Evaluation of Reductions in Fume Emissions (VOCs and SVOCs) from Warm Mix Asphalt Incorporating Natural Zeolite and Reclaimed Asphalt Pavement for Sustainable Pavements
Abstract
:1. Introduction
2. Materials and Methods
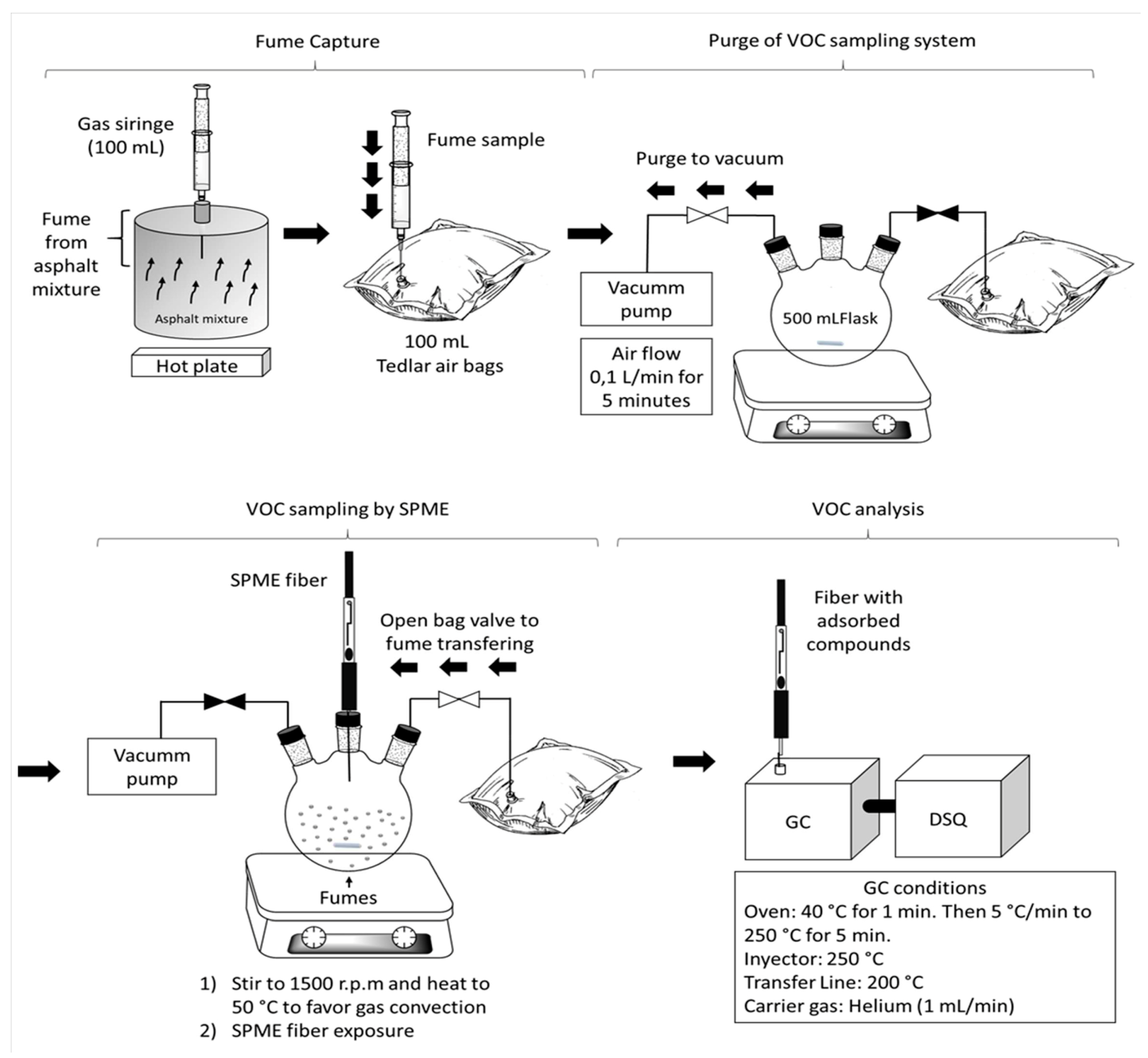
2.1. Asphalt Mixture Preparation and Fume Capture
2.2. Sampling of VOCs and SVOCs from Asphalt Mixture Fumes by SPME
2.3. Analysis of Asphalt Mixture Fumes
2.4. Statistical Analysis
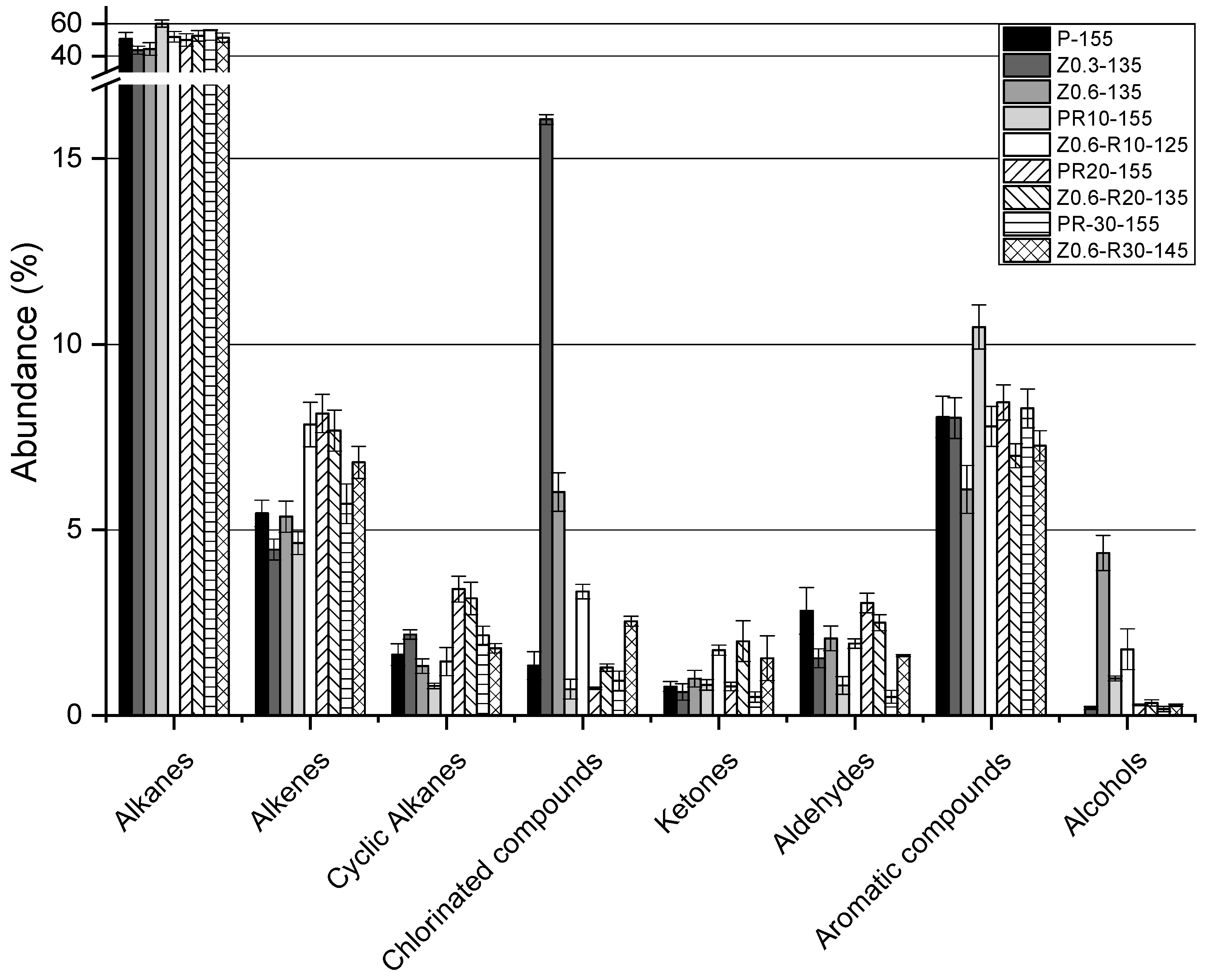
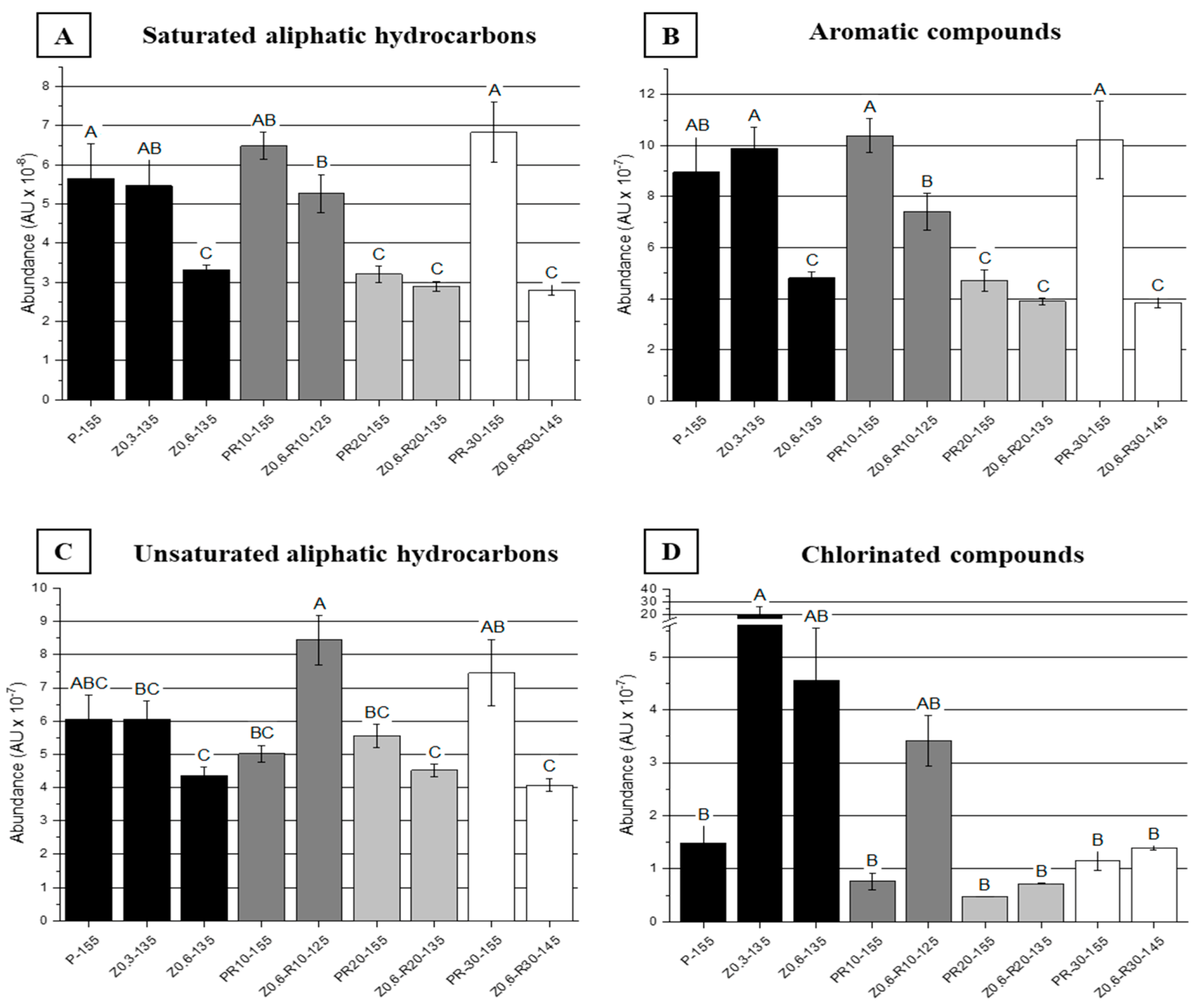
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Leng, Z.; Li, X.; Hu, C. Cold recycling of reclaimed asphalt pavement towards improved engineering performance. J. Clean. Prod. 2018, 171, 1031–1038. [Google Scholar] [CrossRef]
- Raposeiras, A.; Movilla, D.; Vargas, A.; Bilbao, R.; Cifuentes, C. Evaluation of Marshall stiffness, indirect tensile stress and resilient modulus in asphalt mixes with reclaimed asphalt pavement and copper slag. Rev. Ing. Constr. 2017, 32, 15–24. [Google Scholar] [CrossRef] [Green Version]
