Neither Excessive Nitric Oxide Accumulation nor Acute Hyperglycemia Affects the N-Acetylaspartate Network in Wistar Rat Brain Cells
Abstract
:1. Introduction
2. Results and Discussion
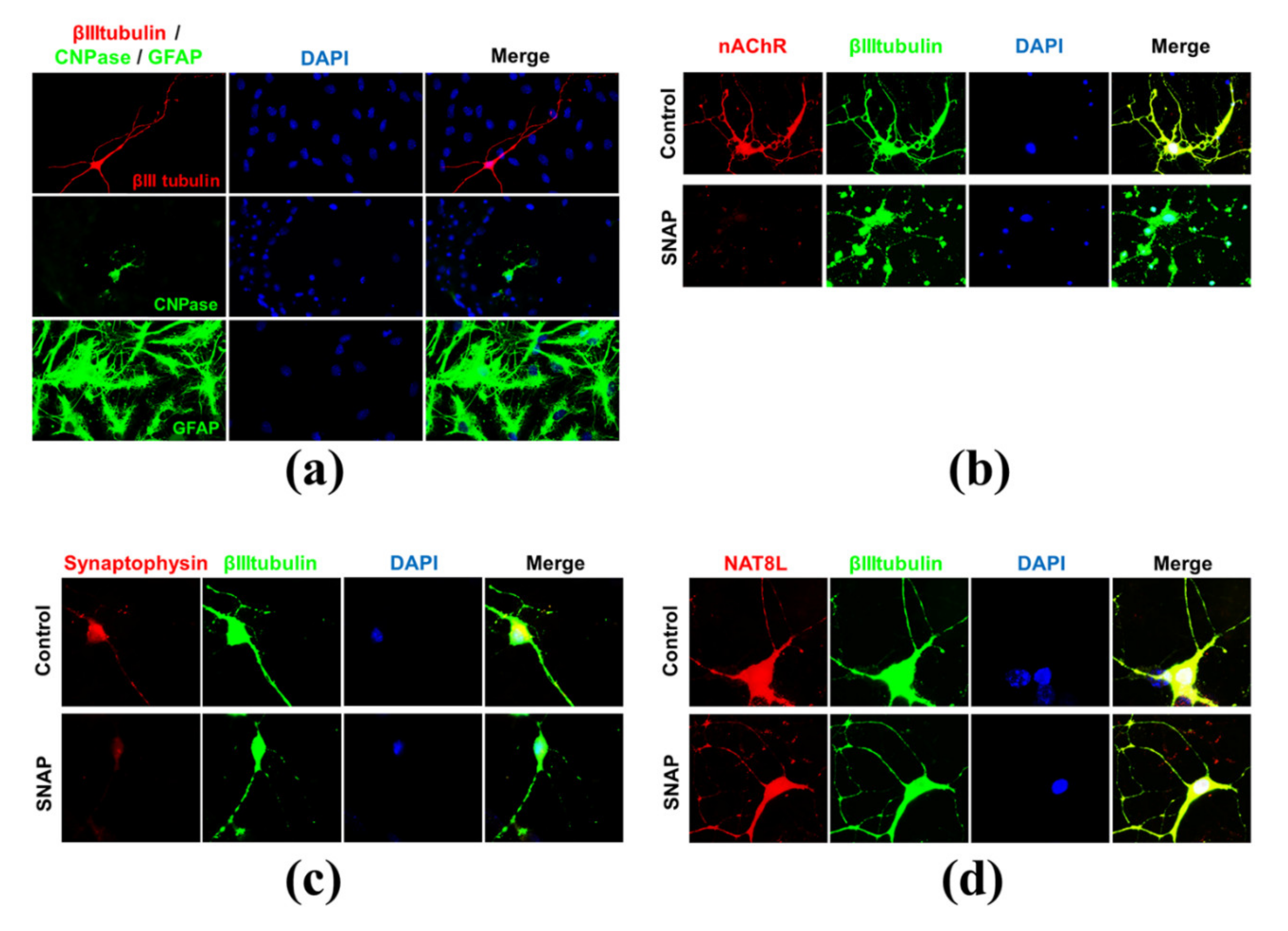
2.1. Primary Cell Cultures
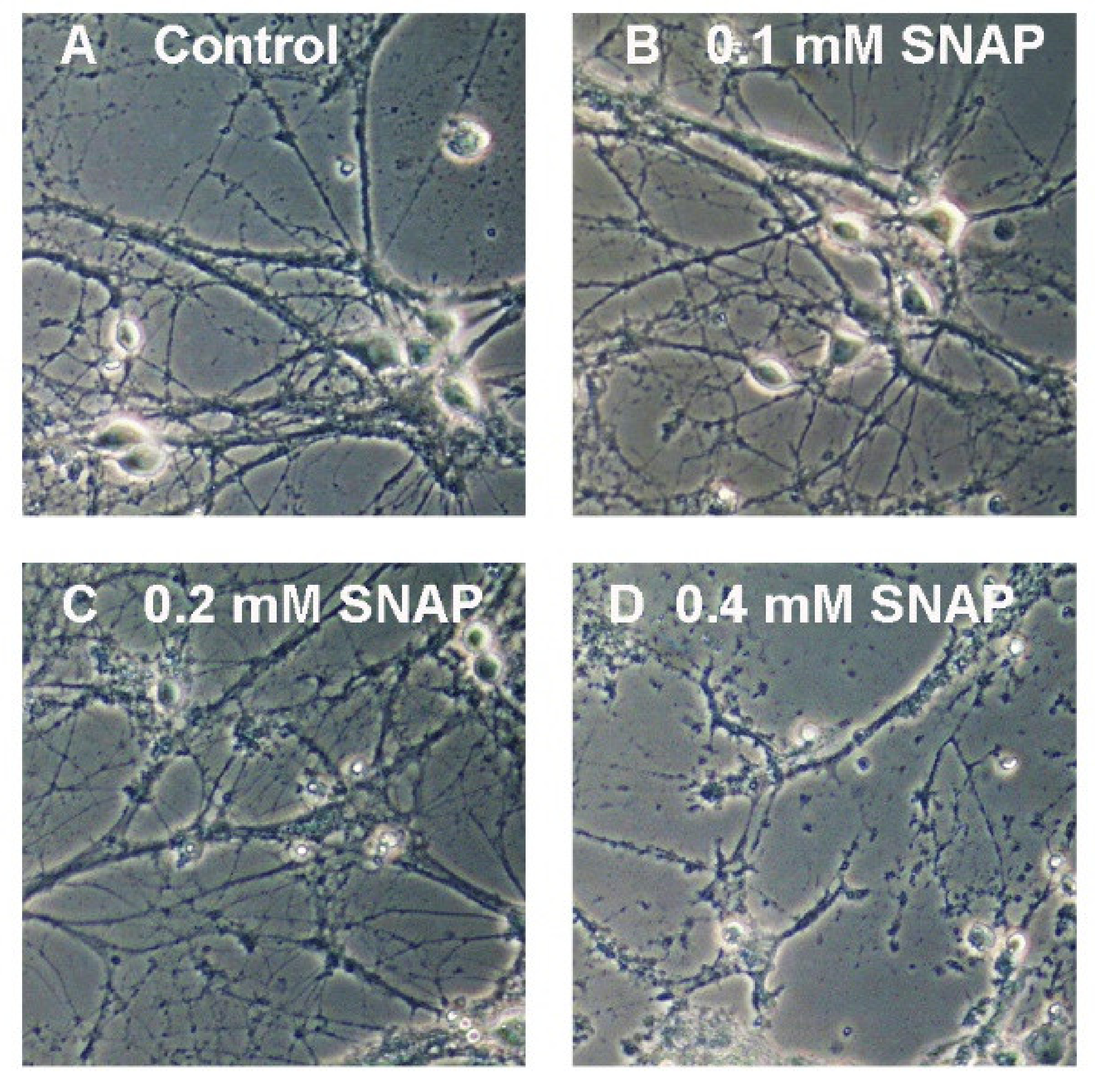
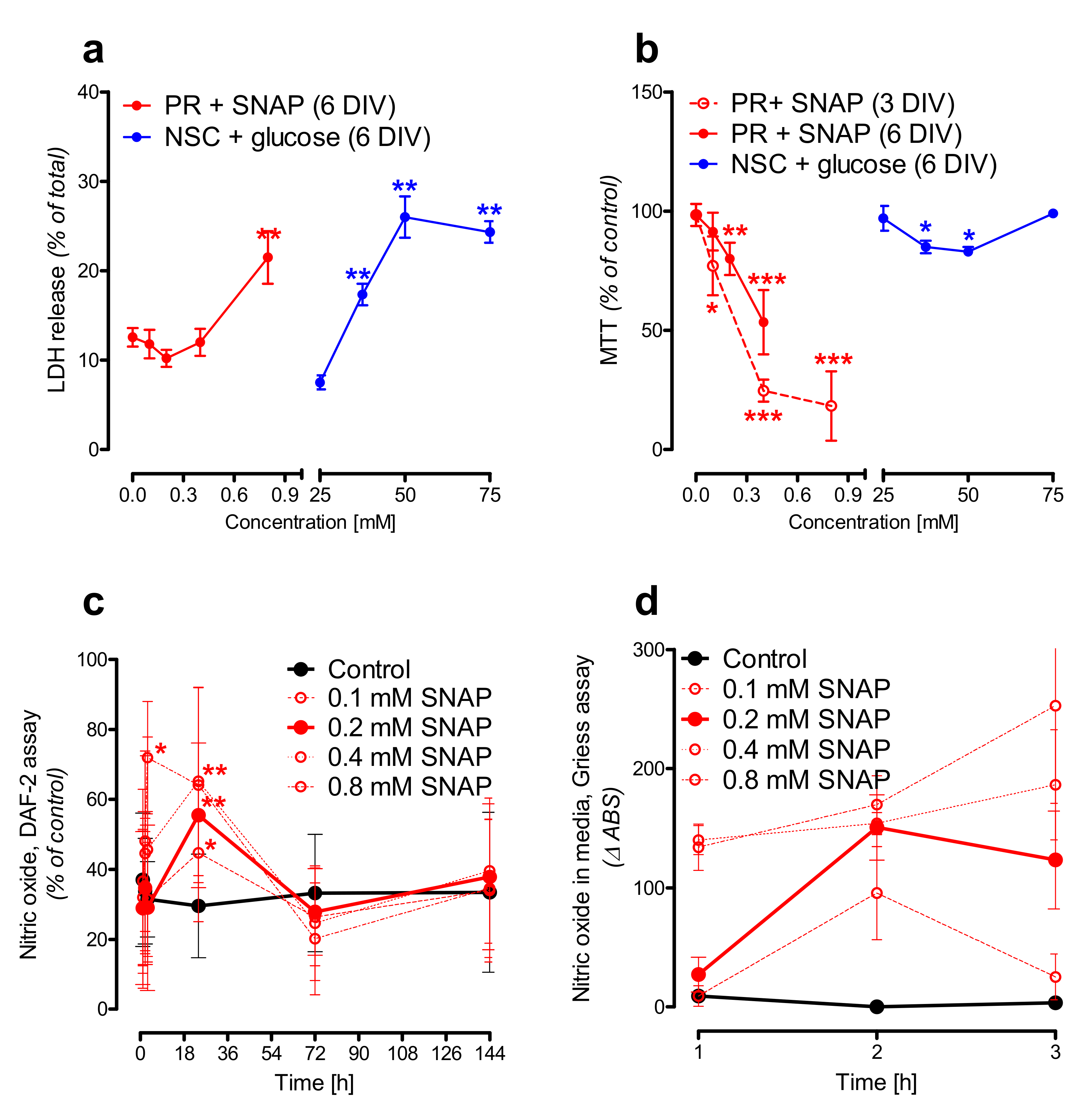
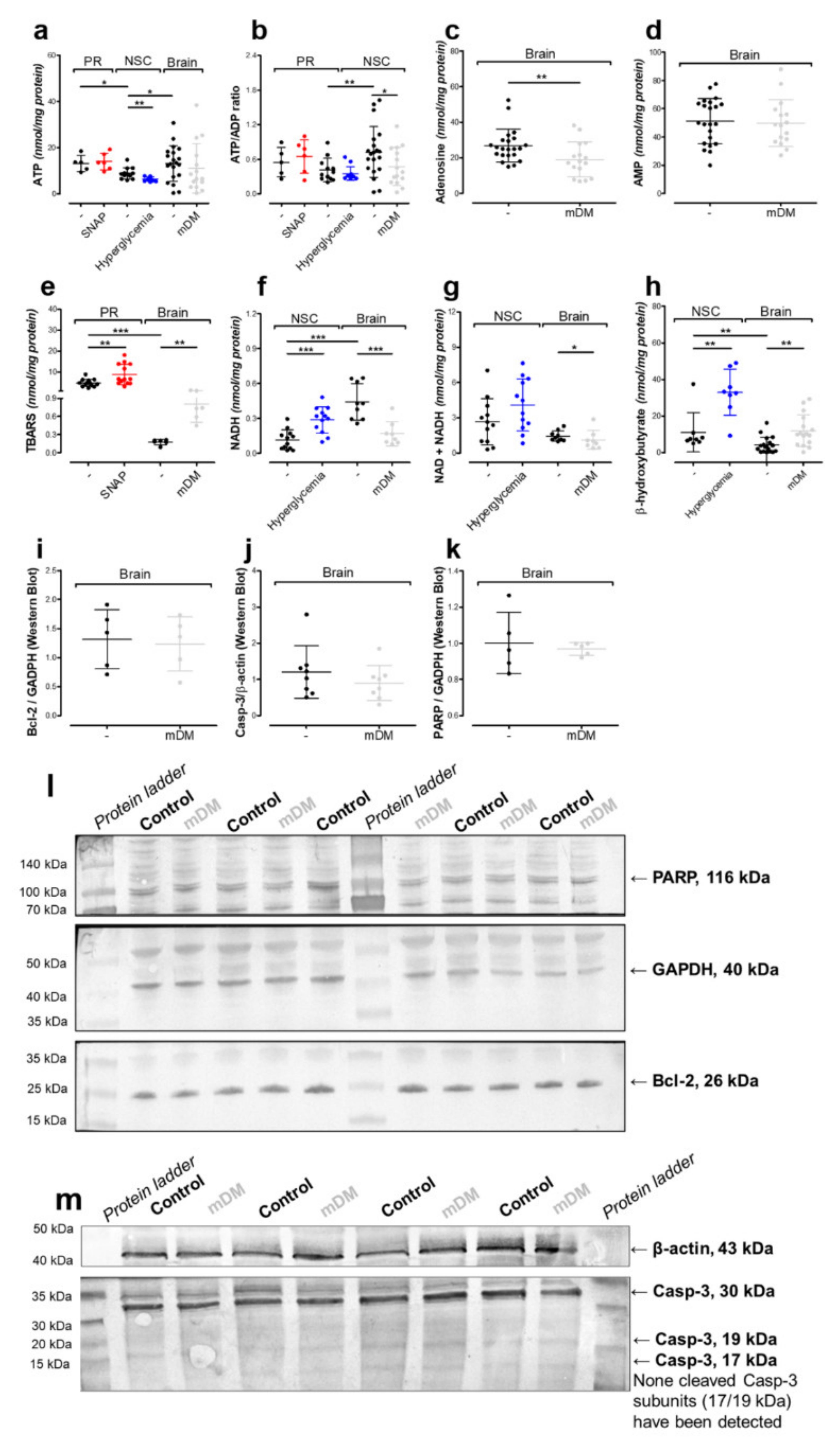
2.2. Nitric Oxide Affects Primary Neurons Morphology and Energy State
2.3. Hyperglycemia Affects Neural Stem Cells Mitochondrial Homeostasis
2.4. In Vivo Model of Hyperglycemia
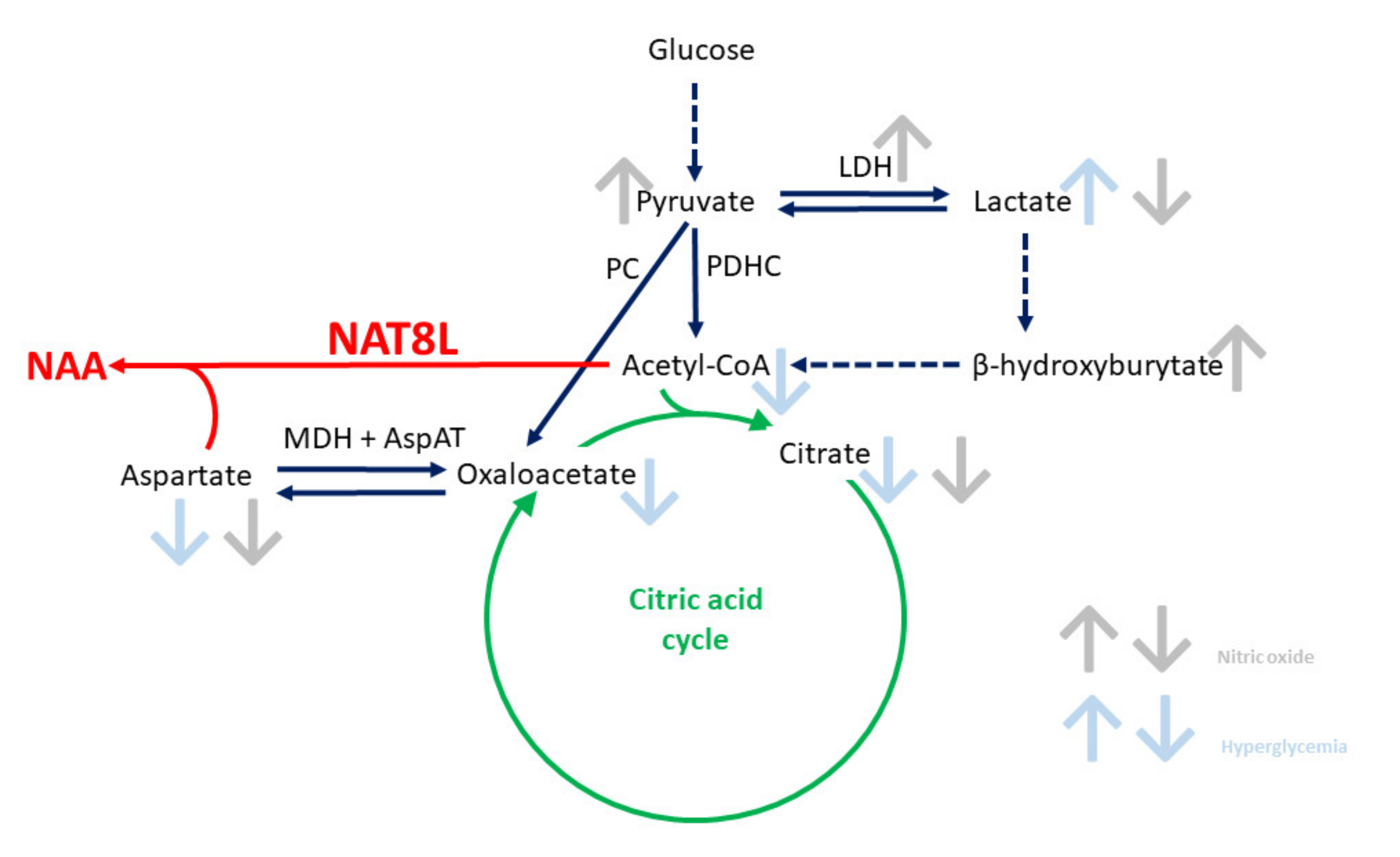
2.5. N-Acetylaspartate Network is Resistant to NO- and Hyperglycemia-Derived Toxicity
3. Materials and Methods
3.1. Materials
3.2. Ethics Approval
3.3. Animals (In Vivo Studies)
3.4. Primary Cultures (In Vitro Studies)
3.5. Embryonic Primary Neurons (PR)
3.6. Embryonic Neural Stem Cells (NSC)
3.7. Sample Preparation
3.8. Morphology Imaging
3.9. Enzymatic Assays
3.10. Metabolic Assays
3.11. Real-Time RT-qPCR Analysis of Nat8l and Chat mRNA Levels
3.12. Double Staining in Immunocytochemistry
3.13. Western Blot Analysis
3.14. Protein Assay
3.15. Statistics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Aco | Aconitase |
| AspAT | Aspartate aminotransferase |
| b.w. | Body weight |
| Casp-3 | Caspase-3 |
| ChAT | Choline acetyltransferase protein |
| Chat | Choline acetyltransferase gene |
| CHT1 | High affinity choline transporter |
| CNPase | 2′,3′-cyclic-nucleotide 3′-phosphodiesterase |
| DIV | Days in vitro |
| DM | Diabetes mellitus |
| GFAP | Glial fibrillary acidic protein |
| IDH | Isocitrate dehydrogenase |
| LDH | Lactate dehydrogenase |
| mDM | Moderate diabetes mellitus |
| MTT | Methylthiazolyldiphenyl-tetrazolium bromide |
| NAA | N-acetylaspartate |
| NAT8L | Aspartate N-acetyltransferase protein |
| Nat8l | N-acetyltransferase 8 Like gene |
| NO | Nitric oxide |
| NSC | Neural stem cells |
| PDHC | Pyruvate dehydrogenase complex |
| SC | Citrate synthase |
| sDM | Severe diabetes mettilus |
| SNAP | S-Nitroso-N-acetylpenicillamine |
| STZ | Streptozotocin |
| TBARS | Thiobarbituric acid reactive substances |
| VAChT | Vasicular acetylcholine transporter |
References
- Ariyannur, P.S.; Madhavarao, C.N.; Namboodiri, A.M. N-acetylaspartate synthesis in the brain: Mitochondria vs. microsomes. Brain Res. 2008, 1227, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Ariyannur, P.S.; Moffett, J.R.; Manickam, P.; Pattabiraman, N.; Arun, P.; Nitta, A.; Nabeshima, T.; Madhavarao, C.N.; Namboodiri, A.M.A. Methamphetamine-induced neuronal protein NAT8L is the NAA biosynthetic enzyme: Implications for specialized acetyl coenzyme A metabolism in the CNS. Brain Res. 2010, 1335, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Arun, P.; Madhavarao, C.N.; Moffett, J.R.; Namboodiri, M.A. Regulation of N-acetylaspartate and N-acetylaspartylglutamate biosynthesis by protein kinase activators. J. Neurochem. 2006, 98, 2034–2042. [Google Scholar] [CrossRef] [PubMed]
- Arun, P.; Moffett, J.R.; Namboodiri, A.M. Evidence for mitochondrial and cytoplasmic N-acetylaspartate synthesis in SH-SY5Y neuroblastoma cells. Neurochem. Int. 2009, 55, 219–225. [Google Scholar] [CrossRef]
