregulation of DKK3 by miR-483-3p plays an important role in the chemoprevention of colorectal cancer mediated by black raspberry anthocyanins. Mol. Carcinog. 2020, 59, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Coates, E.M.; Popa, G.; Gill, C.I.R.; McCann, M.J.; McDougall, G.J.; Stewart, D.; Rowland, I. Colon-available raspberry polyphenols exhibit anti-cancer effects on in vitro models of colon cancer. J. Carcinog. 2007, 6, 1477–3143. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xia, L.; Wang, H.; Oyang, L.; Su, M.; Liu, Q.; Lin, J.; Tan, S.; Tian, Y.; Liao, Q.; et al. Cancer stem cells in progression of colorectal cancer. Oncotarget 2018, 9, 33403–33415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charepalli, V.; Reddivari, L.; Radhakrishnan, S.; Vadde, R.; Agarwal, R.; Vanamala, J.K.P.P. Anthocyanin-containing purple-fleshed potatoes suppress colon tumorigenesis via elimination of colon cancer stem cells. J. Nutr. Biochem. 2015, 26, 1641–1649. [Google Scholar] [CrossRef]
- Shin, D.Y.; Lu, J.N.; Kim, G.Y.; Jung, J.M.; Kang, H.S.; Lee, W.S.; Choi, Y.H. Anti-invasive activities of anthocyanins through modulation of tight junctions and suppression of matrix metalloproteinase activities in HCT-116 human colon carcinoma cells. Oncol. Rep. 2011, 25, 567–572. [Google Scholar]
- Jing, P.; Bomser, J.A.; Schwartz, S.J.; He, J.; Magnuson, B.A.; Giusti, M.M. Structure-function relationships of anthocyanins from various anthocyanin-rich extracts on the inhibition of colon cancer cell growth. J. Agric. Food Chem. 2008, 56, 9391–9398. [Google Scholar] [CrossRef]
- Vishnu, V.R.; Renjith, R.S.; Mukherjee, A.; Anil, S.R.; Sreekumar, J.; Jyothi, A.N. Comparative study on the chemical structure and in vitro antiproliferative activity of anthocyanins in purple root tubers and leaves of sweet potato (Ipomoea batatas). J. Agric. Food Chem. 2019, 67, 2467–2475. [Google Scholar] [CrossRef]
- Wang, L.; Liu, F.; Liu, Y.; Gao, H.; Dong, M. Cyanidin-3-O-glucoside inhibits proliferation of colorectal cancer cells by targeting TOPK. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2019, 35, 1101–1108. [Google Scholar]
- Mazewski, C.; Kim, M.S.; Gonzalez de Mejia, E. Anthocyanins, delphinidin-3-O-glucoside and cyanidin-3-O-glucoside, inhibit immune checkpoints in human colorectal cancer cells in vitro and in silico. Sci. Rep. 2019, 9, 11560. [Google Scholar] [CrossRef] [Green Version]
- Duthie, S.J.; Gardner, P.T.; Morrice, P.C.; Wood, S.G.; Pirie, L.; Bestwick, C.C.; Milne, L.; Duthie, G.G. DNA stability and lipid peroxidation in vitamin E-deficient rats in vivo and colon cells in vitro: Modulation by the dietary anthocyanin, cyanidin-3-glycoside. Eur. J. Nutr. 2005, 44, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Pervin, M.; Hasnat, M.A.; Lim, J.H.; Lee, Y.M.; Kim, E.O.; Um, B.H.; Lim, B.O. Preventive and therapeutic effects of blueberry (Vaccinium corymbosum) extract against DSS-induced ulcerative colitis by regulation of antioxidant and inflammatory mediators. J. Nutr. Biochem. 2016, 28, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Bibi, S.; Du, M.; Zhu, M.J. Dietary red raspberry reduces colorectal inflammation and carcinogenic risk in mice with dextran sulfate sodium-induced colitis. J. Nutr. 2018, 148, 667–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Robertis, M.; Massi, E.; Poeta, M.; Carotti, S.; Morini, S.; Cecchetelli, L.; Signori, E.; Fazio, V. The AOM/DSS murine model for the study of colon carcinogenesis: From pathways to diagnosis and therapy studies. J. Carcinog. 2011, 10, 1477–3163. [Google Scholar]