- Ghanizadeh, A.R.; Rahrovan, M.; Bafghi, K.B. The effect of cement and reclaimed asphalt pavement on the mechanical properties of stabilized base via full-depth reclamation. Constr. Build Mater. 2018, 161, 165–174. [Google Scholar] [CrossRef]
- Gudimettla, J.M.; Cooley, L.A., Jr.; Brown, E.R. Workability of Hot Mix Asphalt. NCAT Rep. 03-03; Auburn Univiversity: Auburn, AL, USA, 2002. [Google Scholar]
- Linch, K.D. Respirable Concrete Dust—Silicosis Hazard in the Construction Industry. Appl. Occup. Environ. Hyg. 2002, 17, 209–221. [Google Scholar] [CrossRef] [PubMed]
- McCLean, M.D.; Rinehart, R.D.; Ngo, L.; Eisen, E.A.; Kelsey, K.T.; Herrick, R.F. Inhalation and dermal exposure among asphalt paving workers. Ann. Occup. Hyg. 2004, 48, 663–671. [Google Scholar]
- European Asphalt Pavement Association (EAPA). EAPA Position Paper on the Use of Warm Mix Asphalt. 2010. Available online: https://eapa.org/wp-content/uploads/2018/07/the_use_of_warm_mix_asphalt_january_2010.pdf (accessed on 10 November 2020).