- Baslow, M.H. Canavan’s spongiform leukodystrophy: A clinical anatomy of a genetic metabolic CNS disease. J. Mol. Neurosci. 2000, 15, 61–69. [Google Scholar] [CrossRef]
- Clark, J.B. N-acetyl aspartate: A marker for neuronal loss or mitochondrial dysfunction. Dev. Neurosci. 1998, 20, 271–276. [Google Scholar] [CrossRef]
- Demougeot, C.; Garnier, P.; Mossiat, C.; Bertrand, N.; Giroud, M.; Beley, A.; Marie, C. N-Acetylaspartate, a marker of both cellular dysfunction and neuronal loss: Its relevance to studies of acute brain injury. J. Neurochem. 2001, 77, 408–415. [Google Scholar] [CrossRef] [Green Version]
- Wiame, E.; Tyteca, D.; Pierrot, N.; Collard, F.; Amyere, M.; Noel, G.; Desmedt, J.; Nassogne, M.C.; Vikkula, M.; Octave, J.O.; et al. Molecular identification of aspartate N-acetyltransferase and its mutation in hypoacetylaspartia. Biochem. J. 2010, 425, 127–136. [Google Scholar] [CrossRef] [Green Version]
- Zyśk, M.; Bielarczyk, H.; Gul-Hinc, S.; Dyś, A.; Gapys, B.; Ronowska, A.; Sakowicz-Burkiewicz, M.; Szutowicz, A. Phenotype-Dependent Interactions between N-acetyl-L-Aspartate and Acetyl-CoA in Septal SN56 Cholinergic Cells Exposed to an Excess of Zinc. J. Alzheimers Dis. 2017, 56, 1145–1158. [Google Scholar] [CrossRef]
- Baslow, M.H. Functions of N-acetyl-L-aspartate and N-acetyl-L-aspartylglutamate in the vertebrate brain: Role in glial cell-specific signaling. J. Neurochem. 2000, 75, 453–459. [Google Scholar] [CrossRef]
- Bates, T.E.; Strangward, M.; Keelan, J.; Davey, G.P.; Munro, P.M.; Clark, J.B. Inhibition of N-acetylaspartate production: Implications for 1H MRS studies in vivo. Neuroreport 1996, 7, 1397–1400. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, G.; Mekala, P.; Yahya, D.; Wu, G.; Ledeen, R.W. Intraneuronal N-acetylaspartate supplies acetyl groups for myelin lipid synthesis: Evidence for myelin-associated aspartoacylase. J. Neurochem. 2001, 78, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Dautry, C.; Vaufrey, F.; Brouillet, E.; Bizat, N.; Henry, P.G.; Condé, F.; Bloch, G.; Hantray, P. Early N-acetylaspartate depletion is a marker of neuronal dysfunction in rats and primates chronically treated with the mitochondrial toxin 3-nitropropionic acid. J. Cereb. Blood Flow Metab. 2000, 20, 789–799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Pietro, V.; Amorini, A.M.; Tavazzi, B.; Vagnozzi, R.; Logan, A.; Vagnozzi, R.; Logan, A.; Lazzarino, G.; Signoretti, S.; Lazzarino, G.; et al. The molecular mechanisms affecting N-acetylaspartate homeostasis following experimental graded traumatic brain injury. Mol. Med. 2014, 20, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, H.; Suzuki, Y.; Huber, V.J.; Nakada, T. N-acetylaspartate decrease in acute stage of ischemic stroke: A perspective from experimental and clinical studies. Magn. Reson. Med. Sci. 2015, 14, 13–24. [Google Scholar] [CrossRef] [Green Version]
- Mangia, S.; Kumar, A.F.; Moheet, A.A.; Roberts, R.J.; Eberly, L.E.; Seaquist, E.R.; Tkáč, I. Neurochemical profile of patients with type 1 diabetes measured by 1H-MRS at 4 T. J. Cereb. Blood Flow Metab. 2013, 33, 754–759. [Google Scholar] [CrossRef] [Green Version]
- Wrighten, S.A.; Piroli, G.G.; Grillo, C.A.; Reagan, L.P. A look inside the diabetic brain: Contributors to diabetes-induced brain aging. Biochim. Biophys. Acta Mol. Basis Dis. 2009, 1792, 444–453. [Google Scholar] [CrossRef] [Green Version]