- Shi, N.; Clinton, S.K.; Liu, Z.; Wang, Y.; Riedl, K.M.; Schwartz, S.J.; Zhang, X.; Pan, Z.; Chen, T. Strawberry phytochemicals inhibit azoxymethane/dextran sodium sulfate-induced colorectal carcinogenesis in Crj: CD-1 mice. Nutrients 2015, 7, 1696–1715. [Google Scholar] [CrossRef] [Green Version]
- Zhou, G.; Chen, L.; Sun, Q.; Mo, Q.G.; Sun, W.C.; Wang, Y.W. Maqui berry exhibited therapeutic effects against DSS-induced ulcerative colitis in C57BL/6 mice. Food Funct. 2019, 10, 6655–6665. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, L.; Cho, S.Y.; Min, K.J.; Oda, T.; Zhang, L.J.; Yu, Q.; Jin, J.O. Ginseng berry extract attenuates dextran sodium sulfate-induced acute and chronic colitis. Nutrients 2016, 8, 199. [Google Scholar] [CrossRef] [Green Version]
- Cai, X.; Han, Y.; Gu, M.; Song, M.; Wu, X.; Li, Z.; Li, F.; Goulette, T.; Xiao, H. Dietary cranberry suppressed colonic inflammation and alleviated gut microbiota dysbiosis in dextran sodium sulfate-treated mice. Food Funct. 2019, 10, 6331–6341. [Google Scholar] [CrossRef]
- Piberger, H.; Oehme, A.; Hofmann, C.; Dreiseitel, A.; Sand, P.G.; Obermeier, F.; Schoelmerich, J.; Schreier, P.; Krammer, G.; Rogler, G. Bilberries and their anthocyanins ameliorate experimental colitis. Mol. Nutr. Food Res. 2011, 55, 1724–1729. [Google Scholar] [CrossRef]
- Xia, Y.; Tian, L.M.; Liu, Y.; Guo, K.S.; Lv, M.; Li, Q.T.; Hao, S.Y.; Ma, C.H.; Chen, Y.X.; Tanaka, M.; et al. Low Dose of cyanidin-3-O-glucoside alleviated dextran sulfate sodium-induced colitis, mediated by CD169+ macrophage pathway. Inflamm. Bowel Dis. 2019, 25, 1510–1521. [Google Scholar] [CrossRef]
- Akiyama, S.; Nesumi, A.; Maeda-Yamamoto, M.; Uehara, M.; Murakami, A. Effects of anthocyanin-rich tea “Sunrouge” on dextran sodium sulfate-induced colitis in mice. BioFactors 2012, 38, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Ju, J.; Song, J.L.; Yang, S.G.; Park, K.Y. Anti-colitic effect of purple carrot on dextran sulfate sodium (DSS)-induced colitis in C57BL/6J Mice. Prev. Nutr. Food Sci. 2018, 23, 77–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mallery, S.R.; Tong, M.; Shumway, B.S.; Curran, A.E.; Larsen, P.E.; Ness, G.M.; Kennedy, K.S.; Blakey, G.H.; Kushner, G.M.; Vickers, A.M.; et al. Topical application of a mucoadhesive freeze-dried black raspberry gel induces clinical and histologic regression and reduces loss of heterozygosity events in premalignant oral intraepithelial lesions: Results from a multicentered, placebo-controlled clin. Clin. Cancer Res. 2014, 20, 1910–1924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shumway, B.S.; Kresty, L.A.; Larsen, P.E.; Zwick, J.C.; Lu, B.; Fields, H.W.; Mumper, R.J.; Stoner, G.D.; Mallery, S.R. Effects of a topically applied bioadhesive berry gel on loss of heterozygosity indices in premalignant oral lesions. Clin. Cancer Res. 2008, 14, 2421–2430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mallery, S.R.; Zwick, J.C.; Pei, P.; Tong, M.; Larsen, P.E.; Shumway, B.S.; Lu, B.; Fields, H.W.; Mumper, R.J.; Stoner, G.D. Topical application of a bioadhesive black raspberry gel modulates gene expression and reduces cyclooxygenase 2 protein in human premalignant oral lesions. Cancer Res. 2008, 68, 4945–4957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kresty, L.A.; Fromkes, J.J.; Frankel, W.L.; Hammond, C.D.; Seeram, N.P.; Baird, M.; Stoner, G.D. A