Antiviral Role of Phenolic Compounds against Dengue Virus: A Review
Abstract
:1. Introduction
2. Geraniin
Anti-DENV Effect of Geraniin
3. Chebulagic Acid and Punicalagin
Anti-DENV Effect of Chebulagic Acid and Punicalagin
4. Flavonoids
Anti-DENV Effect of Flavonoids
5. Resveratrol
Anti-DENV Effect of Resveratrol
6. Nordihydroguaiaretic Acid
Anti-DENV Effect of NDGA
7. Curcumin
Anti-DENV Effect of Curcumin
8. Salidroside
Anti-DENV Effect of Salidroside
9. Verbascoside and Caffeoylcalleryanin
Anti-DENV Effect of Verbascoside and Caffeoylcalleryanin
10. Sodium Salicylate
Anti- Dengue Activity of Sodium Salicylate
11. Cardol Triene
Anti-DENV Effect of Cardol Triene
12. Policresulen
Anti-DENV Effect of Policresulen
13. GW5074
Anti-DENV Effect of GW5074
14. Honokiol
Anti-DENV Effect of Honokiol
15. Materials and Methods
16. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviation
| ADE | Antibody-dependent potentiation |
| AdV | Adenovirus type 5 |
| BBB | Blood–brain barrier |
| CDV | Canine Distemper Virus |
| CHIKV | Chikungunya virus |
| COX | Cyclo-oxygenase enzyme |
| CPE | Cytopathic effect |
| Cscore | Ligand–enzyme consensus score |
| CVB3 | Coxsackie virus |
| DENV | Dengue virus |
| EBOV | Ebola virus |
| EBV | Epstein Barr virus |
| EGCG | Epigallocatechin gallate |
| EMCV | Encephalomyocarditis virus |
| EVA71 | Enterovirus A71 |
| GCRV | Grass carp reovirus |
| HBV | Hepatitis B |
| HCMV | Human cytomegalovirus |
| HCV | Hepatitis C virus |
| HIV | Human immunodeficiency virus |
| HSV | Herpes simplex virus |
| HTLV | Human t-cell lymphotropic virus |
| IAV | Influenza A virus |
| IHNV | Infectious hematopoietic necrosis virus |
| ISG | Stimulated by interferon |
| IV | Influenza virus |
| JEV | Japanese encephalitis virus |
| mCMV | Murine cytomegalovirus |
| MHV | Mouse hepatitis virus |
| MOI | Multiplicity of infection |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| MV | Measles virus |
| NaSal | Sodium salicylate |
| NDGA | Nordihydroguaiaretic acid |
| NKCC1 | Na+–K+–2Cl− cotransporter 1 |
| NSAIDs | Nonsteroidal anti-inflammatory drugs |
| PI-3 | Parainfluenza type-3 |
| PV | Poliovirus |
| RebA | Rebaudioside A |
| ROS | Reactive oxygen species |
| RSV | Respiratory syncytial virus |
| RV | Reovirus |
| SARS-CoV | Severe acute respiratory syndrome-coronavirus |
| SG | Steviol glucosides |
| SIN | Sindbis virus |
| SREBP | Sterol regulatory element binding protein |
| Ste | Extraction process stevioside |
| SVCV | Spring viraemia of carp |
| VACV | Vaccinia virus |
| VHSV | Viral hemorrhagic septicemia |
| VSV | Vesicular stomatitis virus |
| WNV | West Nile virus |
| ZIKV | Zika virus |
References
- Uno, N.; Ross, T.M. Dengue virus and the host innate immune response. Emerg. Microbes Infect. 2018, 7, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martina, B.E.E.; Koraka, P.; Osterhaus, A.D.M.E. Dengue virus pathogenesis: An integrated view. Clin. Microbiol. Rev. 2009, 22, 564–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandoval, E.; Téllez, Y.; Harris, E.; Videa, E.; Amador, J.J.; Gonzalez, A.; Pérez, L.; Campo, L.A.; Pérez, M.L.; Cuadra, R.; et al. Clinical, epidemiologic, and virologic features of dengue in the 1998 epidemic in Nicaragua. Am. J. Trop. Med. Hyg. 2000, 63, 5–11. [Google Scholar] [CrossRef] [Green Version]
- Screaton, G.; Mongkolsapaya, J.; Yacoub, S.; Roberts, C. New insights into the immunopathology and control of dengue virus infection. Nat. Rev. Immunol. 2015, 15, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Pérez, C.; Segura-Carretero, A.; del Mar Contreras, M. Phenolic compounds as natural and multifunctional anti-obesity agents: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1212–1229. [Google Scholar] [CrossRef]
- Thitilertdecha, N.; Teerawutgulrag, A.; Kilburn, J.D.; Rakariyatham, N. Identification of major Phenolic compounds from Nephelium lappaceum L. and their antioxidant activities. Molecules 2010, 15, 1453–1465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sáez, V.; Pastene, E.; Vergara, C.; Mardones, C.; Hermosín-Gutiérrez, I.; Gómez-Alonso, S.; Gómez, M.V.; Theoduloz, C.; Riquelme, S.; von Baer, D. Oligostilbenoids in Vitis vinifera L. Pinot Noir grape cane extract: Isolation, characterization, in vitro antioxidant capacity and anti-proliferative effect on cancer cells. Food Chem. 2018, 265, 101–110. [Google Scholar] [CrossRef]
- Batallán, G.; Torre, R.; Flores, F.; Konigheim, B.; Ludueña-Almeida, F.; Tonn, C.; Contigiani, M.; Almirón, W. Larvicidal activity of crude extracts from Larrea cuneifolia (Zygophyllaceae) and of its metabolite nordihydroguaiaretic acid against the vector Culex quinquefasciatus (Diptera: Culicidae). Rev. Soc. Bras. Med. Trop. 2013, 46, 84–87. [Google Scholar] [CrossRef]
- Wolff, T.; Berrueta, L.A.; Valente, L.M.M.; Barboza, R.S.; Neris, R.L.S.; Guimarães-Andrade, I.P.; Assunção-Miranda, I.; Nascimento, A.C.; Gomes, M.; Gallo, B.; et al. Comprehensive characterisation of polyphenols in leaves and stems of three anti-dengue virus type-2 active Brazilian Faramea species (Rubiaceae) by HPLC-DAD-ESI-MS/MS. Phytochem. Anal. 2019, 30, 62–72. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; He, Y.; She, Y.; Wang, M.; Yan, Z.; Ren, J.H.; Cao, Z.; Shao, Y.; Wang, S.; Abd El-Aty, A.M.; et al. Preparation of molecularly imprinted polymers coupled with high-performance liquid chromatography for the selective extraction of salidroside from Rhodiola crenulata. J. Chromatogr. B 2019, 1118–1119, 180–186. [Google Scholar] [CrossRef]