- Heikkilä, O.; Lundbom, N.; Timonen, M.; Groop, P.H.; Heikkinen, S.; Mäkimattila, S. Hyperglycaemia is associated with changes in the regional concentrations of glucose and myo-inositol within the brain. Diabetologia 2009, 52, 534–540. [Google Scholar] [CrossRef] [Green Version]
- Söllvander, S.; Nikitidou, E.; Gallasch, L.; Zyśk, M.; Söderberg, L.; Sehlin, D.; Lannfelt, L.; Erlandsson, A. The Aβ protofibril selective antibody mAb158 prevents accumulation of Aβ in astrocytes and rescues neurons from Aβ-induced cell death. J. Neuroinflam. 2018, 15, 98. [Google Scholar] [CrossRef]
- Szutowicz, A.; Bielarczyk, H.; Jankowska-Kulawy, A.; Pawełczyk, T.; Ronowska, A. Acetyl-CoA the key factor for survival or death of cholinergic neurons in course of neurodegenerative diseases. Neurochem. Res. 2013, 38, 1523–1542. [Google Scholar] [CrossRef] [Green Version]
- Szutowicz, A.; Bielarczyk, H.; Ronowska, A.; Gul-Hinc, S.; Klimaszewska-Łata, J.; Dyś, A.; Zyśk, M.; Pawełczyk, T. Intracellular redistribution of acetyl-CoA, the pivotal point in differential susceptibility of cholinergic neurons and glial cells to neurodegenerative signals. Biochem. Soc. Trans. 2014, 42, 1101–1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halim, N.D.; McFate, T.; Mohyeldin, A.; Okagaki, P.; Korotchkina, L.G.; Patel, M.S.; Jeoung, N.H.; Harris, R.A.; Schell, M.J.; Verma, A. Phosphorylation status of pyruvate dehydrogenase distinguishes metabolic phenotypes of cultured rat brain astrocytes and neurons. GLIA 2010, 58, 1168–1176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jha, M.K.; Jeon, S.; Suk, K. Pyruvate dehydrogenase kinases in the nervous system: Their principal functions in neuronal-glial metabolic interaction and neuro-metabolic disorders. Curr. Neuropharmacol. 2012, 10, 393–403. [Google Scholar] [PubMed]
- Stacpoole, P.W. Therapeutic Targeting of the Pyruvate Dehydrogenase Complex/Pyruvate Dehydrogenase Kinase (PDC/PDK) Axis in Cancer. J. Natl. Cancer. Inst. 2017, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zyśk, M.; Sakowicz-Burkiewicz, M.; Pikul, P.; Kowalski, R.; Michno, A.; Pawełczyk, T. The impact of acetyl-CoA and aspartate shortages on the N-acetylaspartate level in different models of cholinergic neurons. Antioxidants 2020, 13, 522. [Google Scholar] [CrossRef]
- Adela, R.; Nethi, S.K.; Bagul, P.K.; Barui, A.K.; Mattapally, S.; Kuncha, M.; Patra, C.R.; Reddy, P.N.C.; Banerjee, S.K. Hyperglycaemia enhances nitric oxide production in diabetes: A study from South Indian patients. PLoS ONE 2015, 10, e0125270. [Google Scholar] [CrossRef] [Green Version]
- Yang, P.; Cao, Y.; Li, H. Hyperglycemia induces inducible nitric oxide synthase gene expression and consequent nitrosative stress via c-Jun N-terminal kinase activation. Am. J. Obstet. Gynecol. 2010, 203, e5–e185. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Fu, Y.; Xu, X.; Li, M.; Du, L.; Han, Y.; Ge, Y. PERK pathway are involved in NO-induced apoptosis in endothelial cells cocultured with RPE under high glucose conditions. Nitric Oxide Biol. Chem. 2014, 40, 10–16. [Google Scholar] [CrossRef]
- Assmann, T.S.; Brondani, L.A.; Bouças, A.P.; Rheinheimer, J.; de Souza, B.M.; Canani, L.H.; Bauer, A.C.; Crispim, D. Nitric oxide levels in patients with diabetes mellitus: A systematic review and meta-analysis. Nitric Oxide Biol. Chem. 2016, 61, 1–9. [Google Scholar] [CrossRef]