phase I pilot study evaluating the beneficial effects of black raspberries in patients with Barrett’s esophagus. Oncotarget 2018, 9, 35356–35372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.; Subar, A.F.; Bosire, C.; Dawsey, S.M.; Kahle, L.L.; Zimmerman, T.P.; Abnet, C.C.; Heller, R.; Graubard, B.I.; Cook, M.B.; et al. Dietary flavonoid intake reduces the risk of head and neck but not esophageal or gastric cancer in US men and women. J. Nutr. 2017, 147, 1729–1738. [Google Scholar]
- Pan, P.; Skaer, C.W.; Stirdivant, S.M.; Young, M.R.; Stoner, G.D.; Lechner, J.F.; Huang, Y.W.; Wang, L.S. Beneficial regulation of metabolic profiles by black raspberries in human colorectal cancer patients. Cancer Prev. Res. 2015, 8, 743–750. [Google Scholar] [CrossRef] [Green Version]
- Wajed, S.A.; Laird, P.W.; DeMeester, T.R. DNA methylation: An alternative pathway to cancer. Ann. Surg. 2001, 234, 10–20. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Okamura, S.; Yamaji, T.; Iwasaki, M.; Tsugane, S.; Shetty, V.; Koizumi, T. Plasma cytokine levels and the presence of colorectal cancer. PLoS ONE 2019, 14, e0213602. [Google Scholar] [CrossRef] [Green Version]
- Mentor-Marcel, R.A.; Bobe, G.; Sardo, C.; Wang, L.S.; Kuo, C.T.; Stoner, G.; Colburn, N.H. Plasma cytokines as potential response indicators to dietary freeze-dried black raspberries in colorectal cancer patients. Nutr. Cancer 2012, 64, 820–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.S.; Burke, C.A.; Hasson, H.; Kuo, C.T.; Molmenti, C.L.S.; Seguin, C.; Liu, P.; Huang, T.H.M.; Frankel, W.L.; Stoner, G.D. A phase Ib study of the effects of black raspberries on rectal polyps in patients with familial adenomatous polyposis. Cancer Prev. Res. 2014, 7, 666–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molan, A.-L.; Liu, Z.; Plimmer, G. Evaluation of the effect of blackcurrant products on gut microbiota and on markers of risk for colon cancer in humans. Phyther. Res. 2014, 28, 416–422. [Google Scholar] [CrossRef] [PubMed]
| Type of the Cancer | Syndrome | Associated Germline Mutations | Reference | |
|---|---|---|---|---|
| Esophageal | Familial Barrett’s esophagus, Familial esophageal adenocarcinoma | MSR1, ASCC1 and CTHRC1 | [29] | |
| Tylosis with esophageal cancer-squamous cell carcinoma | RHBDF2 | [30] | ||
| Gastric | Diffuse hereditary gastric cancer-adenocarcinoma | CDH1 (E-cadherin) | [31] | |
| Pancreatic | Hereditary pancreatitis | PRSS1, CFTR, SPINK1, CTRC | [32] | |
| Hereditary breast and ovarian cancer | BRCA1/2 | |||
| Peutz-Jeghers syndrome | STK11/LKB1 | |||
| Familial atypical multiple mole melanoma syndrome | CDKN2A/p16 | |||
| Familial adenomatous polyposis | APC | |||
| Colorectal | Familial adenomatous polyposis | APC | [33,34] | |
| Lynch syndrome | EPCAM, MLH1, MSH2, MSH6, PMS2 | |||
| MYH associated polyposis | MUTYH | |||
| Hamartomatous polyposis syndrome | Peutz-Jeghers syndrome | STK11 | ||
| Juvenile polyposis syndrome | SMAD4, BMPR1A | |||
| Attenuated Familial adenomatous polyposis | APC | |||
| Small intestine | Familial adenomatous polyposis | APC | [35] | |
| Lynch syndrome | Mutations in mismatch repair genes | |||
| Juvenile polyposis syndrome | SMAD4 | |||
| Peutz-Jeghers syndrome | STK11 | |||
| Liver | α-1 antitrypsin deficiency | SERPINA1 | [36,37,38,39,40] | |
| Hereditary hemochromatosis | HFE | |||
| Hereditary tyrosinemia type 1 | FAH | |||