- Barbieri, M.; Heard, C.M. Isolation of punicalagin from Punica granatum rind extract using mass-directed semi-preparative ESI-AP single quadrupole LC-MS. J. Pharm. Biomed. Anal. 2019, 166, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Çevik, D.; Kan, Y.; Kırmızıbekmez, H. Mechanisms of action of cytotoxic phenolic compounds from Glycyrrhiza iconica roots. Phytomedicine 2019, 58, 152872. [Google Scholar] [CrossRef] [PubMed]
- Cianciosi, D.; Forbes-Hernández, T.; Afrin, S.; Gasparrini, M.; Reboredo-Rodriguez, P.; Manna, P.; Zhang, J.; Bravo Lamas, L.; Martínez Flórez, S.; Agudo Toyos, P.; et al. Phenolic compounds in honey and their associated health benefits: A review. Molecules 2018, 23, 2322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiang, J.; Apea-Bah, F.B.; Ndolo, V.U.; Katundu, M.C.; Beta, T. Profile of phenolic compounds and antioxidant activity of finger millet varieties. Food Chem. 2019, 275, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J. Antioxidant activities of flavonoids as bioactive components of food. Biochem. Soc. Trans. 1996, 24, 790–795. [Google Scholar] [CrossRef]
- Saud, S.M.; Li, W.; Morris, N.L.; Matter, M.S.; Colburn, N.H.; Kim, Y.S.; Young, M.R. Resveratrol prevents tumorigenesis in mouse model of Kras activated sporadic colorectal cancer by suppressing oncogenic Kras expression. Carcinogenesis 2014, 35, 2778–2786. [Google Scholar] [CrossRef] [Green Version]
- Boakye, Y.D.; Agyare, C.; Abotsi, W.K.M.; Ayande, P.G.; Ossei, P.P.S. Anti-inflammatory activity of aqueous leaf extract of Phyllanthus muellerianus (Kuntze) Exell. and its major constituent, geraniin. J. Ethnopharmacol. 2016, 187, 17–27. [Google Scholar] [CrossRef]
- Izui, S.; Sekine, S.; Maeda, K.; Kuboniwa, M.; Takada, A.; Amano, A.; Nagata, H. Antibacterial activity of curcumin against Periodontopathic bacteria. J. Periodontol. 2016, 87, 83–90. [Google Scholar] [CrossRef]
- Andrade, J.T.; Fantini de Figueiredo, G.; Cruz, L.F.; Eliza de Morais, S.; Souza, C.D.F.; Pinto, F.C.H.; Ferreira, J.M.S.; de Freitas Araújo, M.G. Efficacy of curcumin in the treatment of experimental vulvovaginal candidiasis. Rev. Iberoam. Micol. 2019, 36, 192–199. [Google Scholar] [CrossRef]
- Zhang, X.-L.; Guo, Y.-S.; Wang, C.-H.; Li, G.-Q.; Xu, J.-J.; Chung, H.Y.; Ye, W.-C.; Li, Y.-L.; Wang, G.-C. Phenolic compounds from Origanum vulgare and their antioxidant and antiviral activities. Food Chem. 2014, 152, 300–306. [Google Scholar] [CrossRef]
- Weber, C.; Sliva, K.; von Rhein, C.; Kümmerer, B.M.; Schnierle, B.S. The green tea catechin, epigallocatechin gallate inhibits chikungunya virus infection. Antiviral Res. 2015, 113, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Gu, W.; Li, C.; Li, X.; Xing, G.; Li, Y.; Song, Y.; Zheng, W. Epigallocatechin gallate inhibits hepatitis B virus via farnesoid X receptor alpha. J. Nat. Med. 2016, 70, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.-F.; Bai, L.-P.; Huang, W.; Li, X.-Z.; Zhao, S.-S.; Zhong, N.-S.; Jiang, Z.-H. Comparison of in vitro antiviral activity of tea polyphenols against influenza A and B viruses and structure–activity relationship analysis. Fitoterapia 2014, 93, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Tyagi, A.K. Curcumin and its analogues: A potential natural compound against HIV infection and AIDS. Food Funct. 2015, 6, 3412–3419. [Google Scholar] [CrossRef]
- Kim, K.; Kim, K.H.; Kim, H.Y.; Cho, H.K.; Sakamoto, N.; Cheong, J. Curcumin inhibits hepatitis C virus replication via suppressing the Akt-SREBP-1 pathway. FEBS Lett. 2010, 584, 707–712. [Google Scholar] [CrossRef]
- Horne, J.R.; Vohl, M.-C. Biological plausibility for interactions between dietary fat, resveratrol, ACE2, and SARS-CoV illness severity. Am. J. Physiol. Metab. 2020, 318, E830–E833. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.-C.; Ho, C.-T.; Chuo, W.-H.; Li, S.; Wang, T.T.; Lin, C.-C. Effective inhibition of MERS-CoV infection by resveratrol. BMC Infect. Dis. 2017, 17, 144. [Google Scholar] [CrossRef] [Green Version]
- Merino-Ramos, T.; Jiménez de Oya, N.; Saiz, J.-C.; Martín-Acebes, M.A. Antiviral activity of Nordihydroguaiaretic acid and its derivative Tetra-O-Methyl Nordihydroguaiaretic acid against West Nile Virus and Zika Virus. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef] [Green Version]
- Houston, D.M.J.; Bugert, J.J.; Denyer, S.P.; Heard, C.M. Potentiated virucidal activity of pomegranate rind extract (PRE) and punicalagin against Herpes simplex virus (HSV) when co-administered with zinc (II) ions, and antiviral activity of PRE against HSV and aciclovir-resistant HSV. PLoS ONE 2017, 12, e0179291. [Google Scholar] [CrossRef] [Green Version]
- Krylova, N.V.; Popov, A.M.; Leonova, G.N. Antioxidants as potential antiviral agents for Flavivirus Infections. Antibiot. khimioterapiia = Antibiot. Chemoterapy [sic] 2016, 61, 25–31. [Google Scholar]