- Bradley, S.A.; Steinert, J.R. Characterisation and comparison of temporal release profiles of nitric oxide generating donors. J. Neurosci. Meth. 2015, 245, 116–124. [Google Scholar] [CrossRef] [Green Version]
- Bielarczyk, H.; Jankowska, A.; Madziar, B.; Matecki, A.; Michno, A.; Szutowicz, A. Differential toxicity of nitric oxide, aluminum, and amyloid beta-peptide in SN56 cholinergic cells from mouse septum. Neurochem. Int. 2003, 42, 323–331. [Google Scholar] [CrossRef]
- Palmieri, E.M.; Gonzalez-Cotto, M.; Baseler, W.A.; Davies, L.C.; Ghesquière, B.; Maio, N.; Rice, C.M.; Rouault, T.A.; Cassel, T.; Higashi, R.M.; et al. Nitric oxide orchestrates metabolic rewiring in M1 macrophages by targeting aconitase 2 and pyruvate dehydrogenase. Nat. Commun. 2020, 11, 698. [Google Scholar] [CrossRef] [PubMed]
- Feldman, E.L.; Callaghan, B.C.; Pop-Busui, R.; Zochodne, D.W.; Wright, D.E.; Bennett, D.L.; Bril, V.; Russell, J.W.; Viswanathan, V. Diabetic neuropathy. Nature Rev. Dis. Primers 2019, 5, 42. [Google Scholar] [CrossRef] [PubMed]
- Benarroch, E.E. Brain glucose transporters: Implications for neurologic disease. Neurology 2014, 82, 1374–1379. [Google Scholar] [CrossRef]
- Zhao, L.; Dong, M.; Wang, D.; Ren, M.; Zheng, Y.; Zheng, H.; Li, C.; Gao, H. Characteristic metabolic alterations identified in primary neurons under high glucose exposure. Front. Cell Neurosci. 2018, 12, 207. [Google Scholar] [CrossRef]
- Turner, D.A.; Adamson, D.C. Neuronal-astrocyte metabolic interactions: Understanding the transition into abnormal astrocytoma metabolism. J. Neuropath. Exp. Neur. 2011, 70, 167–176. [Google Scholar] [CrossRef]
- Campbell, I.; Campbell, H. A pyruvate dehydrogenase complex disorder hypothesis for bipolar disorder. Med. Hypotheses 2019, 130, 109263. [Google Scholar] [CrossRef]
- Yellen, G. Fueling thought: Management of glycolysis and oxidative phosphorylation in neuronal metabolism. J. Cell. Biol. 2018, 217, 2235–2246. [Google Scholar] [CrossRef]
- Edmond, J.; Higa, T.A.; Korsak, R.A.; Bergner, E.A.; Lee, W.N.P. Fatty acid transport and utilization for the developing brain. J. Neurochem. 1998, 70, 1227–1234. [Google Scholar] [CrossRef] [Green Version]
- Blázquez, C.; Sánchez, C.; Daza, A.; Galve-Roperh, I.; Guzmán, M. The stimulation of ketogenesis by cannabinoids in cultured astrocytes defines carnitine palmitoyltransferase I as a new ceramide-activated enzyme. J. Neurochem. 1999, 72, 1759–1768. [Google Scholar] [CrossRef]
- Mahinpour, R.; Riazi, G.; Shokrgozar, M.A.; Sarbolouki, M.N.; Ahmadian, S.; Douraghi, M.; Alijanvand, H.H.; Azadmanesh, K.; Heidari, M.; Gheshlaghi, Z.N.; et al. Disruption of tubulin polymerization and cell proliferation by 1-naphthylarsonic acid. Cell Biol. Int. 2012, 36, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Jin, Z.; Zheng, H.; Yan, L.J. Sources and implications of NADH/NAD+ redox imbalance in diabetes and its complications. Diabetes Metab. Syndr. Obes. 2016, 9, 145–153. [Google Scholar] [PubMed] [Green Version]