| Glycogen storage disease type 1 | G6PC, SLC37A4 | |||
| Wilson’s disease | ATP7B | |||
| Niemann-park disease | SMPD1 AND NPC1 OR NPC2 | |||
| Biliary | Bile salt export pump deficiency | ABCB11 | [41] | |
| Source of Anthocyanin | Dosage | Cell Line/Animal Model | Observations | Reference |
|---|---|---|---|---|
| Oral Cancer | ||||
| Blueberry and malvidin | 50 µg/mL | Human oral SCC131 cells | Reduced STAT-3 phosphorylation and nuclear translocation Induced cell cycle arrest at G1/S phase and apoptosis | [152] |
| Cranberry extracts | 25–200 µg/mL | Human oral epidermal KB, CAL-27 cancer cells | Inhibited cell proliferation | [169] |
| Black rice (Oryza Sativa L.) | 100–500 µg/mL | Human tongue epithelial CAL 27 cells | Inhibited cell migration and invasion Inhibited activity of MMP-2 Inhibited NF-κB p65 protein expression Suppressed Pl3K/Akt pathway | [170] |
| Commercial anthocyanin | 250 µg/mL | Human oral SCC | Reduced cell viability, Inhibited migration, and invasion abilities Increased NLRP3, caspase-1, IL-1β protein expression | [171] |
| Grape skin extract | 2.5 mg/kg of body weight | Male Wistar rats; 4-nitroquinoline 1-oxide induced tongue carcinogenesis | Reduced epithelial dysplasia Reduced p-NF-κB p50 and MyD88 protein expression No change in copper-zinc superoxide dismutase, manganese superoxide dismutase, and catalase gene expression | [172] |
| Lyophilized strawberry | 5% or 10% w/w for 12 weeks | Hamster cheek pouch (HCP) model of oral cancer | Reduced number of tumors Mild and severe dysplasia | [173] |
| Esophageal Cancer | ||||
| Lyophilized black raspberry | 100 μg/mL | Human esophageal microvascular endothelial cells (HEMEC) | Inhibited TNF-α/IL-1β-induced NFκB p65 nuclear translocation, PGE2 production Reduced COX-2, ICAM-1 and VCAM-1 mRNA and protein expression and leukocyte binding Inhibited Akt, MAPK and JNK phosphorylation | [174] |
| Lyophilized black raspberry, C3G, C3R | 10–50 µg/mL | RE-149DHD and RE-149 rat esophageal cancer cell lines | Inhibited cell growth Induced apoptosis Reduced COX-2, iNOS mRNA expression | [175] |
| Lyophilized black raspberry | 2.5% w/w of the diet | Male Sprague-Dawley rats, EDA surgery-induced carcinogenesis | No change in COX-2 level Reduced MnSOD levels Not effective in the prevention of reflux-induced esophageal adenocarcinoma | [176] |
| Lyophilized black raspberry | 5% w/w for 10 weeks | NMBA-induced carcinogenesis in F344 rats | Influenced the metabolic activation and detoxification of NMBA Reduced cell proliferation, inflammation, and angiogenesis Inhibited CYP2a2 mRNA expression | [177] |
| Lyophilized black raspberry | 5% w/w for 30 weeks | NMBA induced carcinogenesis in F344 rats | Reduced NF-κB protein expression Reduced number and volume of NMBA-induced papillomas Inhibited cell proliferation and, inflammation Induced apoptosis | [178] |
| Either black or red raspberries, strawberries, blueberries, noni, açaí or wolfberry | 5% w/w for 35 weeks | NMBA induced carcinogenesis in F344 rats | Reduced serum cytokines, IL-5, and GRO/KC protein expression No change in serum IL-1ß, IL-4, IL-13, and TNF-α protein expression Increased IFN-γ protein expression | [179] |
| Lyophilized black raspberry, anthocyanin extract, PCA | 6.1% w/w, 0.35 ppm and 500 ppm respectively | NMBA induced carcinogenesis in F344 rats | Reduced IL-1β protein expression Increased IL-10, IL-12 protein expression Increased infiltration of both macrophages and neutrophils into the esophagus | [180] |