- Yamada, H.; Wakamori, S.; Hirokane, T.; Ikeuchi, K.; Matsumoto, S. Structural revisions in Natural Ellagitannins. Molecules 2018, 23, 1901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elendran, S.; Wang, L.W.; Prankerd, R.; Palanisamy, U.D. The physicochemical properties of geraniin, a potential antihyperglycemic agent. Pharm. Biol. 2015, 53, 1719–1726. [Google Scholar] [CrossRef] [PubMed]
- Sudjaroen, Y.; Hull, W.E.; Erben, G.; Würtele, G.; Changbumrung, S.; Ulrich, C.M.; Owen, R.W. Isolation and characterization of ellagitannins as the major polyphenolic components of Longan (Dimocarpus longan Lour) seeds. Phytochemistry 2012, 77, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, Z.; Li, X.; Jiang, Z.; Zhao, Y.; Ping, F. Geraniin suppresses ovarian cancer growth through inhibition of NF-κB activation and downregulation of Mcl-1 expression. J. Biochem. Mol. Toxicol. 2017, 31, e21929. [Google Scholar] [CrossRef] [PubMed]
- Lipińska, L.; Klewicka, E.; Sójka, M. The structure, occurrence and biological activity of ellagitannins: A general review. Acta Sci. Pol. Technol. Aliment. 2014, 13, 289–299. [Google Scholar] [CrossRef] [Green Version]
- Ndjonka, D.; Bergmann, B.; Agyare, C.; Zimbres, F.M.; Lüersen, K.; Hensel, A.; Wrenger, C.; Liebau, E. In vitro activity of extracts and isolated polyphenols from West African medicinal plants against Plasmodium falciparum. Parasitol. Res. 2012, 111, 827–834. [Google Scholar] [CrossRef]
- Vassallo, A.; Vaccaro, M.C.; De Tommasi, N.; Dal Piaz, F.; Leone, A. Identification of the plant compound Geraniin as a novel Hsp90 inhibitor. PLoS ONE 2013, 8, e74266. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Zhang, L.; Fan, X.; Qin, C.; Liu, J. Antiviral effect of geraniin on human enterovirus 71 in vitro and in vivo. Bioorg. Med. Chem. Lett. 2012, 22, 2209–2211. [Google Scholar] [CrossRef]
- Yang, C.-M.; Cheng, H.-Y.; Lin, T.-C.; Chiang, L.-C.; Lin, C.-C. The in vitro activity of geraniin and 1,3,4,6-tetra-O-galloyl-β-d-glucose isolated from Phyllanthus urinaria against herpes simplex virus type 1 and type 2 infection. J. Ethnopharmacol. 2007, 110, 555–558. [Google Scholar] [CrossRef]
- Notka, F.; Meier, G.; Wagner, R. Inhibition of wild-type human immunodeficiency virus and reverse transcriptase inhibitor-resistant variants by Phyllanthus amarus. Antivir. Res. 2003, 58, 175–186. [Google Scholar] [CrossRef]
- Li, J.; Huang, H.; Feng, M.; Zhou, W.; Shi, X.; Zhou, P. In vitro and in vivo anti-hepatitis B virus activities of a plant extract from Geranium carolinianum L. Antivir. Res. 2008, 79, 114–120. [Google Scholar] [CrossRef]
- Chen Liu, K.C.S.; Lin, M.-T.; Lee, S.-S.; Chiou, J.-F.; Ren, S.; Lien, E.J. Antiviral Tannins from two Phyllanthus species. Planta Med. 1999, 65, 043–046. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yu, S.; Liu, D.; Proksch, P.; Lin, W. Inhibitory effects of polyphenols toward HCV from the mangrove plant Excoecaria agallocha L. Bioorg. Med. Chem. Lett. 2012, 22, 1099–1102. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Tang, Y.Q.; Rathkrishnan, A.; Wang, S.M.; Ong, K.C.; Manikam, R.; Payne, B.J.; Jaganath, I.B.; Sekaran, S.D. Effects of cocktail of four local Malaysian medicinal plants (Phyllanthus spp.) against dengue virus 2. BMC Complement Altern. Med. 2013, 13, 192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdul Ahmad, S.A.; Palanisamy, U.D.; Tejo, B.A.; Chew, M.F.; Tham, H.W.; Syed Hassan, S. Geraniin extracted from the rind of Nephelium lappaceum binds to dengue virus type-2 envelope protein and inhibits early stage of virus replication. Virol. J. 2017, 14, 229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paes, M.V.; Pinhão, A.T.; Barreto, D.F.; Costa, S.M.; Oliveira, M.P.; Nogueira, A.C.; Takiya, C.M.; Farias-Filho, J.C.; Schatzmayr, H.G.; Alves, A.M.B.; et al. Liver injury and viremia in mice infected with dengue-2 virus. Virology 2005, 338, 236–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdul Ahmad, S.A.; Palanisamy, U.D.; Khoo, J.J.; Dhanoa, A.; Syed Hassan, S. Efficacy of geraniin on dengue virus type-2 infected BALB/c mice. Virol. J. 2019, 16, 26. [Google Scholar] [CrossRef]
- Chen, F.; Tang, Q.; Ma, H.; Bian, K.; Seeram, N.P.; Li, D. Hydrolyzable Tannins are iron chelators that inhibit DNA repair enzyme ALKBH2. Chem. Res. Toxicol. 2019, 32, 1082–1086. [Google Scholar] [CrossRef]
- Yoshida, T.; Amakura, Y.; Yoshimura, M. Structural features and biological properties of Ellagitannins in some plant families of the order Myrtales. Int. J. Mol. Sci. 2010, 11, 79–106. [Google Scholar] [CrossRef] [Green Version]
- Heber, D. Pomegranate ellagitannins. In Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2011. [Google Scholar]
- Reddy, D.B.; Reddanna, P. Chebulagic acid (CA) attenuates LPS-induced inflammation by suppressing NF-κB and MAPK activation in RAW 264.7 macrophages. Biochem. Biophys. Res. Commun. 2009, 381, 112–117. [Google Scholar] [CrossRef]