- Autelitano, F.; Bianchi, F.; Giuliani, F. Airborne emissions of asphalt/wax blends for warm mix asphalt production. J. Clean. Prod. 2017, 164, 749–756. [Google Scholar] [CrossRef]
- Capitão, S.; Picado-Santos, L.; Martinho, F. Pavement engineering materials: Review on the use of warm-mix asphalt. Constr. Build Mater. 2012, 36, 1016–1024. [Google Scholar] [CrossRef]
- Rubio, M.D.C.; Moreno, F.; Martínez-Echevarría, M.J.; Martínez, G.; Vásquez, J.M. Warm mix asphalt: An overview. J. Clean. Prod. 2013, 41, 1–6. [Google Scholar] [CrossRef]
- Rubio, M.D.C.; Martinez, G.; Baena, L.; Moreno, F. Warm mix asphalt: An overview. J. Clean. Prod. 2012, 24, 76–84. [Google Scholar] [CrossRef]
- Kheradmand, B.; Muniandy, R.; Hua, L.T.; Yunus, R.B.; Solouki, A. An overview of the emerging warm mix asphalt technology. Int. J. Pavement Eng. 2014, 15, 79–94. [Google Scholar] [CrossRef]
- Woszuk, A.; Franus, W. Properties of the Warm Mix Asphalt involving clinoptilolite and Na-P1 zeolite additives. Constr. Build. Mater. 2016, 114, 556–563. [Google Scholar] [CrossRef]
- Pérez-Martínez, M.; Moreno-Navarro, F.; Martín-Marín, J.; Ríos-Losada, C.; Rubio-Gámez, M.C. Analysis of cleaner technologies based on waxes and surfactant additives in road construction. J. Clean. Prod. 2014, 65, 374–379. [Google Scholar] [CrossRef]
- Qin, Q.; Farrar, M.J.; Pauli, A.T.; Adams, J.J. Morphology, thermal analysis and rheology of Sasobit modified warm mix asphalt binders. Fuel 2014, 115, 416–425. [Google Scholar] [CrossRef]
- Sharma, A.; Lee, B. Novel nanocomposite of Ca(OH)2-incorporated zeolite as an additive to reduce atmospheric emissions of PM and VOCs during asphalt production. Environ. Sci. Nano 2017, 4, 613–624. [Google Scholar] [CrossRef]
- Sengoz, B.; Topal, A.; Gorkem, C. Evaluation of natural zeolite as warm mix asphalt additive and its comparison with other warm mix additives. Constr. Build. Mater. 2013, 43, 242–252. [Google Scholar] [CrossRef]
- Topal, A.; Sengoz, B.; Kok, B.V.; Yilmaz, M.; Dokandari, P.A.; Oner, J.; Kaya, D. Evaluation of mixture characteristics of warm mix asphalt involving natural and synthetic zeolite additives. Constr. Build. Mater. 2014, 57, 38–44. [Google Scholar] [CrossRef]
- Woszuk, A.; Franus, W. A Review of the Application of Zeolite Materials in Warm Mix Asphalt Technologies. Appl. Sci. 2017, 7, 293. [Google Scholar] [CrossRef] [Green Version]
- Woszuk, A.; Zofka, A.; Bandura, L.; Franus, W. Effect of zeolite properties on asphalt foaming. Constr. Build. Mater. 2017, 139, 247–255. [Google Scholar] [CrossRef]
- Franus, W.; Wdowin, M.; Franus, M. Synthesis and characterization of zeolites prepared from industrial fly ash. Environ. Monit. Assess. 2014, 186, 5721–5729. [Google Scholar] [CrossRef] [Green Version]
- Franus, M.; Wdowin, M.; Bandura, L.; Franus, W. Removal of environmental pollutions using zeolites from fly ash: A review. Fresenius Environ. Bull. 2015, 24, 854–866. [Google Scholar]
- Boczkaj, G.; Przyjazny, A.; Kamiński, M. Characteristics of volatile organic compounds emissions profiles from hot road bitumens. Chemosphere 2014, 107, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Boczkaj, G.; Kamiński, M.; Przyjazny, A. Process control and investigation of oxidation kinetics of postoxidative effluents using gas chromatography with pulsed flame photometric detector (GC-PFPD). Ind. Eng. Chem. Res. 2010, 49, 12654–12662. [Google Scholar] [CrossRef]
- Boczkaj, G.; Przyjazny, A.; Kamiński, M. New procedures for control of industrial effluents treatment processes. Ind. Eng. Chem. Res. 2014, 53, 1503–1514. [Google Scholar] [CrossRef]
- Deygout, F. Volatile emissions from hot bitumen storage tanks. Environ. Prog. Sustain. Energy 2011, 30, 102–112. [Google Scholar] [CrossRef]
- Davie, F.M.; Mores, S.; Nolan, P.F.; Hoban, T.W.S. Evidence of the oxidation of deposits in heated bitumen storage tanks. J. Loss Prev. Process Ind. 1993, 6, 145–150. [Google Scholar] [CrossRef]
- Davie, F.M.; Nolan, P.F.; Hoban, T.W.S. Study of iron sulfide as a possible ignition source in the storage of heated bitumen. J. Loss Prev. Process Ind. 1993, 6, 139–143. [Google Scholar] [CrossRef]
- Trumbore, D.C. Estimates of air emissions from asphalt storage tanks and truck loading. Environ. Prog. 1999, 18, 250–259. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency (EPA). Control of Volatile Organic Compounds from Use of Cutback Asphalt. 2000. Available online: https://nepis.epa.gov/Exe/ZyPDF.cgi?Dockey=2000UKB7.pdf (accessed on 10 November 2020).
- U.S. Environmental Protection Agency (EPA). Hot Mix Asphalt Plants—Emission Assessment Report. 2000. Available online: https://www3.epa.gov/ttnchie1/ap42/ch11/related/ea-report.pdf (accessed on 10 November 2020).