| Gastric Cancer | ||||
| Malvidin | 50–200 µg/mL | Human AGS cells | Induced apoptosis-arrest G0/G1 phase Loss of mitochondrial membrane potential Increased BAX/Bcl-2 ration and P38 kinase expression Inhibited ERK activity | [157] |
| Black soybean anthocyanin | 12.5–50 µg/mL | H. pylori-induced inflammation in AGS cells | Reduced H. pylori-induced ROS production Inhibited phosphorylation of mitogen-activated protein kinases, translocation of NF-κB, iNOS, Cox-2 mRNA expressions, IL-8 production | [181] |
| Liver Cancer | ||||
| Black currant | 100, 500 mg/kg body weight for 22 weeks | DENA-induced carcinogenesis in rats | Reduced abnormal lipid peroxidation, protein oxidation and expression of iNOS, 3-nitrotyrosine, Nrf-2 | [133] |
| Malvidin-3-galactoside | 50–200 µg/mL | Human HepG2 cells | Reduced P-AKT level, MMP-2 and, MMP-9 protein expression Induced apoptosis Increased cyclin-D1, B, E, Caspase-3 protein expression | [182] |
| Meoru anthocyanin | 400 µg/mL | Human Hep3B cells | Reduced MMP-2, MMP-9 protein expression Activated NF-κB Promoted anti-invasive effects | [183] |
| Isolated anthocyanins | 100 or 500 µg/mL | Rat hepatoma cells (MH1C1)-DNA damaged induced by TBHP | Reduced DNA single-strand formation and lipid peroxidation No change in redox state | [184] |
| Meoru anthocyanin | 400 µg/mL | Human Hep3B cells | Reduced cell proliferation, invasion Induced mitochondrial dysfunction Reduced Bcl-2, XlAP, ClAP-1, ClAP-2 protein expression | [154] |
| Berry anthocyanin | 0.001–0.1 mg/mL | Human HCC cell lines PLC/PRF/5 | Increased Bax, cytochrome c, caspase 3 and, elF2-α protein expression Reduced mTOR, Bcl-2 protein expression | [185] |
| Delphinidin, cyanidin, and malvidin | 100 µg/mL | Human HepG2 cells | Reduced cell growth Induced apoptosis-internucleosomal DNA fragmentation Increased Bax: Bcl-2 protein expression Activated c-Jun-N-terminal cascade | [186] |
| Black currant | 0.125%, 0.625% w/w for 22 weeks | DENA-induced carcinogenesis in Sprague-Dawley rats | Increased incidence, total number, multiplicity, size, and volume of preneoplastic hepatic nodules Abnormal cell proliferation Induced apoptosis Increased Bax: Bcl-2 protein expression | [187] |
| Colorectal Cancer | ||||
| Anthocyanin metabolites (gallic acid, 3-O-methylgallic acid, and 2,4,6-trihydroxybenzaldehyde | 10–100 µmol/L | Human Caco-2 cells | Reduced cell viability Induced cell cycle arrest at G0/G1 Increased caspase-3 activation Inhibited transcription factors NF-κB, AP-1, STAT-1, and OCT-1 | [22] |
| Standardized anthocyanin-rich extract | 50–500 μg/mL | Human Caco-2 cells | Inhibited cell proliferation Caspase-3 activation Induced apoptosis Increased cellular ROS | [188] |
| Lyophilized blueberry | 70–100 μg/mL 50–100 μg/mL | Human HT-29 Human Caco-2 cells | Inhibited cell proliferation 2–7 times increased DNA fragmentation Induced apoptosis | [189] |
| Lyophilized black raspberries | 0%, 2.5%, 5%, or 10% wt/wt for 33 weeks | AOM-induced carcinogenesis in F344 rats | Reduced ACF, tumor multiplicity, adenocarcinoma multiplicity by the dose-depended manner | [190] |
| Purple fleshed sweet potato | 10% w/w of potato skin, potato flesh & 0.12% w/w anthocyanin-rich extracted for 18 weeks | C57BL/6J-APCMIN/+ mice | Reduced adenoma number (0.12% w/w anthocyanin-rich extracted more effective) | [191] |