- Dell’Agli, M.; Galli, G.V.; Bulgari, M.; Basilico, N.; Romeo, S.; Bhattacharya, D.; Taramelli, D.; Bosisio, E. Ellagitannins of the fruit rind of pomegranate (Punica granatum) antagonize in vitro the host inflammatory response mechanisms involved in the onset of malaria. Malar. J. 2010, 9, 208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adaramoye, O.; Erguen, B.; Nitzsche, B.; Höpfner, M.; Jung, K.; Rabien, A. Punicalagin, a polyphenol from pomegranate fruit, induces growth inhibition and apoptosis in human PC-3 and LNCaP cells. Chem. Biol. Interact. 2017, 274, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Silva, O.; Viegas, S.; de Mello-Sampayo, C.; Costa, M.J.P.; Serrano, R.; Cabrita, J.; Gomes, E.T. Anti-Helicobacter pylori activity of Terminalia macroptera root. Fitoterapia 2012, 83, 872–876. [Google Scholar] [CrossRef] [PubMed]
- Endo, E.H.; Garcia Cortez, D.A.; Ueda-Nakamura, T.; Nakamura, C.V.; Dias Filho, B.P. Potent antifungal activity of extracts and pure compound isolated from pomegranate peels and synergism with fluconazole against Candida albicans. Res. Microbiol. 2010, 161, 534–540. [Google Scholar] [CrossRef]
- Li, P.; Du, R.; Wang, Y.; Hou, X.; Wang, L.; Zhao, X.; Zhan, P.; Liu, X.; Rong, L.; Cui, Q. Identification of Chebulinic Acid and Chebulagic Acid as novel Influenza Viral Neuraminidase inhibitors. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.-T.; Chen, T.-Y.; Chung, C.-Y.; Noyce, R.S.; Grindley, T.B.; McCormick, C.; Lin, T.-C.; Wang, G.-H.; Lin, C.-C.; Richardson, C.D. Hydrolyzable Tannins (Chebulagic Acid and Punicalagin) target viral Glycoprotein-Glycosaminoglycan interactions to inhibit herpes simplex Virus 1 entry and cell-to-cell spread. J. Virol. 2011, 85, 4386–4398. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.-T.; Chen, T.-Y.; Lin, S.-C.; Chung, C.-Y.; Lin, T.-C.; Wang, G.-H.; Anderson, R.; Lin, C.-C.; Richardson, C.D. Broad-spectrum antiviral activity of chebulagic acid and punicalagin against viruses that use glycosaminoglycans for entry. BMC Microbiol. 2013, 13, 187. [Google Scholar] [CrossRef] [Green Version]
- Blanco, E.; Sabetta, W.; Danzi, D.; Negro, D.; Passeri, V.; De Lisi, A.; Paolocci, F.; Sonnante, G. Isolation and characterization of the flavonol regulator CcMYB12 From the Globe Artichoke [Cynara cardunculus var. scolymus (L.) Fiori]. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Ravishankar, D.; Rajora, A.K.; Greco, F.; Osborn, H.M.I. Flavonoids as prospective compounds for anti-cancer therapy. Int. J. Biochem. Cell Biol. 2013, 45, 2821–2831. [Google Scholar] [CrossRef]
- Airoldi, C.; La Ferla, B.; D‘Orazio, G.; Ciaramelli, C.; Palmioli, A. Flavonoids in the treatment of Alzheimer’s and other neurodegenerative diseases. Curr. Med. Chem. 2018, 25, 3228–3246. [Google Scholar] [CrossRef]
- Ding, Y.; Li, C.; Zhang, Y.; Ma, P.; Zhao, T.; Che, D.; Cao, J.; Wang, J.; Liu, R.; Zhang, T.; et al. Quercetin as a Lyn kinase inhibitor inhibits IgE-mediated allergic conjunctivitis. Food Chem. Toxicol. 2020, 135, 110924. [Google Scholar] [CrossRef] [PubMed]
- Goh, F.Y.; Upton, N.; Guan, S.; Cheng, C.; Shanmugam, M.K.; Sethi, G.; Leung, B.P.; Wong, W.S.F. Fisetin, a bioactive flavonol, attenuates allergic airway inflammation through negative regulation of NF-κB. Eur. J. Pharmacol. 2012, 679, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Wang, G.; Kandhare, A.D.; Mukherjee-Kandhare, A.A.; Bodhankar, S.L. Neuroprotective effect of naringin, a flavone glycoside in quinolinic acid-induced neurotoxicity: Possible role of PPAR-γ, Bax/Bcl-2, and caspase-3. Food Chem. Toxicol. 2018, 121, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Pingili, R.B.; Challa, S.R.; Pawar, A.K.; Toleti, V.; Kodali, T.; Koppula, S. A systematic review on hepatoprotective activity of quercetin against various drugs and toxic agents: Evidence from preclinical studies. Phyther. Res. 2020, 34, 5–32. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Vicente, L.; González-Calle, D.; Casanova, A.G.; Hernández-Sánchez, M.T.; Prieto, M.; Rama-Merchán, J.C.; Martín-Moreiras, J.; Martín-Herrero, F.; Sánchez, P.L.; López-Hernández, F.J.; et al. Quercetin, a promising clinical candidate for the prevention of contrast-induced Nephropathy. Int. J. Mol. Sci. 2019, 20, 4961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ezzati, M.; Yousefi, B.; Velaei, K.; Safa, A. A review on anti-cancer properties of Quercetin in breast cancer. Life Sci. 2020, 248, 117463. [Google Scholar] [CrossRef]
- Wang, S.; Yao, J.; Zhou, B.; Yang, J.; Chaudry, M.T.; Wang, M.; Xiao, F.; Li, Y.; Yin, W. Bacteriostatic effect of Quercetin as an Antibiotic Alternative in vivo and its antibacterial mechanism in vitro. J. Food Prot. 2018, 81, 68–78. [Google Scholar] [CrossRef]
- Pal, A.; Tripathi, A. Quercetin potentiates meropenem activity among pathogenic carbapenem-resistant Pseudomonas aeruginosa and Acinetobacter baumannii. J. Appl. Microbiol. 2019, 127, 1038–1047. [Google Scholar] [CrossRef]
- Pendota, S.C.; Aderogba, M.A.; Ndhlala, A.R.; Van Staden, J. Antimicrobial and acetylcholinesterase inhibitory activities of Buddleja salviifolia (L.) Lam. leaf extracts and isolated compounds. J. Ethnopharmacol. 2013, 148, 515–520. [Google Scholar] [CrossRef]
- Chattopadhyay, D.; Naik, T. Antivirals of ethnomedicinal origin: Structure-activity relationship and scope. Mini-Reviews Med. Chem. 2007, 7, 275–301. [Google Scholar] [CrossRef]