- Ventura, A.; Jullien, A.; Moneron, P. Polycyclic aromatic hydrocarbons emitted from a hot-mix drum, asphalt plant: Study of the influence from use of recycled bitumen. J. Environ. Eng. Sci. 2007, 6, 727–734. [Google Scholar] [CrossRef] [Green Version]
- Ruehl, R.; Musanke, U.; Kolmsee, K.; Priess, R.; Breuer, D. Bitumen emissions on workplaces in Germany. J. Occup. Environ. Hyg. 2007, 4, 77–86. [Google Scholar] [CrossRef]
- Gasthauer, E.; Mazé, M.; Marchand, J.P.; Amouroux, J. Characterization of asphalt fume composition by GC/MS and effect of temperature. Fuel 2008, 87, 1428–1434. [Google Scholar] [CrossRef]
- Chauhan, S.K.; Sharma, S.; Shukla, A.; Gangopadhyay, S. Recent trends of the emission characteristics from the road construction industry. Environ. Sci. Pollut. Res. 2010, 17, 1493–1501. [Google Scholar] [CrossRef]
- Jullien, A.; Gaudefroy, V.; Ventura, A.; de la Roche, C.; Paranhos, R.; Moneron, P. Airborne emissions assessment of hot asphalt mixing methods and limitations. Road Mater. Pavement Des. 2010, 11, 149–169. [Google Scholar] [CrossRef]
- Rogge, W.F.; Hildemann, L.M.; Mazurek, M.A.; Cass, G.R.; Simoneit, B.R.T. Sources of fine organic aerosol 7. Hot asphalt roofing tar pot fumes. Environ. Sci. Technol. 1997, 31, 2726–2730. [Google Scholar] [CrossRef]
- Trumbore, D.C. The magnitude and source of air emissions from asphalt blowing operations. Environ. Prog. 1998, 17, 53–59. [Google Scholar] [CrossRef]
- Franzen, M.R.; Trumbore, D.C. Reduction of asphalt fumes in roofing kettles. Environ. Sci. Technol. 2000, 34, 2582–2586. [Google Scholar] [CrossRef]
- Kriech, A.J.; Osborn, L.V.; Trumbore, D.C.; Kurek, J.T.; Wissel, H.L.; Rosinski, K.D. Evaluation of worker exposure to asphalt roofing fumes: Influence of work practices and materials. J. Occup. Environ. Hyg. 2004, 1, 88–98. [Google Scholar] [CrossRef]
- Parker, C.M.; Schreiner, C.A.; Hallmark, N.; Kriech, A.J.; Osborn, L.V.; Fuhst, R.; Buschmann, J.; Ernst, H.; Hansen, T.; Pohlmann, G.; et al. Evaluation of reproductive/developmental and repeated dose (subchronic) toxicity and cytogenetic effects in rats of a roofing asphalt fume condensate by nose-only inhalation. Regul. Toxicol. Pharm. 2011, 59, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Brandt, H.C.A.; Degroot, P.C.; Molyneux, M.K.B.; Tindle, P.E. Sampling and analysis of bitumen fumes. Ann. Occup. Hyg. 1985, 29, 27–30. [Google Scholar] [PubMed]
- Monarca, S.; Pasquini, R.; Scassellati Sforzolini, G.; Savino, A.; Bauleo, F.A.; Angeli, G. Environmental monitoring of mutagenic/carcinogenic hazards during road paving operations with bitumens. Int. Arch. Occup. Environ. Health 1987, 59, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Greenspan, C.A.; Moure-Eraso, R.; Wegman, D.H.; Oliver, L.C. Occupational hygiene characterization of a highway construction project: A pilot study. Appl. Occup. Environ. Hyg. 1995, 10, 50–58. [Google Scholar] [CrossRef]
- Hicks, J.B. Asphalt industry cross-sectional exposure assessment study. Appl. Occup. Environ. Hyg. 1995, 10, 840–848. [Google Scholar] [CrossRef]
- Gamble, J.F.; Nicolich, M.J.; Barone, N.J.; Vincent, W.J. Exposure-response of asphalt fumes with changes in pulmonary function and symptoms. Scand. J. Work Environ. Health 1999, 25, 186–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burstyn, I.; Kromhout, H. Are all the members of a paving crew uniformly exposed to bitumen fume, organic vapour and benzo(a)pyrene? Risk Anal. 2000, 20, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Burstyn, I.; Boffetta, P.; Kauppinen, T.; Heikkila, P.; Svane, O.; Partanen, T.; Stucker, I.; Frentzel-Beyme, R.; Ahrens, W.; Merzenich, H.; et al. Estimating exposures in the asphalt industry for an international epidemiological cohort study of cancer risk. Am. J. Ind. Med. 2003, 43, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Posniak, M. Polycyclic aromatic hydrocarbons in the occupational environment during exposure to bitumen fume. Pol. J. Environ. Stud. 2005, 14, 809–815. [Google Scholar]
- Preiss, A.; Koch, W.; Kock, H.; Elend, M.; Raabe, M.; Pohlmann, G. Collection, validation and generation of bitumen fumes for inhalation studies in rats—Part 1: Workplace samples and validation criteria. Ann. Occup. Hyg. 2006, 50, 789–804. [Google Scholar] [PubMed] [Green Version]
- Hugener, M.; Emmenegger, L.; Mattrel, P. Hot-recycling of tar-containing asphalt pavements emission measurements in the laboratory and in the field. Road Mater. Pavement Des. 2010, 11, 29–46. [Google Scholar] [CrossRef]
- Breuer, D.; Hahn, J.-U.; Hober, D.; Emmel, C.; Musanke, U.; Ruhl, R.; Spickenheuer, A.; Raulf-Heimsoth, M.; Bramer, R.; Seidel, A.; et al. Air sampling and determination of vapours and aerosols of bitumen and polycyclic aromatic hydrocarbons in the human bitumen study. Arch. Toxicol. 2011, 85, S11–S20. [Google Scholar] [CrossRef]
- Spickenheuer, A.; Ruhl, R.; Hober, D.; Raulf-Heimsoth, M.; Marczynski, B.; Welge, P.; Breuer, D.; Gabriel, S.; Musanke, U.; Rode, P.; et al. Levels and determinants of exposure to vapours and aerosols of bitumen. Arch. Toxicol. 2011, 85, S21–S28. [Google Scholar] [CrossRef]
- Raulf-Heimsoth, M.; Pesch, B.; Rühl, R.; Brüning, T. The human bitumen study: Executive summary. Arch. Toxicol. 2011, 85, S3–S9. [Google Scholar] [CrossRef]
- Boczkaj, G.; Makoś, P.; Przyjazny, A. Application of dispersive liquid–liquid microextraction and gas chromatography with mass spectrometry for the determination of oxygenated volatile organic compounds in effluents from the production of petroleum bitumen. J. Sep. Sci. 2016, 39, 2604–2615. [Google Scholar] [CrossRef] [PubMed]
- Boczkaj, G.; Makoś, P.; Przyjazny, A. Application of dynamic headspace and gas chromatography coupled to mass spectrometry (DHS-GC-MS) for the determination of oxygenated volatile organic compounds in refinery effluents. Anal. Methods 2016, 8, 3570–3577. [Google Scholar] [CrossRef] [Green Version]
- Thives, L.P.; Ghisi, E. Asphalt mixtures emission and energy consumption: A review. Renew. Sustain. Energy Rev. 2017, 72, 473–484. [Google Scholar] [CrossRef]
- Kitto, A.M.; Pirbazari, M.; Badriyha, B.N.; Ravindran, V.; Tyner, R.; Synolakis, C.E. Emissions of volatile and semi-volatile organic compounds and particulate matter from hot asphalts. Environ. Technol. 1997, 18, 121–138. [Google Scholar] [CrossRef]
- Tang, B.; Isacsson, U. Chemical characterization of oil-based asphalt release agents and their emissions. Fuel 2006, 85, 1232–1241. [Google Scholar] [CrossRef]
- Wang, J.; Lewis, D.M.; Castranova, V.; Frazer, D.G.; Goldsmith, T.; Tomblyn, S.; Simpson, J.; Stone, S.; Afshari, A.; Siegel, P.D. Characterization of asphalt fume composition under simulated road paving conditions by GC/MS and microflow LC/Quadrupole Time-of-Flight MS. Anal. Chem. 2001, 73, 3691–3700. [Google Scholar] [CrossRef]
- Boczkaj, G.; Makoś, P.; Fernandes, A.; Przyjazny, A. New procedure for the control of the treatment of industrial effluents to remove volatile organosulfur compounds. J. Sep. Sci. 2016, 39, 3946–3956. [Google Scholar] [CrossRef]
- Boczkaj, G.; Makoś, P.; Fernandes, A.; Przyjazny, A. New procedure for the examination of the degradation of volatile organonitrogen compounds during the treatment of industrial effluents. J. Sep. Sci. 2017, 40, 1301–1309. [Google Scholar] [CrossRef]
- Makoś, P.; Fernandes, A.; Boczkaj, G. Method for the determination of carboxylic acids in industrial effluents using dispersive liquid-liquid microextraction with injection port derivatization gas chromatography–mass spectrometry. J. Chromatogr. A 2017, 1517, 26–34. [Google Scholar] [CrossRef]
- Makoś, P.; Fernandes, A.; Boczkaj, G. Method for the simultaneous determination of monoaromatic and polycyclic aromatic hydrocarbons in industrial effluents using dispersive liquid–liquid microextraction with gas chromatography–mass spectrometry. J. Sep. Sci. 2018, 41, 2360–2367. [Google Scholar] [CrossRef]
- American Society for Testing and Materials (ASTM D3549). Standard Test Method for Thickness or Height of Compacted Bituminous Paving Mixture Specimens; American Society for Testing and Materials: West Conshohocken, PA, USA, 2011. [Google Scholar]
- Solaimanian, M.; Tahmoressi, M. Variability analysis of hot-mix asphalt concrete containing high percentage of reclaimed asphalt pavement. Transp. Res. Rec. J. Transp. Res. Board 1996, 1543, 13–20. [Google Scholar] [CrossRef]
- Valdes-Vidal, G.; Calabi-Floody, A.; Sanchez-Alonso, E. Performance evaluation of warm mix asphalt involving natural zeolite and reclaimed asphalt pavement (RAP) for sustainable pavement. Constr. Build. Mater. 2018, 174, 576–585. [Google Scholar] [CrossRef]
- Ouyang, G. 8-SPME and Environmental Analysis. In Handbook of Solid Phase Microextraction, 1st ed.; Pawliszyn, J., Ed.; Elsevier: Waltham, MA, USA, 2012; pp. 251–290. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Kovats, E.; Keulemans, A.I.M. The Kováts Retention Index System. Anal. Chem. 1964, 36, 31A–41A. [Google Scholar]
- Wang, H.; Geppert, H.; Fischer, T.; Wieprecht, W.; Möller, D. Determination of Volatile Organic and Polycyclic Aromatic Hydrocarbons in Crude Oil with Efficient Gas-Chromatographic Methods. J. Chromatogr. Sci. 2014, 53, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Benzene; Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2007.