- Jeong, J.Y.; Jeoung, N.H.; Park, K.G.; Lee, I.K. Transcriptional regulation of pyruvate dehydrogenase kinase. Diabetes Metab. J. 2016, 36, 328–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paidi, R.K.; Nthenge-Ngumbau, D.N.; Singh, R.; Kankanala, T.; Mehta, H.; Mehta, H.; Mohanakumar, K.P. Mitochondrial deficits accompany cognitive decline following single bilateral intracerebroventricular streptozotocin. Cur. Alzheimer Res. 2015, 12, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zheng, W.; Xie, J.W.; Wang, T.; Wang, S.L.; Teng, W.P.; Wang, Z.Y. Insulin deficiency exacerbates cerebral amyloidosis and behavioral deficits in an Alzheimer transgenic mouse model. Mol. Neurodegener. 2010, 5, 46. [Google Scholar] [CrossRef] [Green Version]
- Pohanka, M. Alpha7 nicotinic acetylcholine receptor is a target in pharmacology and toxicology. Int. J. Mol. Sci. 2012, 13, 2219–2238. [Google Scholar] [CrossRef] [Green Version]
- Brandon, E.P.; Mellott, T.; Pizzo, D.P.; Coufal, N.; D’Amour, K.A.; Gobeske, K.; Lortie, M.; López-Coviella, I.; Berse, B.; Thal, L.J.; et al. Choline transporter 1 maintains cholinergic function in choline acetyltransferase haploinsufficiency. J. Neurosci. 2004, 24, 5459–5466. [Google Scholar] [CrossRef] [Green Version]
- Sherin, A.; Peeyush, K.T.; Jayanarayanan, S.; Amee, K.K.; Paulose, C.S. Decreased cholinergic receptor expression in the striatum: Motor function deficit in hypoglycemic and diabetic rats. Cell Mol. Neurobiol. 2012, 32, 83–93. [Google Scholar] [CrossRef]
- Chandrasekharan, B.; Anitha, M.; Blatt, R.; Shahnavaz, N.; Kooby, D.; Staley, C.; Mwangi, S.; Jones, D.P.; Sitaraman, S.V.; Srinivasan, S. Colonic motor dysfunction in human diabetes is associated with enteric neuronal loss and increased oxidative stress. Neurogastroenterol. Motil. 2011, 23, e126–e131. [Google Scholar] [CrossRef] [Green Version]
- Boison, D.; Chen, J.F.; Fredholm, B.B. Adenosine signaling and function in glial cells. Cell Death. Differ. 2010, 17, 1071–1082. [Google Scholar] [CrossRef] [Green Version]
- Bathina, S.; Srinivas, N.; Das, U.N. Streptozotocin produces oxidative stress, inflammation and decreases BDNF concentrations to induce apoptosis of RIN5F cells and type 2 diabetes mellitus in Wistar rats. Biochem. Biophys. Res. Commun. 2017, 486, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Fan, S.; Song, D.; Wang, Z.; Ma, S.; Li, S.; Li, X.; Xu, M.; Xu, M.; Wang, X. Long-term streptozotocin-induced diabetes in rats leads to severe damage of brain blood vessels and neurons via enhanced oxidative stress. Mol. Med. Rep. 2013, 7, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Zaroff, S.; Leone, P.; Markov, V.; Francis, J.S. Transcriptional regulation of N-acetylaspartate metabolism in the 5xFAD model of Alzheimer’s disease: Evidence for neuron-glia communication during energetic crisis. Mol. Cell Neurosci. 2015, 65, 143–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tahay, G.; Wiame, E.; Tyteca, D.; Courtoy, P.J.; van Schaftingen, E. Determinants of the enzymatic activity and the subcellular localization of aspartate N-acetyltransferase. Biochem. J. 2012, 441, 105–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyamoto, Y.; Ishikawa, Y.; Iegaki, N.; Sumi, K.; Fu, K.; Sato, K.; Furukawa-Hibi, Y.; Muramatsu, S.I.; Nabeshima, T.; Uno, K.; et al. Overexpression of Shati/Nat8l, an N-acetyltransferase, in the nucleus accumbens attenuates the response to methamphetamine via activation of group II mGluRs in mice. Int. J. Neuropsychopharmacol. 