- Huang, H.-C.; Tao, M.-H.; Hung, T.-M.; Chen, J.-C.; Lin, Z.-J.; Huang, C. (−)-Epigallocatechin-3-gallate inhibits entry of hepatitis B virus into hepatocytes. Antivir. Res. 2014, 111, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Chiow, K.H.; Phoon, M.C.; Putti, T.; Tan, B.K.H.; Chow, V.T. Evaluation of antiviral activities of Houttuynia cordata Thunb. extract, quercetin, quercetrin and cinanserin on murine coronavirus and dengue virus infection. Asian Pac. J. Trop. Med. 2016, 9, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, C.; Xi, C.; Hu, K.; Gao, W.; Cai, X.; Qin, J.; Lv, S.; Du, C.; Wei, Y. Inhibition of enterovirus 71 replication and viral 3C protease by quercetin. Virol. J. 2018, 15, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De González-Búrquez, M.J.; González-Díaz, F.R.; García-Tovar, C.G.; Carrillo-Miranda, L.; Soto-Zárate, C.I.; Canales-Martínez, M.M.; Penieres-Carrillo, J.G.; Crúz-Sánchez, T.A.; Fonseca-Coronado, S. Comparison between in vitro antiviral effect of Mexican propolis and three commercial Flavonoids against Canine Distemper Virus. Evid. Based Complement. Altern. Med. 2018, 2018, 7092416. [Google Scholar] [CrossRef] [Green Version]
- Salehi, B.; Fokou, P.; Sharifi-Rad, M.; Zucca, P.; Pezzani, R.; Martins, N.; Sharifi-Rad, J. The therapeutic potential of Naringenin: A review of clinical trials. Pharmaceuticals 2019, 12, 11. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.-G.; Lee, H.; Kim, Y.S.; Hwang, Y.-H.; Oh, Y.-C.; Lee, B.; Moon, K.M.; Cho, W.-K.; Ma, J.Y. Aloe vera and its components inhibit influenza a virus-induced autophagy and replication. Am. J. Chin. Med. 2019, 47, 1307–1324. [Google Scholar] [CrossRef]
- Marunaka, Y. Actions of quercetin, a flavonoid, on ion transporters: Its physiological roles. Ann. N. Y. Acad. Sci. 2017, 1398, 142–151. [Google Scholar] [CrossRef]
- Lin, Y.-J.; Chang, Y.-C.; Hsiao, N.-W.; Hsieh, J.-L.; Wang, C.-Y.; Kung, S.-H.; Tsai, F.-J.; Lan, Y.-C.; Lin, C.-W. Fisetin and rutin as 3C protease inhibitors of enterovirus A71. J. Virol. Methods 2012, 182, 93–98. [Google Scholar] [CrossRef]
- Özçelik, B.; Kartal, M.; Orhan, I. Cytotoxicity, antiviral and antimicrobial activities of alkaloids, flavonoids, and phenolic acids. Pharm. Biol. 2011, 49, 396–402. [Google Scholar] [CrossRef]
- He, W. Epigallocatechin gallate inhibits HBV DNA synthesis in a viral replication—inducible cell line. World J. Gastroenterol. 2011, 17, 1507. [Google Scholar] [CrossRef]
- Zhong, L.; Hu, J.; Shu, W.; Gao, B.; Xiong, S. Epigallocatechin-3-gallate opposes HBV-induced incomplete autophagy by enhancing lysosomal acidification, which is unfavorable for HBV replication. Cell Death Dis. 2015, 6, e1770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colpitts, C.C.; Schang, L.M. A Small molecule inhibits virion attachment to Heparan Sulfate- or Sialic Acid-containing Glycans. J. Virol. 2014, 88, 7806–7817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Xu, Z.; Zheng, W. A review of the antiviral role of green tea catechins. Molecules 2017, 22, 1337. [Google Scholar] [CrossRef] [Green Version]
- Jasso-Miranda, C.; Herrera-Camacho, I.; Flores-Mendoza, L.K.; Dominguez, F.; Vallejo-Ruiz, V.; Sanchez-Burgos, G.G.; Pando-Robles, V.; Santos-Lopez, G.; Reyes-Leyva, J. Antiviral and immunomodulatory effects of polyphenols on macrophages infected with dengue virus serotypes 2 and 3 enhanced or not with antibodies. Infect. Drug Resist. 2019, 12, 1833–1852. [Google Scholar] [CrossRef] [PubMed]
- Igbe, I.; Shen, X.-F.; Jiao, W.; Qiang, Z.; Deng, T.; Li, S.; Liu, W.-L.; Liu, H.-W.; Zhang, G.-L.; Wang, F. Dietary quercetin potentiates the antiproliferative effect of interferon-α in hepatocellular carcinoma cells through activation of JAK/STAT pathway signaling by inhibition of SHP2 phosphatase. Oncotarget 2017, 8, 113734–113748. [Google Scholar] [CrossRef]
- Trujillo-Correa, A.I.; Quintero-Gil, D.C.; Diaz-Castillo, F.; Quiñones, W.; Robledo, S.M.; Martinez-Gutierrez, M. In vitro and in silico anti-dengue activity of compounds obtained from Psidium guajava through bioprospecting. BMC Complement. Altern. Med. 2019, 19, 298. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, V.D.; Bharadwaj, S.; Afroz, S.; Khan, N.; Ansari, M.A.; Yadava, U.; Tripathi, R.C.; Tripathi, I.P.; Mishra, S.K.; Kang, S.G. Anti-dengue infectivity evaluation of bioflavonoid from Azadirachta indica by dengue virus serine protease inhibition. J. Biomol. Struct. Dyn. 2020, 1–14. [Google Scholar] [CrossRef]
- Ismail, N.A.; Jusoh, S.A. Molecular docking and molecular dynamics simulation studies to predict flavonoid binding on the surface of DENV2 E protein. Interdiscip. Sci. Comput. Life Sci. 2017, 9, 499–511. [Google Scholar] [CrossRef]
- Qamar, M.; Mumtaz, A.; Naseem, R.; Ali, A.; Fatima, T.; Jabbar, T.; Ahmad, Z.; Ashfaq, U.A. Molecular docking based screening of plant flavonoids as Dengue NS1 inhibitors. Bioinformation 2014, 10, 460–465. [Google Scholar] [CrossRef]
- De Sousa, L.R.F.; Wu, H.; Nebo, L.; Fernandes, J.B.; das Graças Fernandes da Silva, M.F.; Kiefer, W.; Kanitz, M.; Bodem, J.; Diederich, W.E.; Schirmeister, T.; et al. Flavonoids as noncompetitive inhibitors of Dengue virus NS2B-NS3 protease: Inhibition kinetics and docking studies. Bioorg. Med. Chem. 2015, 23, 466–470. [Google Scholar] [CrossRef]