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Toluene; Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2017.
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Xylenes; Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2007.
- Henschler, D. Toxicity of Chlorinated Organic Compounds: Effects of the Introduction of Chlorine in Organic Molecules. Angew. Chem. Int. Ed. Engl. 1994, 33, 1920–1935. [Google Scholar] [CrossRef]
- Ábalos, M.; Bayona, J.M.; Ventura, F. Development of a solid-phase microextraction GC-NPD procedure for the determination of free volatile amines in wastewater and sewage-polluted waters. Anal. Chem. 1999, 71, 3531–3537. [Google Scholar] [CrossRef]
- Tomkin, B.A.; Griest, W.H.; Higgins, C.E. Determination of N-nitrosodimethylamine at part-per-trillion levels in drinking waters and contaminated groundwaters. Anal. Chem. 1995, 67, 4387–4395. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, S.; Nagamura, F.; Sasaki, M.; Fujii, T. Gas chromatography with surface ionization detection of nitro pesticides. Chem. Pap. 2009, 63, 613–616. [Google Scholar] [CrossRef]
- Hwang, Y.; Matsuo, T.; Hanaki, K.; Suzuki, N. Identification and quantification of sulfur and nitrogen containing odorous compounds in wastewater. Water Res. 1995, 29, 711–718. [Google Scholar] [CrossRef]
- Lu, X.; Fan, C.; Shang, J.; Deng, J.; Yin, H. Headspace solid-phase microextraction for the determination of volatile sulfur compounds in odorous hyper-eutrophic freshwater lakes using gas chromatography with flame photometric detection. Microchem. J. 2012, 104, 26–32. [Google Scholar] [CrossRef]
- Lestremau, F.; Desauziers, V.; Fanlo, J.-L. Headspace SPME followed by GC/PFPD for the analysis of malodorous sulfur compounds in liquid industrial effluents. Anal. Bioanal. Chem. 2004, 378, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Hu, S.; Sharma, K.R.; Keller-Lehmann, B.; Yuan, Z. An efficient method for measuring dissolved VOSCs in wastewater using GC-SCD with static headspace technique. Water Res. 2014, 52, 208–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedersen-Bjergaard, S.; Asp, T.N.; Vedde, J.; Carlberg, G.E.; Greibrokk, T. Identification of chlorinated sulfur compounds in pulp mill effluents by GC-MS and GCAED. Chromatographia 1993, 35, 193–198. [Google Scholar] [CrossRef]
- Chong, D.; Wang, Y.P.E.; Guo, H.; Lu, Y.A.M. Volatile Organic Compounds Generated in Asphalt Pavement Construction and Their Health Effects on Workers. J. Constr. Eng. Manag. 2014, 140, 04013051. [Google Scholar] [CrossRef]
- Song, Y.R.; Wang, C.X.; Zhang, Y.Z. Determination of the chemical composition in asphalt aqueous solutions by SPE and GC/MS. Petrol. Sci. Technol. 2011, 29, 1590–1595. [Google Scholar] [CrossRef]
- Sulej-Suchomska, A.M.; Polkowska, Z.; Chmiel, T.; Dymerski, T.M.; Kokot, Z.J.; Namieśnik, J. Solid phase microextraction–comprehensive two-dimensional gas chromatography–time-of-flight mass spectrometry: A new tool for determining PAHs in airport runoff water samples. Anal. Methods 2016, 8, 4509–4520. [Google Scholar] [CrossRef] [Green Version]
- Baimatova, N.; Koziel, J.A.; Kenessov, B. Passive sampling and analysis of naphthalene in internal combustion engine exhaust with retracted SPME device and GC-MS. Atmosphere 2017, 8, 130. [Google Scholar] [CrossRef] [Green Version]
- Mandalakis, M.; Tsapakis, M.; Tsoga, A.; Stephanou, E.G. Gas-particle concentrations and distribution of aliphatic hydrocarbons, PAHs, PCBs and PCDD/Fs in the atmosphere of Athens (Greece). Atmos. Environ. 2002, 36, 4023–4035. [Google Scholar] [CrossRef]
- Tsapakis, M.; Stephanou, E.G. Occurrence of gaseous and particulate polycyclic aromatic hydrocarbons in the urban atmosphere: Study of sources and ambient temperature effect on the gas/particle concentration and distribution. Environ. Pollut. 2005, 133, 147–156. [Google Scholar] [CrossRef]
- Possanzini, M.; di Palo, V.; Gigliucci, P.; Tomasi Scianò, M.C.; Cecinato, A. Determination of phase-distributed PAH in Rome ambient air by denuder/GC-MS method. Atmos. Environ. 2004, 38, 1727–1734. [Google Scholar] [CrossRef]
- Bezabeh, D.Z. Screening of aerosol filter samples for PAHs and Nitro-PAHs by laser desorption ionization TOF Mass Spectrometry. Aerosol Sci. Technol. 1999, 30, 288–299. [Google Scholar] [CrossRef]
- Bertoni, G.; Tappa, R.; Cecinato, A. Environmental monitoring of semi-volatile polycyclic aromatic hydrocarbons by means of diffusive sampling devices and GC-MS analysis. Chromatographia 2001, 53, 312–316. [Google Scholar] [CrossRef]