2014, 17, 1283–1294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sumi, K.; Uno, K.; Noike, H.; Tomohiro, T.; Hatanaka, Y.; Sumi, K.; Uno, K.; Noike, H.; Tomohiro, T.; Hatanaka, Y.; et al. Behavioral impairment in SHATI/NAT8L knockout mice via dysfunction of myelination development. Sci. Rep. 2017, 7, 16872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jessen, F.; Lewczuk, P.; Gür, O.; Block, W.; Ende, G.; Frölich, L.; Hammen, T.; Arlt, S.; Kornhuber, J.; Kucinski, T.; et al. Association of N-acetylaspartate and cerebrospinal fluid Aβ42 in dementia. J. Alzheimers Dis. 2011, 27, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Kantarci, K.; Jack, C.R. Neuroimaging in Alzheimer disease. an evidence-based review. Neuroimaging Clin. N. Am. 2003, 3, 197–209. [Google Scholar] [CrossRef]
- Wang, H.; Tan, L.; Wang, H.F.; Liu, Y.; Yin, R.H.; Wang, W.Y.; Chang, X.L.; Jiang, T.; Yu, J.T. Magnetic Resonance Spectroscopy in Alzheimer’s Disease: Systematic Review and Meta-Analysis. J. Alzheimers Dis. 2015, 46, 1049–1070. [Google Scholar] [CrossRef]
- Watanabe, T.; Shiino, A.; Akiguchi, I. Hippocampal metabolites and memory performances in patients with amnestic mild cognitive impairment and Alzheimer’s disease. Neurobiol. Learn. Mem. 2012, 97, 289–293. [Google Scholar] [CrossRef]
- Ma, M.W.; Wang, J.; Zhang, Q.; Wang, R.; Dhandapani, K.M.; Vadlamudi, R.K.; Brann, D.W. NADPH oxidase in brain injury and neurodegenerative disorders. Mol. Neurodegener. 2017, 12, 7. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Lu, J.; Xie, W.; Lu, X.; Liang, Y.; Li, M.; Wang, Z.; Huang, X.; Tang, M.; Pfaff, D.W.; et al. Maternal diabetes induces autism-like behavior by hyperglycemia-mediated persistent oxidative stress and suppression of superoxide dismutase 2. Proc. Natl. Acad. Sci. USA. 2019, 116, 23743–23752. [Google Scholar] [CrossRef] [Green Version]
- Kreft, E.; Kowalski, R.; Jankowski, M.; Szczepańska-Konkel, M. Renal vasculature reactivity to agonist of P2X7 receptor is increased in streptozotocin-induced diabetes. Pharmacol. Rep. 2016, 68, 71–74. [Google Scholar] [CrossRef]
- Almandoz-Gil, L.; Persson, E.; Lindström, V.; Ingelsson, M.; Erlandsson, A.; Bergström, J. In situ proximity ligation assay reveals co-localization of alpha-synuclein and SNARE proteins in murine primary neurons. Front. Neurol. 2018, 9, 180. [Google Scholar] [CrossRef] [Green Version]
- Slein, M.W.; Cori, G.T.; ori, C.F. A comparative study of hexokinase from yeast and animal tissues. J. Biol. Chem. 1950, 186, 763–780. [Google Scholar]
- Koh, J.Y.; Choi, D.W. Quantitative determination of glutamate mediated cortical neuronal injury in cell culture by lactate dehydrogenase efflux assay. J. Neurosci. Methods 1987, 20, 83–90. [Google Scholar] [CrossRef]
- Szutowicz, A.; Bielarczyk, H. Elimination of CoASH interference from acetyl-CoA cycling assay by maleic anhydride. Anal. Biochem. 1987, 164, 292–296. [Google Scholar] [CrossRef]