- Senthilvel, P.; Lavanya, P.; Kumar, K.M.; Swetha, R.; Anitha, P.; Bag, S.; Sarveswari, S.; Vijayakumar, V.; Ramaiah, S.; Anbarasu, A. Flavonoid from Carica papaya inhibits NS2B-NS3 protease and prevents Dengue 2 viral assembly. Bioinformation 2013, 9, 889–895. [Google Scholar] [CrossRef]
- Chappell, K.; Stoermer, M.; Fairlie, D.; Young, P. West Nile Virus NS2B/NS3 protease as an antiviral target. Curr. Med. Chem. 2008, 15, 2771–2784. [Google Scholar] [CrossRef]
- Kim, Y.M.; Gayen, S.; Kang, C.; Joy, J.; Huang, Q.; Chen, A.S.; Wee, J.L.K.; Ang, M.J.Y.; Lim, H.A.; Hung, A.W.; et al. NMR Analysis of a novel enzymatically active unlinked Dengue NS2B-NS3 protease complex. J. Biol. Chem. 2013, 288, 12891–12900. [Google Scholar] [CrossRef] [Green Version]
- Keivan, Z.; Teoh, B.-T.; Sam, S.-S.; Wong, P.-F.; Mustafa, M.R.; AbuBakar, S. In vitro antiviral activity of fisetin, rutin and naringenin against dengue virus type-2. J. Med. Plants Res. 2011, 5, 5534–5539. [Google Scholar]
- Zandi, K.; Teoh, B.-T.; Sam, S.-S.; Wong, P.-F.; Mustafa, M.; AbuBakar, S. Antiviral activity of four types of bioflavonoid against dengue virus type-2. Virol. J. 2011, 8, 560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raekiansyah, M.; Buerano, C.C.; Luz, M.A.D.; Morita, K. Inhibitory effect of the green tea molecule EGCG against dengue virus infection. Arch. Virol. 2018, 163, 1649–1655. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Calvo, Á.; Jiménez de Oya, N.; Martín-Acebes, M.A.; Garcia-Moruno, E.; Saiz, J.-C. Antiviral properties of the natural Polyphenols Delphinidin and Epigallocatechin Gallate against the Flaviviruses West Nile Virus, Zika Virus, and Dengue Virus. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Johari, J.; Kianmehr, A.; Mustafa, M.; Abubakar, S.; Zandi, K. Antiviral activity of Baicalein and Quercetin against the Japanese Encephalitis Virus. Int. J. Mol. Sci. 2012, 13, 16785–16795. [Google Scholar] [CrossRef]
- Zandi, K.; Teoh, B.-T.; Sam, S.-S.; Wong, P.-F.; Mustafa, M.R.; AbuBakar, S. Novel antiviral activity of baicalein against dengue virus. BMC Complement. Altern. Med. 2012, 12, 1185. [Google Scholar] [CrossRef] [Green Version]
- Shukla, Y.; Singh, R. Resveratrol and cellular mechanisms of cancer prevention. Ann. N. Y. Acad. Sci. 2011, 1215, 1–8. [Google Scholar] [CrossRef]
- Shen, T.; Wang, X.-N.; Lou, H.-X. Natural stilbenes: An overview. Nat. Prod. Rep. 2009, 26, 916. [Google Scholar] [CrossRef] [PubMed]
- Ito, T. Resveratrol oligomer structure in Dipterocarpaceaeous plants. J. Nat. Med. 2020, 74, 619–637. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, W.; Zhou, Z.; Deng, S.; Ma, X.; Ma, X.; Li, C.; Shu, X. Therapeutic versatility of resveratrol derivatives. Nutrients 2017, 9, 1188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thapa, S.B.; Pandey, R.P.; Park, Y., II; Sohng, J.K. Biotechnological advances in resveratrol production and its chemical diversity. Molecules 2019, 24, 2571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abba, Y.; Hassim, H.; Hamzah, H.; Noordin, M.M. Antiviral activity of resveratrol against human and animal viruses. Adv. Virol. 2015, 2015, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohd, A.; Zainal, N.; Tan, K.-K.; AbuBakar, S. Resveratrol affects Zika virus replication in vitro. Sci. Rep. 2019, 9, 14336. [Google Scholar] [CrossRef]
- Nakamura, M. An antioxidant resveratrol significantly enhanced replication of hepatitis C virus. World J. Gastroenterol. 2010, 16, 184. [Google Scholar] [CrossRef]
- Paemanee, A.; Hitakarun, A.; Roytrakul, S.; Smith, D.R. Screening of melatonin, α-tocopherol, folic acid, acetyl-l-carnitine and resveratrol for anti-dengue 2 virus activity. BMC Res. Notes 2018, 11, 307. [Google Scholar] [CrossRef] [Green Version]
- Zainal, N.; Chang, C.-P.; Cheng, Y.-L.; Wu, Y.-W.; Anderson, R.; Wan, S.-W.; Chen, C.-L.; Ho, T.-S.; AbuBakar, S.; Lin, Y.-S. Resveratrol treatment reveals a novel role for HMGB1 in regulation of the type 1 interferon response in dengue virus infection. Sci. Rep. 2017, 7, 42998. [Google Scholar] [CrossRef] [Green Version]
- Ong, S.P.; Lee, L.M.; Leong, Y.F.I.; Ng, M.L.; Chu, J.J.H. Dengue virus infection mediates HMGB1 release from monocytes involving PCAF acetylase complex and induces vascular leakage in Endothelial cells. PLoS ONE 2012, 7, e41932. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.-S.; Penthala, N.R.; Oliveira, M.; Mesplède, T.; Xu, H.; Quan, Y.; Crooks, P.A.; Wainberg, M.A. Identification of resveratrol analogs as potent anti-dengue agents using a cell-based assay. J. Med. Virol. 2017, 89, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, M.; Garcia-Blanco, M. Targeting host factors to treat west Nile and Dengue Viral infections. Viruses 2014, 6, 683–708. [Google Scholar] [CrossRef] [PubMed]
- De Wispelaere, M.; LaCroix, A.J.; Yang, P.L. The Small Molecules AZD0530 and Dasatinib Inhibit Dengue Virus RNA Replication via Fyn Kinase. J. Virol. 2013, 87, 7367–7381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lü, J.-M.; Nurko, J.; Weakley, S.M.; Jiang, J.; Kougias, P.; Lin, P.H.; Yao, Q.; Chen, C. Molecular mechanisms and clinical applications of nordihydroguaiaretic acid (NDGA) and its derivatives: An update. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2010, 16, RA93. [Google Scholar]