| Lyophilized black raspberries | 5% w/w for 8 weeks | APCMIN/+ mice | Reduced intestinal and colonic polyp number and size Reversed 23 APC-regulated metabolites, including 13 colonic mucosa, 8 liver and 2 fecal metabolites Reduced putrescine and linolenate levels | [192] |
| Cocoplum anthocyanin | 1 to 20 μg/mL | TNF-α stimulated Human HT-29 cells, CCD-18Co non-malignant colonic fibroblasts | Inhibited cell proliferation Increased cellular ROS Reduced TNF-α, IL-1β, IL-6, and NF-κB1 mRNA expression | [117] |
| Purple-sweet potato anthocyanin | 0–40 μM | Human colonic SW480 cancer cells | Inhibited cell proliferation Cell cycle arrest at G1 phase | [193] |
| Purple fleshed potato | 10–30 μg/mL | Human HCT-116 and HT-29 cells | Inhibited cell proliferation Induced apoptosis | [194] |
| Cyanidin chloride | 0–50 µM | TNF-α stimulated Human HCT116, HT29, and SW620 | Suppressed NF-κB signaling Activated the Nrf2 pathway Increased Bax: Bcl-2 protein and mRNA expression Reduced protein and mRNA expression of TNF-α, IL-6, and IL-8 | [195] |
| Black raspberry powder | 0.5,5,25 μg/mL | Human HCT116, Caco2 and SW480 cells | Increased protein expression of DNMT1 and DNMT3B Reduced mRNA expression of β-catenin Inhibited cell proliferation Induced apoptosis | [196] |
| Anthocyanin-rich extract from Hull blackberries | 0–40 μg/mL | Human HT-29 cells | Inhibited cell proliferation Increased release of IL-12 | [197] |
| Anthocyanin-rich extracts from bilberry, chokeberry, grape | 3.85 g/kg for 4 weeks | AOM-induced carcinogenesis in F344 rats | Reduced ACF, fecal bile acids and, colonic cellular proliferation Reduced COX-2 mRNA expression (bilberry, grape diets) | [198] |
| Anthocyanin-rich extracts from bilberry | 10% w/w supplementation for 9 weeks | AOM/DSS-induced colitis-associated carcinogenesis in Balb/c mice | Less reduced colon length Less inflammation Less mean tumor number | [199] |
| Source of Anthocyanin | Dosage | Treatment | Observations | Reference |
|---|---|---|---|---|
| Black rice anthocyanin-rich extract | 25, 50, and 100 mg/kg of body weight | 8 weeks old female C57BL/6 mice: administration of 3% DSS for 5 consecutive days in drinking water | Reduced DAI and the histological score of colons, myeloperoxidase (MPO) and nitric oxide (NO) levels and, mRNA expression of IL-6, IL-1β, TNF-α, iNOS, and COX-2 | [119] |
| Malvidin 3-glucoside | 24 mg/kg of feed weight | 4–5 weeks old C57BL/6J male mice: 2 cycles (7 days of 2.5% DSS and 14 days of fresh tap water) | Improved histopathological scores mRNA expression of IL-10 Promoted microbial interactions and restored the Firmicutes/Bacteroidetes ratio repressed by DSS Reduced abundance of Ruminococcus gnavus | [167] |
| Blueberry extract | 50 mg/kg body weight | Female Balb/C mice: administration of 3% DSS for 1 week in drinking water | Reduced DAI and improved the macroscopic and histological score of colons Reduced myeloperoxidase accumulation and malondialdehyde in the colon Increased prostaglandin E2 level in serum Reduced levels of superoxide dismutase and catalase Reduced mRNA expression of COX-2 and IL-1β in colonic tissue Reduced nuclear translocation of NF-kB | [237] |
| Dietary red raspberry | 5% w/w of feed weight | Six-week-old male C57BL/6J mice: administration of 2 repeated cycles of 1% DSS (7-d DSS treatment plus 14-d recovery) | Reduced DAI score and histologic damage Reduced expression of inflammatory mediators Facilitated epithelial repair Reduced β-catenin, STAT3 signaling | [238] |