- Lesellier, E. Extraction and analysis of polycyclic aromatic hydrocarbons (PAHs) by solid phase micro-extraction/supercritical fluid chromatography (SPME/SFC). Analusis 1999, 27, 363–368. [Google Scholar] [CrossRef]
- Cam, D.; Gagni, S.; Meldolesi, L.; Galletti, G. Determination of polycyclic aromatic hydrocarbons in sediment using solid-phase microextraction with gas chromatography-mass spectrometry. J. Chromatogr. Sci. 2000, 38, 55–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doong, R.; Chang, S.; Sun, Y. Solid-phase microextraction and headspace solid-phase microextraction for the determination of high molecular-weight polycyclic aromatic hydrocarbons in water and soil samples. J. Chromatogr. Sci. 2000, 38, 528–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Zhang, J.; Ding, Y.; Zhou, J.; Ni, L.; Sun, C. Quantitative determination of 16 polycyclic aromatic hydrocarbons in soil samples using solid-phase microextraction. J. Sep. Sci. 2009, 32, 3951–3957. [Google Scholar] [CrossRef] [PubMed]
- Tuncel, S.G.; Topal, T. Multifactorial optimization approach for determination of polycyclic aromatic hydrocarbons in sea sediments of Turkish Mediterranean coast. Am. J. Anal. Chem. 2011, 2, 783–794. [Google Scholar] [CrossRef] [Green Version]
- Pacenti, M.; Lofrumento, C.; Dugheri, S.; Zoppi, A.; Borsi, I.; Speranza, A.; Boccalon, P.; Arcangeli, G.; Antoniucci, A.; Castellucci, E.M. Physicochemical characterization of exhaust particulates from gasoline and diesel engines by solid-phase micro extraction sampling and combined Raman microspectroscopic/fast gas-chromotography mass spectrometry analysis. Eur. J. Inflamm. 2009, 7, 25–37. [Google Scholar] [CrossRef]
- Ballesteros, R.; Hernández, J.J.; Lyons, L.L. Determination of PAHs in diesel particulate matter using thermal extraction and solid phase micro-extraction. Atmos. Environ. 2009, 43, 655–662. [Google Scholar] [CrossRef]
- Koziel, J.A.; Odziemkowski, M.; Pawliszyn, J. Sampling and analysis of airborne particulate matter and aerosols using in-needle trap and SPME fiber devices. Anal. Chem. 2001, 73, 47–54. [Google Scholar] [CrossRef]
- Marinković, M.; Milović, T.; Matić, V. Zeolite as Additive in Warm Mix Asphalt. In Proceedings of the 5th International Conference, Contemporary Achievements in Civil Engineering (SERBIA), Subotica, Serbia, 21 April 2017. [Google Scholar] [CrossRef] [Green Version]
- Sargand, S.; Figueroa, J.L.; Edwards, W.; Al-Rawashdeh, A.S. Performance Assessment of Warm Mix Asphalt (WMA) Pavements (Report FHWA/OH-2009/08); Ohio University: Athens, OH, USA, 2009. [Google Scholar]
- Prakash, D.S.; Athota, K.V.; Greene, H.L.; Vogel, C.A. Sorption and Catalytic Destruction of Chlorinated VOCs Using Fresh and Dealuminated Y and ZSM-5 Zeolites. AlChE Symp. Ser. 1995, 309, 1–17. [Google Scholar]
- Farrell, J.; Manspeaker, C.; Luo, J. Understanding competitive adsorption of water and trichloroethylene in a high-silica Y zeolite. Microporous Mesoporous Mater. 2003, 59, 205–214. [Google Scholar] [CrossRef]
| Mixture | Composition (g) | Natural Aggregates Temperature (°C) | Asphalt Binder Temperature (°C) | RAP Temperature (°C) | Production Temperature of Mixes (°C) | |||
|---|---|---|---|---|---|---|---|---|
| Natural Aggregates | Asphalt Binder Added | Natural Zeolite | Reclaimed Asphalt Pavement (RAP) | |||||
| P-155 | 4.000 | 216 | 0 | 0 | 155 | 155 | - | 155 |
| Z0.3-135 | 4.000 | 216 | 12 | 0 | 135 | 155 | - | 135 |
| Z0.6-135 | 4.000 | 216 | 24 | 0 | 135 | 155 | - | 135 |
| PR10-155 | 3600 | 207.6 | 0 | 400 (10% of the aggregates) | 142 | 155 | 25 | 155 |
| Z0.6-R10-125 | 3600 | 207.6 | 24 | 400 (10% of the aggregates) | 142 | 155 | 25 | 125 |
| PR20-155 | 3200 | 199.2 | 0 | 800 (20% of the aggregates) | 169 | 155 | 25 | 155 |
| Z0.6-R20-135 | 3200 | 199.2 | 24 | 800 (20% of the aggregates) | 169 | 155 | 25 | 135 |
| PR30-155 | 2800 | 194.4 | 0 | 1200 (30% of the aggregates) | 202 | 155 | 25 | 155 |
| Z0.6-R30-145 | 2800 | 194.4 | 24 | 1200 (30% of the aggregates) | 202 | 155 | 25 | 145 |
| N° | RT | RI calc | Compound | Concentration in Mixtures (ppm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P-155 | Z0.3-135 | Z0.6-135 | PR10-155 | Z0.6-R10-125 | PR20-155 | Z0.6-R20-135 | PR-30-155 | Z0.6-R30-145 | ||||
| 1 | 1.38 | - | Pentane | - | - | - | - | 3.86 | - | 2.86 | - | 2.41 |
| 2 | 1.66 | - | 2-Methylpentane | - | 1.28 | 2.97 | - | - | 1.38 | 2.24 | 1.40 | 1.25 |
| 3 | 1.73 | - | 3-Methylpentane | - | - | 1.09 | - | 0.47 | - | 0.63 | - | 0.53 |