- Manda, G.; Rojo, A.I.; Martínez-Klimova, E.; Pedraza-Chaverri, J.; Cuadrado, A. Nordihydroguaiaretic Acid: From herbal medicine to clinical development for cancer and chronic diseases. Front. Pharmacol. 2020, 11. [Google Scholar] [CrossRef]
- Hernández-Damián, J.; Andérica-Romero, A.C.; Pedraza-Chaverri, J. Paradoxical cellular effects and biological role of the multifaceted compound Nordihydroguaiaretic Acid. Arch. Pharm. (Weinheim) 2014, 347, 685–697. [Google Scholar] [CrossRef]
- Iranpour, M.; Moghadam, A.R.; Yazdi, M.; Ande, S.R.; Alizadeh, J.; Wiechec, E.; Lindsay, R.; Drebot, M.; Coombs, K.M.; Ghavami, S. Apoptosis, autophagy and unfolded protein response pathways in Arbovirus replication and pathogenesis. Expert Rev. Mol. Med. 2016, 18, e1. [Google Scholar] [CrossRef]
- Hwu, J.R.; Hsu, M.-H.; Huang, R.C.C. New nordihydroguaiaretic acid derivatives as anti-HIV agents. Bioorg. Med. Chem. Lett. 2008, 18, 1884–1888. [Google Scholar] [CrossRef]
- Uchide, N.; Ohyama, K.; Bessho, T.; Toyoda, H. Inhibition of Influenza-Virus-Induced Apoptosis in Chorion cells of human fetal membranes by Nordihydroguaiaretic Acid. Intervirology 2005, 48, 336–340. [Google Scholar] [CrossRef]
- Syed, G.H.; Siddiqui, A. Effects of hypolipidemic agent nordihydroguaiaretic acid on lipid droplets and hepatitis C virus. Hepatology 2011, 54, 1936–1946. [Google Scholar] [CrossRef] [Green Version]
- Samsa, M.M.; Mondotte, J.A.; Iglesias, N.G.; Assunção-Miranda, I.; Barbosa-Lima, G.; Da Poian, A.T.; Bozza, P.T.; Gamarnik, A.V. Dengue virus Capsid protein usurps lipid droplets for viral particle formation. PLoS Pathog. 2009, 5, e1000632. [Google Scholar] [CrossRef] [PubMed]
- Soto-Acosta, R.; Bautista-Carbajal, P.; Syed, G.H.; Siddiqui, A.; Del Angel, R.M. Nordihydroguaiaretic acid (NDGA) inhibits replication and viral morphogenesis of Dengue virus. Antivir. Res. 2014, 109, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.A.; Carneiro, F.A.; Martins, I.C.; Assuncao-Miranda, I.; Faustino, A.F.; Pereira, R.M.; Bozza, P.T.; Castanho, M.A.R.B.; Mohana-Borges, R.; Da Poian, A.T.; et al. Dengue Virus Capsid protein binding to Hepatic Lipid Droplets (LD) is Potassium Ion dependent and is mediated by LD surface proteins. J. Virol. 2012, 86, 2096–2108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Gutierrez, M.; Castellanos, J.E.; Gallego-Gómez, J.C. Statins reduce Dengue virus production via decreased Virion assembly. Intervirology 2011, 54, 202–216. [Google Scholar] [CrossRef] [PubMed]
- Martín-Acebes, M.A.; Vázquez-Calvo, Á.; Saiz, J.-C. Lipids and flaviviruses, present and future perspectives for the control of dengue, Zika, and West Nile viruses. Prog. Lipid Res. 2016, 64, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Villareal, V.A.; Rodgers, M.A.; Costello, D.A.; Yang, P.L. Targeting host lipid synthesis and metabolism to inhibit dengue and hepatitis C viruses. Antivir. Res. 2015, 124, 110–121. [Google Scholar] [CrossRef] [Green Version]
- Merino-Ramos, T.; Blázquez, A.-B.; Escribano-Romero, E.; Cañas-Arranz, R.; Sobrino, F.; Saiz, J.-C.; Martín-Acebes, M.A. Protection of a single dose West Nile Virus recombinant subviral particle vaccine against Lineage 1 or 2 strains and analysis of the cross-reactivity with Usutu virus. PLoS ONE 2014, 9, e108056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manolova, Y.; Deneva, V.; Antonov, L.; Drakalska, E.; Momekova, D.; Lambov, N. The effect of the water on the curcumin tautomerism: A quantitative approach. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 132, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Katsuyama, Y.; Kita, T.; Funa, N.; Horinouchi, S. Curcuminoid Biosynthesis by two type III Polyketide Synthases in the Herb Curcuma longa. J. Biol. Chem. 2009, 284, 11160–11170. [Google Scholar] [CrossRef] [Green Version]
- Kocaadam, B.; Şanlier, N. Curcumin, an active component of turmeric ( Curcuma longa ), and its effects on health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2889–2895. [Google Scholar] [CrossRef] [PubMed]
- Joe, B.; Vijaykumar, M.; Lokesh, B.R. Biological properties of curcumin-cellular and molecular mechanisms of action. Crit. Rev. Food Sci. Nutr. 2004, 44, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Ingolfsson, H.I.; Koeppe, R.E.; Andersen, O.S. Curcumin is a modulator of Bilayer material properties †. Biochemistry 2007, 46, 10384–10391. [Google Scholar] [CrossRef] [PubMed]
- Si, X.; Wang, Y.; Wong, J.; Zhang, J.; McManus, B.M.; Luo, H. Dysregulation of the Ubiquitin-Proteasome system by curcumin suppresses Coxsackievirus B3 replication. J. Virol. 2007, 81, 3142–3150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dutta, K.; Ghosh, D.; Basu, A. Curcumin protects neuronal cells from Japanese Encephalitis Virus-mediated cell death and also inhibits infective viral particle formation by Dysregulation of Ubiquitin–Proteasome system. J. Neuroimmune Pharmacol. 2009, 4, 328–337. [Google Scholar] [CrossRef]
- Kutluay, S.B.; Doroghazi, J.; Roemer, M.E.; Triezenberg, S.J. Curcumin inhibits herpes simplex virus immediate-early gene expression by a mechanism independent of p300/CBP histone acetyltransferase activity. Virology 2008, 373, 239–247. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.; Ammosova, T.; Kumari, N.; Nekhai, S. Protein Phosphatase-1 –targeted small molecules, Iron Chelators and Curcumin analogs as HIV-1 antivirals. Curr. Pharm. Des. 2017, 23. [Google Scholar] [CrossRef] [Green Version]