| Maqui berry water extract | 50–200 mg/kg of body weight | 6 weeks old wild-type C57BL/6 male mice: administration of 3% (w/v) DSS for 1 week in drinking water | Reduced protein expression of COX2 and IL-6 in LPS-stimulated RAW 264.7 cells Reduced inflammatory bowel disease index, MDA, NO, i-NOS, COX-2 protein expression in colon tissue Reduced MPO, TNF-α, and IL-1β protein expression in blood serums Increased protein expression of occludin (Dose-dependent manner) | [241] |
| Ginseng berry extract | 50 mg/kg of body weight | C57BL/6 mice: administration of 3% DSS for 8 days in drinking water | Reduced DAI score and histologic damage Reduced numbers and inhibited the activation of colon-infiltrating T cells, neutrophils, intestinal CD103−CD11c+ dendritic cells and macrophages | [242] |
| Cranberry extract | 1.5% w/w of feed weight | 6 weeks old male CD-1 mice: 1.5% DSS for 4 cycles (4 days/cycle, with a 7-day recovery after each of the first 3 DSS cycles) | Inhibited reduction in colon length Reduced DAI and histologic score Increased colonic levels of IL-1β, IL-6, and TNF-α proteins Altered the microbial structure of fecal microbiota in mice Reduced DSS-induced decline in α-diversity Increased abundance of Lactobacillus and Bifidobacterium Reduced abundance of Sutterella and Bilophila | [243] |
| Dried bilberries | 10% w/w of feed weight | Balb/c mice: 2.5% DSS for 1 week in drinking water | Reduced DAI and histologic score Reduced secretion of IFN-γ and TNF-α from mesenteric lymph node cells Intestinal inflammation Prevented inflammation-induced apoptosis in colonic epithelial cells | [244] |
| C3G | Intraperitoneal injected with 1ug C3G every 2 days, a total of 3 times | 8–12 weeks old C57BL/6 mice: 3.5% DSS for 1 week in drinking water | No change in body weight and colon length Reduced mRNA expression of IL- 6, IL-1β, IL-18, TNF-α, IFN-γ in colons and mesenteric lymph nodes Reduced CCL22 levels and Tregs induction | [245] |
| Anthocyanin-rich tea | 0.13 or 0.16 mg/day by gavage | 5 weeks old female ICR mice: 3% DSS for 2 weeks in drinking water | Lowered body weight loss, spleen hypertrophy, and shortening of the colon Reduced deteriorations in survival rate, liver function, colon mucosal IL-1β level (mRNA) | [246] |
| Purple carrot extract | 5% w/w of feed weight | 6–7 weeks old C57BL/6 mice: 2% DSS for 1 week in drinking water | Reduced DSS-induced colon shortening and inflammatory cell infiltration Reduced serum levels of TNF-α and IL-6 (protein) Inhibited colonic mRNA expression of iNOS, COX-2 | [247] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dharmawansa, K.V.S.; Hoskin, D.W.; Rupasinghe, H.P.V. Chemopreventive Effect of Dietary Anthocyanins against Gastrointestinal Cancers: A Review of Recent Advances and Perspectives. Int. J. Mol. Sci. 2020, 21, 6555. https://doi.org/10.3390/ijms21186555
Dharmawansa KVS, Hoskin DW, Rupasinghe HPV. Chemopreventive Effect of Dietary Anthocyanins against Gastrointestinal Cancers: A Review of Recent Advances and Perspectives. International Journal of Molecular Sciences. 2020; 21(18):6555. https://doi.org/10.3390/ijms21186555
Chicago/Turabian StyleDharmawansa, K.V. Surangi, David W. Hoskin, and H. P. Vasantha Rupasinghe. 2020. "Chemopreventive Effect of Dietary Anthocyanins against Gastrointestinal Cancers: A Review of Recent Advances and Perspectives" International Journal of Molecular Sciences 21, no. 18: 6555. https://doi.org/10.3390/ijms21186555