| 4 | 1.75 | - | 2-Methyl-1-pentene | - | 2.56 | 2.51 | - | 1.33 | - | - | 1.56 | - |
| 5 | 1.79 | - | cis-1-Ethyl-2-methyl cyclopropane | - | - | - | - | - | 2.62 | 1.40 | 1.83 | - |
| 6 | 1.84 | - | Hexane | 6.28 | 8.66 | 8.14 | 3.94 | 7.79 | 5.49 | 6.00 | 6.38 | 4.68 |
| 7 | 1.88 | - | Trichloromethane | 10.06 | 5.99 | 12.12 | 5.15 | 23.00 | 2.55 | 4.27 | 7.77 | 9.43 |
| 8 | 2.45 | - | 2-Methylhexane | 3.99 | 2.60 | 5.50 | 12.55 | 2.29 | 6.29 | 8.47 | 2.48 | 2.57 |
| 9 | 2.58 | - | 3-Methylhexane | 5.80 | 3.68 | 4.77 | 6.56 | 4.06 | 4.71 | 3.88 | 5.62 | 2.75 |
| 10 | 2.75 | - | 1-Heptene | 6.09 | 4.55 | 5.77 | 3.88 | 4.92 | 4.71 | 4.40 | 5.00 | 3.09 |
| 11 | 2.91 | - | Heptane | 18.92 | 12.64 | 16.44 | 10.98 | 13.46 | 13.43 | 12.35 | 14.53 | 11.03 |
| 12 | 2.99 | - | 2-Heptene | - | 2.26 | 1.39 | - | 2.34 | 1.60 | 1.24 | 2.15 | 1.68 |
| 13 | 3.12 | - | 3-Heptene | - | 0.57 | 0.59 | - | 0.53 | 0.21 | 0.44 | 0.59 | 0.43 |
| 14 | 3.22 | - | Methylcyclohexane | 2.38 | 1.80 | 2.07 | 1.54 | 1.83 | 2.00 | 1.64 | 2.13 | 1.57 |
| 15 | 3.29 | - | Methyl isobutyl ketone | 1.06 | 1.20 | 1.24 | - | 0.63 | 0.39 | 0.44 | 0.66 | 0.35 |
| 16 | 3.57 | - | 1,2,4-Trimethyl cyclopentane | - | 0.06 | - | - | - | 4.06 | 3.86 | - | - |
| 17 | 3.77 | - | 3-Methylpentanal | 7.25 | 4.78 | 4.44 | - | 4.46 | 5.49 | 3.67 | - | - |
| 18 | 3.87 | - | Toluene | 6.20 | 6.05 | 4.48 | 5.62 | 5.59 | 3.89 | 3.43 | 6.95 | 3.40 |
| 19 | 3.99 | - | 3-Ethyl-2-methyl pentane | 4.41 | 4.55 | 4.96 | 4.23 | 4.06 | 4.63 | 3.76 | 5.38 | 4.16 |
| 20 | 4.10 | - | 2-Methylheptane | 16.47 | 12.21 | 14.25 | 10.52 | 12.18 | 13.47 | 11.72 | 14.65 | 11.23 |
| 21 | 4.26 | - | 3-Methylheptane | 9.62 | 7.51 | 7.97 | 2.46 | 7.22 | 8.20 | 5.70 | 9.60 | 6.97 |
| 22 | 4.40 | - | Hexanal | 4.03 | 1.85 | 1.35 | 5.89 | 2.19 | 1.98 | 1.36 | 4.16 | 1.23 |
| 23 | 4.50 | - | 2-Methyl-1-heptene | 3.19 | 2.66 | 2.40 | 2.01 | 2.59 | 2.35 | 2.03 | 3.44 | 2.00 |
| 24 | 4.60 | - | 1-Octene | 7.89 | 6.34 | 5.65 | 5.29 | 6.21 | 5.58 | 4.77 | 6.90 | 4.52 |
| 25 | 4.82 | - | Tetrachloroethylene | - | 130.40 | 18.63 | - | - | 0.66 | 0.56 | - | - |
| 26 | 4.87 | - | Octane | 33.63 | 22.23 | 24.74 | 24.05 | 27.88 | 23.25 | 19.63 | 29.48 | 18.97 |
| 27 | 5.02 | - | 3-Octene | - | - | - | - | 3.40 | 2.94 | 2.23 | - | 2.34 |
| 28 | 5.15 | - | 2-Octene | 1.79 | 1.44 | 1.04 | - | 1.19 | 1.18 | 0.83 | 1.56 | 0.94 |
| 29 | 5.32 | - | 4-Hydroxy-4-methyl-2-pentanone | 4.79 | 4.16 | 3.82 | 5.99 | 11.48 | 3.04 | 7.06 | 3.48 | 5.39 |
| 30 | 5.44 | - | 2,4-Dimethylheptane | 2.22 | 1.64 | 1.72 | - | 1.70 | 1.91 | 1.72 | 2.12 | 1.82 |
| 31 | 5.60 | - | 2,6-Dimethylheptane | 7.90 | 2.90 | 2.76 | - | 3.14 | 2.98 | 2.71 | 3.48 | 2.55 |
| 32 | 5.70 | - | 1,1,3-Trimethyl cyclohexane | 5.64 | 4.56 | 3.54 | - | 3.53 | 3.73 | 2.78 | 4.85 | 3.39 |
| 33 | 5.81 | - | 3,5-Dimethylheptane | - | 1.09 | - | - | 0.96 | 1.16 | 0.91 | 1.19 | 1.03 |
| 34 | 6.31 | - | 2,3-Dimethylheptane | 23.27 | 19.55 | 13.21 | 19.39 | 17.64 | 14.84 | 12.12 | 22.24 | 12.53 |
| 35 | 6.54 | - | 4-Methyloctane | 15.06 | 11.52 | 8.96 | 6.31 | 10.51 | 9.93 | 8.14 | 12.89 | 8.37 |
| 36 | 6.74 | - | 3-Methyloctane | 12.70 | 8.51 | 7.45 | 9.71 | 9.16 | 8.55 | 6.82 | 11.59 | 7.13 |
| 37 | 6.90 | - | p-Xylene | 5.31 | 4.63 | 2.64 | 5.00 | 4.49 | 2.84 | 2.13 | 5.49 | 2.25 |
| 38 | 7.07 | - | Heptanal | 9.88 | 6.45 | 4.83 | - | 6.70 | 5.80 | 4.34 | - | 4.75 |
| 39 | 7.21 | - | 1-Nonene | 9.25 | 7.24 | 4.74 | 7.04 | 7.88 | 5.55 | 4.55 | 8.84 | 4.48 |
| 40 | 7.36 | - | 2-Butoxyethanol | - | 1.68 | 22.34 | 7.25 | 12.29 | 1.24 | 1.27 | 1.46 | 1.02 |
| 41 | 7.55 | 900 | Nonane | 44.14 | 30.57 | 21.98 | 28.07 | 30.83 | 23.21 | 20.67 | 42.23 | 22.32 |
| 42 | 7.65 | 904 | 4-Nonene | 4.58 | 2.70 | 1.63 | - | 2.91 | 2.05 | 1.81 | 4.40 | 2.25 |
| 43 | 7.83 | 911 | 3,7-Dimethyl- 1-octene | 1.54 | - | - | 1.55 | 1.27 | 1.00 | 1.88 | 1.09 | - |
| 44 | 8.26 | 928 | Propylcyclohexane | 2.47 | 2.88 | 1.19 | - | 1.64 | 1.18 | 1.02 | 2.83 | 1.10 |
| 45 | 8.31 | 930 | 3,5-Dimethyloctane | 2.82 | 2.03 | 1.37 | - | 2.07 | 1.69 | 1.36 | 3.12 | 1.41 |
| 46 | 8.56 | 939 | 2,6-Dimethyloctane | 12.73 | 9.60 | 6.90 | 10.51 | 9.62 | 7.66 | 6.16 | 14.37 | 6.59 |
| 47 | 8.76 | 946 | 3-Ethyl-2-methyl heptane | 16.58 | 13.42 | 8.90 | 12.88 | 13.45 | 9.94 | 8.02 | 18.03 | 8.62 |
| 48 | 8.88 | 950 | 4-Methyl-1-octene | 3.67 | 3.04 | 1.82 | 3.89 | 3.15 | 2.06 | 1.67 | 4.17 | 1.81 |
| 49 | 8.93 | 952 | 1-Ethyl-3-methyl benzene | 3.42 | 10.97 | 1.42 | 6.09 | 3.96 | 1.37 | 1.21 | 3.89 | 1.33 |