- Contreras-Sanz, A.; Scott-Ward, T.S.; Gill, H.S.; Jacoby, J.C.; Birch, R.E.; Malone-Lee, J.; Taylor, K.M.G.; Peppiatt-Wildman, C.M.; Wildman, S.S.P. Simultaneous quantification of 12 different nucleotides and nucleosides released from renal epithelium and in human urine samples using ion-pair reversed-phase HPLC. Purinergic. Signal. 2012, 8, 741–751. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; He, P. Improved measurements of intracellular nitric oxide in intact microvessels using 4,5-diaminofluorescein diacetate. Am. J. Phys. Heart. Circ. Physiol. 2011, 301, H108–H114. [Google Scholar] [CrossRef]
- Miranda, K.M.; Espey, M.G.; Wink, D.A. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 2001, 5, 62–71. [Google Scholar] [CrossRef]
- Gibon, Y.; Larher, F. Cycling assay for nicotinamide adenine dinucleotides: NaCl precipitation and ethanol solubilization of the reduced tetrazolium. Anal. Biochem. 1997, 251, 153–157. [Google Scholar] [CrossRef]
- Zyśk, M.; Gapys, B.; Ronowska, A.; Gul-Hinc, S.; Erlandsson, A.; Iwanicki, A.; Sakowicz-Burkiewicz, M.; Szutowicz, A.; Bielarczyk, H. Protective effects of voltage-gated calcium channel antagonists against zinc toxicity in SN56 neuroblastoma cholinergic cells. PLoS ONE 2018, 13, e0209363. [Google Scholar]


| Parameter | Added | Primary Neurons | Neural Stem Cells | Wistar Rat’s Brain Tissue |
|---|---|---|---|---|
| Pyruvate dehydrogenase complex nmol/min/mg protein | Control/Sham | 17.7 ± 4.9 | 7.0 ± 3.0 | 23.8 ± 9.9 |
| 0.2 mM SNAP | 22.8 ± 6.8 | |||
| 50 mM glucose | 6.2 ± 2.0 | |||
| mDM | 17.4 ± 4.9 | |||
| Lactate dehydrogenase µmol/min/mg protein | Control/Sham | 0.4 ± 0.1 | 1.3 ± 0.4 | |
| 50 mM glucose | 0.5 ± 0.6 ** | |||
| mDM | 1.0 ± 0.6 | |||
| Aspartate aminotransferase nmol/min/mg protein | Control/Sham | 0.2 ± 0.05 | 0.4 ± 0.2 | |
| 50 mM glucose | 0.2 ± 0.05 | |||
| mDM | 0.5 ± 0.2 | |||
| Hexokinase µmol/min/mg protein | Control/Sham | 26.0 ± 7.0 | ||
| mDM | 59.0 ± 33.0 | |||
| Citrate synthase µmol/min/mg protein | Control/Sham | 34.3 ± 4.0 | 44.4 ± 19.6 | |
| 50 mM glucose | 41.8 ± 5.3 ** | |||
| mDM | 35.4 ± 15.4 | |||
| Aconitase µmol/min/mg protein | Control/Sham | 36.4 ± 9.3 | 23.0 ± 7.2 | 31.5 ± 11.1 |
| 0.2 mM SNAP | 48.4 ± 12.7 * | |||
| 50 mM glucose | 30.0 ± 6.5 | |||
| mDM | 26.8 ± 16.9 | |||
| Isocitrate dehydrogenase µmol/min/mg protein | Control/Sham | 68.3 ± 22.3 | 56.7 ± 11.1 | 14.7 ± 7.1 |
| 0.2 mM SNAP | 80.2 ± 34.7 | |||
| 50 mM glucose | 61.9 ± 8.4 | |||
| mDM | 9.6 ± 5.8 |
| Parameter | Added | Primary Neurons | Neural Stem Cells | Wistar Rat’s Brain Tissue |
|---|---|---|---|---|
| Pyruvate nmol/mg protein | Control/Sham | 50.7 ± 27.0 | 24.9 ± 8.5 | 13.2 ± 7.4 |
| 0.2 mM SNAP | 25.3 ± 15.5 ** | |||
| 50 mM glucose | 75.2 ± 23.7 ** | |||
| mDM | 10.3 ± 5.5 | |||
| Lactate nmol/mg protein | Control/Sham | 15.3 ± 4.3 | 10.7 ± 5.5 | 25.0 ± 11.3 |
| 0.2 mM SNAP | 49.5 ± 15.2 *** | |||
| 50 mM glucose | 5.7 ± 5.1 | |||
| mDM | 35.3 ± 12.3 ** | |||
| Citrate nmol/mg protein | Control/Sham | 45.8 ± 13. | 26.4 ± 9.3 | |
| 0.2 mM SNAP | 16.9 ± 11.2 ** | |||
| 50 mM glucose | 10.4 ± 4.7 *** | |||
| Oxaloacetate nmol/mg protein | Control/Sham | 5.1 ± 0.6 | 2.3 ± 0.9 | 0.5 ± 0.4 |
| 0.2 mM SNAP | 3.1 ± 2.5 * | |||
| 50 mM glucose | 2.8 ± 1.5 | |||
| mDM | 0.4 ± 0.1 |
| Parameters | Sham Control | Moderate DM | Severe DM |
|---|---|---|---|
| Body weight (g) | 307 ± 33 | 210 ± 16 *** | 240 ± 50 * |
| Blood glucose (mg/dL) | 127 ± 13 | 513 ± 56 *** | 523 ± 92 *** |
| Urine acetoacetate (µmol/24 h) | 0.3 ± 0.1 | 1.9 ± 1.0 ** | 5.8 ± 2.4 *** |