| 50 | 9.04 | 955 | 1-Ethyl-2-methyl benzene | 10.03 | 2.80 | 2.02 | 10.95 | 2.92 | 2.04 | 1.65 | 5.38 | 1.91 |
| 51 | 9.37 | 966 | 4-Methylnonane | 11.69 | 12.11 | 5.71 | 11.96 | 10.88 | 5.16 | 3.55 | 14.52 | 4.00 |
| 52 | 9.47 | 970 | 2-Methylnonane | 10.76 | 10.44 | 5.82 | 11.22 | 10.42 | 5.84 | 5.05 | 11.85 | 4.87 |
| 53 | 9.65 | 975 | 3-Methylnonane | 6.69 | 6.92 | 3.15 | 6.32 | 6.13 | 3.35 | 2.77 | 8.49 | 2.69 |
| 54 | 9.89 | 983 | 1-Ethyl-2-methyl benzene | 7.49 | 10.43 | 5.13 | 13.01 | 9.15 | 4.08 | 3.53 | 14.44 | 2.62 |
| 55 | 10.15 | 991 | 2-Methyl-1-nonene | - | - | - | - | 3.17 | 2.07 | 1.26 | - | - |
| 56 | 10.16 | 991 | 1-Decene | 3.72 | 5.18 | 1.50 | 6.18 | 4.58 | 1.71 | 1.33 | 4.35 | 1.35 |
| 57 | 10.29 | 995 | 1-Methyl-2-propyl cyclohexane | - | 5.29 | - | - | 1.31 | 0.69 | 0.55 | 2.56 | 0.61 |
| 58 | 10.41 | 999 | cis-3-Decene | - | - | - | 4.20 | 2.98 | 1.32 | 1.14 | 4.23 | 1.08 |
| 59 | 10.54 | 1003 | Decane | 38.29 | 37.00 | 19.42 | 42.08 | 38.18 | 16.47 | 14.80 | 57.26 | 14.48 |
| 60 | 10.73 | 1011 | 1,2,3-Trimethyl benzene | 2.81 | - | - | 1.27 | 0.84 | 0.72 | 3.54 | 0.69 | - |
| 61 | 11.24 | 1029 | 2,6-Dimethylnonane | 11.31 | 11.11 | 6.02 | 12.30 | 10.02 | 4.45 | 3.59 | 22.17 | 4.59 |
| 62 | 11.46 | 1037 | Pentylcyclopentane | 1.52 | 2.91 | - | 3.60 | 1.33 | 0.50 | 0.46 | 2.98 | 0.00 |
| 63 | 11.67 | 1044 | 3,7-Dimethylnonane | 2.24 | 3.65 | 1.10 | 6.24 | 2.19 | - | 0.69 | 5.73 | 0.68 |
| 64 | 12.26 | 1064 | 5-Methyldecane | 4.41 | 5.75 | 1.04 | 7.19 | 4.78 | 1.61 | 1.32 | 7.98 | 1.45 |
| 65 | 12.35 | 1067 | 2,3-Dimethylnonane | 4.53 | 6.68 | 1.75 | 8.98 | 5.30 | 1.54 | 1.25 | 8.48 | 1.38 |
| 66 | 12.46 | 1070 | 2-Methyldecane | 5.94 | 8.58 | 1.96 | 11.39 | 6.65 | 1.97 | 1.21 | 11.37 | 1.81 |
| 67 | 12.64 | 1076 | 3-Methyldecane | 5.58 | 7.59 | 2.29 | 10.16 | 5.99 | 2.18 | 1.70 | 9.77 | 1.79 |
| 68 | 13.30 | 1096 | 1-Undecene | - | - | - | - | 5.82 | 1.85 | 1.08 | - | - |
| 69 | 13.51 | 1103 | Undecane | 24.91 | 35.09 | 7.14 | 49.90 | 27.63 | 9.31 | 7.48 | 41.81 | 7.79 |
| 70 | 14.37 | 1135 | 2,3-Dimethyldecane | - | - | - | 11.16 | 5.47 | 1.54 | 1.25 | 7.50 | 1.13 |
| 71 | 14.51 | 1140 | 1,2,4,5-Tetramethyl benzene | 2.28 | 5.77 | 1.10 | 6.12 | 2.86 | 0.98 | 0.69 | 3.77 | 0.52 |
| 72 | 15.17 | 1163 | 2,6-Dimethyl undecane | - | - | - | 6.44 | 2.83 | - | 0.48 | - | 0.42 |
| 73 | 15.26 | 1166 | 4-Methylundecane | - | 3.39 | - | 5.97 | 2.76 | 0.75 | 0.64 | 2.45 | 0.40 |
| 74 | 15.37 | 1169 | 2-Methylundecane | 1.87 | 3.25 | 0.79 | 5.01 | 2.17 | 0.67 | 0.50 | 2.40 | 0.37 |
| 75 | 16.05 | 1191 | 2,3-Dimethyl undecane | - | 3.82 | - | 3.32 | 1.63 | 0.73 | 0.53 | - | - |
| 76 | 16.21 | 1197 | 1,2-Benzisothiazole | - | 1.27 | 0.10 | 4.94 | 2.95 | 4.15 | - | - | 0.85 |
| 77 | 16.43 | 1204 | Dodecane | 9.02 | 13.71 | 2.29 | 23.43 | 10.79 | 1.00 | 3.43 | 10.23 | 3.14 |
| 78 | 16.77 | 1218 | 2,6-Dimethyl undecane | 3.06 | 5.38 | 2.69 | 5.58 | 3.73 | - | 0.63 | 0.98 | 0.60 |
| 79 | 18.43 | 1278 | 2-Butyl-1-octanol | 1.59 | 0.61 | 3.08 | 1.33 | 0.59 | 0.23 | 0.49 | 0.21 | - |
| 80 | 19.09 | 1301 | Tridecane | 2.41 | 2.98 | 0.83 | 5.52 | 2.38 | 1.17 | 0.73 | 1.19 | 0.47 |
| 81 | 21.66 | 1400 | Tetradecane | 0.33 | 0.35 | 0.26 | 0.86 | 0.46 | - | 0.25 | 0.13 | 0.06 |
| 82 | 24.11 | 1500 | Pentadecane | - | 0.12 | 0.17 | 0.37 | 0.20 | - | 0.28 | - | 0.34 |
| 83 | 26.38 | 1597 | Hexadecane | - | - | - | - | 0.08 | - | - | - | 0.24 |
| Identified compounds (%) | 70.79 | 76.76 | 70.63 | 79.32 | 77.86 | 74.76 | 76.52 | 74.34 | 73.29 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espinoza, J.; Medina, C.; Calabi-Floody, A.; Sánchez-Alonso, E.; Valdés, G.; Quiroz, A. Evaluation of Reductions in Fume Emissions (VOCs and SVOCs) from Warm Mix Asphalt Incorporating Natural Zeolite and Reclaimed Asphalt Pavement for Sustainable Pavements. Sustainability 2020, 12, 9546. https://doi.org/10.3390/su12229546
Espinoza J, Medina C, Calabi-Floody A, Sánchez-Alonso E, Valdés G, Quiroz A. Evaluation of Reductions in Fume Emissions (VOCs and SVOCs) from Warm Mix Asphalt Incorporating Natural Zeolite and Reclaimed Asphalt Pavement for Sustainable Pavements. Sustainability. 2020; 12(22):9546. https://doi.org/10.3390/su12229546
Chicago/Turabian StyleEspinoza, Javier, Cristian Medina, Alejandra Calabi-Floody, Elsa Sánchez-Alonso, Gonzalo Valdés, and Andrés Quiroz. 2020. "Evaluation of Reductions in Fume Emissions (VOCs and SVOCs) from Warm Mix Asphalt Incorporating Natural Zeolite and Reclaimed Asphalt Pavement for Sustainable Pavements" Sustainability 12, no. 22: 9546. https://doi.org/10.3390/su12229546