| Gene Transcript | Primers | TaqMan Probe | Transcript of Reference Gene |
|---|---|---|---|
| Nat8l NM_001191681.1 | (F) tggctgacattgaacagtactaca (R) cacaacattgccgtccag | Universal ProbeLibrary Probe #83 (Roche, Cat #04689062001) | Universal ProbeLibrary Rat Actb Gene Assay |
| Chat NM_001170593.1 | (F) gaagcttccaagccactttc (R) gtagtagagcctcagacgacgac | Universal ProbeLibrary Probe #66 (Roche, Cat #04688651001) | (Roche, Cat #05046203001) |
| Target Protein | Type of Antibody | Company | Cat# |
|---|---|---|---|
| β-actin | mouse primary monoclonal | Sigma Aldrich | A2228 |
| NAT8L | rabbit primary polyclonal | Thermo Fisher Sc. | PA5-49536 |
| Bcl-2 | rabbit primary polyclonal | Abcam | Ab59348 |
| β-III-tubulin | rabbit primary monoclonal | Cell Signaling | 5568T |
| Caspase-3 | rabbit primary polyclonal | Cell Signaling | 9662s |
| ChAT | rabbit primary polyclonal | MyBioSource | MBS127981 |
| CNPase | mouse primary monoclonal | Sigma Aldrich | C5922 |
| GFAP | rabbit primary polyclonal | DAKO | Z0334 |
| GAPDH | mouse primary monoclonal | Abcam | ab8245 |
| Goat IgG | rabbit secondary polyclonal AP–conjugated | Sigma Aldrich | A4187 |
| CHT-1 | rabbit primary polyclonal | MyBioSource | MBS129733 |
| Mouse IgG | goat secondary polyclonal AP–conjugated | Sigma Aldrich | A3562 |
| Mouse IgG1 | goat secondary polyclonal 488–conjugated | Thermo Fisher Sc. | A21121 |
| NeuN | rabbit primary monoclonal | Cell Signaling | 24307T |
| nAChR | rabbit primary polyclonal | Santa Cruz SCBT | sc-5591 |
| PARP | rabbit primary polyclonal | Millipore | AB16661 |
| Rabbit IgG | goat secondary polyclonal AP–conjugated | Santa Cruz SCBT | sc-2007 |
| Rabbit IgG | goat secondary polyclonal 555–conjugated | Thermo Fisher Sc. | A21428 |
| Synaptophysin | rabbit primary polyclonal | Abcam | ab14692 |
| VAChT | goat primary polyclonal | Thermo Fisher Sc. | OSG00003W |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zyśk, M.; Pikul, P.; Kowalski, R.; Lewandowski, K.; Sakowicz-Burkiewicz, M.; Pawełczyk, T. Neither Excessive Nitric Oxide Accumulation nor Acute Hyperglycemia Affects the N-Acetylaspartate Network in Wistar Rat Brain Cells. Int. J. Mol. Sci. 2020, 21, 8541. https://doi.org/10.3390/ijms21228541
Zyśk M, Pikul P, Kowalski R, Lewandowski K, Sakowicz-Burkiewicz M, Pawełczyk T. Neither Excessive Nitric Oxide Accumulation nor Acute Hyperglycemia Affects the N-Acetylaspartate Network in Wistar Rat Brain Cells. International Journal of Molecular Sciences. 2020; 21(22):8541. https://doi.org/10.3390/ijms21228541
Chicago/Turabian StyleZyśk, Marlena, Piotr Pikul, Robert Kowalski, Krzysztof Lewandowski, Monika Sakowicz-Burkiewicz, and Tadeusz Pawełczyk. 2020. "Neither Excessive Nitric Oxide Accumulation nor Acute Hyperglycemia Affects the N-Acetylaspartate Network in Wistar Rat Brain Cells" International Journal of Molecular Sciences 21, no. 22: 8541. https://doi.org/10.3390/ijms21228541
APA StyleZyśk, M., Pikul, P., Kowalski, R., Lewandowski, K., Sakowicz-Burkiewicz, M., & Pawełczyk, T. (2020). Neither Excessive Nitric Oxide Accumulation nor Acute Hyperglycemia Affects the N-Acetylaspartate Network in Wistar Rat Brain Cells. International Journal of Molecular Sciences, 21(22), 8541. https://doi.org/10.3390/ijms21228541