- Anggakusuma; Colpitts, C.C.; Schang, L.M.; Rachmawati, H.; Frentzen, A.; Pfaender, S.; Behrendt, P.; Brown, R.J.P.; Bankwitz, D.; Steinmann, J.; et al. Turmeric curcumin inhibits entry of all hepatitis C virus genotypes into human liver cells. Gut 2014, 63, 1137–1149. [Google Scholar] [CrossRef]
- Mounce, B.C.; Cesaro, T.; Carrau, L.; Vallet, T.; Vignuzzi, M. Curcumin inhibits Zika and chikungunya virus infection by inhibiting cell binding. Antivir. Res. 2017, 142, 148–157. [Google Scholar] [CrossRef]
- Fernandez-Garcia, M.-D.; Meertens, L.; Bonazzi, M.; Cossart, P.; Arenzana-Seisdedos, F.; Amara, A. Appraising the roles of CBLL1 and the Ubiquitin/Proteasome system for Flavivirus entry and replication. J. Virol. 2011, 85, 2980–2989. [Google Scholar] [CrossRef] [Green Version]
- Padilla-S, L.; Rodríguez, A.; Gonzales, M.M.; Gallego-G, J.C.; Castaño-O, J.C. Inhibitory effects of curcumin on dengue virus type 2-infected cells in vitro. Arch. Virol. 2014, 159, 573–579. [Google Scholar] [CrossRef]
- Chen, T.-Y.; Chen, D.-Y.; Wen, H.-W.; Ou, J.-L.; Chiou, S.-S.; Chen, J.-M.; Wong, M.-L.; Hsu, W.-L. Inhibition of enveloped viruses infectivity by Curcumin. PLoS ONE 2013, 8, e62482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, T.T.H.; Si, J.; Kang, C.; Chung, B.; Chung, D.; Kim, D. Facile preparation of water soluble curcuminoids extracted from turmeric ( Curcuma longa L.) powder by using steviol glucosides. Food Chem. 2017, 214, 366–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torrens-Spence, M.P.; Pluskal, T.; Li, F.-S.; Carballo, V.; Weng, J.-K. Complete pathway elucidation and heterologous reconstitution of Rhodiola Salidroside biosynthesis. Mol. Plant 2018, 11, 205–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, Z.; Han, J.; Zhang, J.; Xiao, Q.; Hu, J.; Chen, L. Pharmacological activities, mechanisms of action, and safety of salidroside in the central nervous system. Drug Des. Devel. Ther. 2018, 12, 1479–1489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sa, L.; Wei, X.; Huang, Q.; Cai, Y.; Lu, D.; Mei, R.; Hu, X. Contribution of salidroside to the relieve of symptom and sign in the early acute stage of osteoarthritis in rat model. J. Ethnopharmacol. 2020, 259, 112883. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Peng, L.; Li, C.; Ji, Q.; Li, P. Salidroside alleviates cartilage degeneration through NF-κB pathway in Osteoarthritis Rats. Drug Des. Devel. Ther. 2020, 14, 1445–1454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shati, A.A. Salidroside ameliorates diabetic nephropathy in rats by activating renal AMPK/SIRT1 signaling pathway. J. Food Biochem. 2020, 44. [Google Scholar] [CrossRef]
- Sun, A.; Ju, X.-L. Advances in research on anticancer properties of Salidroside. Chin. J. Integr. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Agbo, M.O.; Odimegwu, D.C.; Okoye, F.B.C.; Osadebe, P.O. Antiviral activity of Salidroside from the leaves of Nigerian mistletoe (Loranthus micranthus Linn) parasitic on Hevea brasiliensis against respiratory syncytial virus. Pak. J. Pharm. Sci. 2017, 30, 1251–1256. [Google Scholar] [PubMed]
- Wang, H.; Ding, Y.; Zhou, J.; Sun, X.; Wang, S. The in vitro and in vivo antiviral effects of salidroside from Rhodiola rosea L. against coxsackievirus B3. Phytomedicine 2009, 16, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-L.; Lin, Y.-S.; Chen, C.-L.; Wan, S.-W.; Ou, Y.-D.; Yu, C.-Y.; Tsai, T.-T.; Tseng, P.-C.; Lin, C.-F. Dengue virus infection causes the activation of distinct NF- κ B pathways for inducible Nitric Oxide synthase and TNF- α expression in RAW264.7 Cells. Mediators Inflamm. 2015, 2015, 274025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.; Jung, J.-C.; Jang, S.; Kim, J.; Ali, Z.; Khan, I.A.; Oh, S. Anti-inflammatory and neuroprotective effects of constituents isolated from Rhodiola rosea. Evidence-Based Complement. Altern. Med. 2013, 2013, 514049. [Google Scholar] [CrossRef] [Green Version]
- Zuo, G.; Li, Z.; Chen, L.; Xu, X. Activity of compounds from Chinese herbal medicine Rhodiola kirilowii (Regel) Maxim against HCV NS3 serine protease. Antivir. Res. 2007, 76, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Mishra, K.P.; Ganju, L. Salidroside exhibits anti-dengue virus activity by upregulating host innate immune factors. Arch. Virol. 2016, 161, 3331–3344. [Google Scholar] [CrossRef]
- Gack, M.U. Mechanisms of RIG-I-Like Receptor activation and manipulation by Viral Pathogens. J. Virol. 2014, 88, 5213–5216. [Google Scholar] [CrossRef] [Green Version]
- Navarro-Sánchez, E.; Desprès, P.; Cedillo-Barrón, L. Innate immune responses to Dengue virus. Arch. Med. Res. 2005, 36, 425–435. [Google Scholar] [CrossRef]
- Sadler, A.J.; Williams, B.R.G. Structure and function of the protein Kinase R. In Interferon: The 50th Anniversary; Springer: Berlin/Heidelberg, Germany, 2007; pp. 253–292. [Google Scholar]
- Beltrán, D.; López-Vergès, S. NK Cells during Dengue disease and their recognition of Dengue virus-infected cells. Front. Immunol. 2014, 5. [Google Scholar] [CrossRef] [Green Version]
- Martins, F.O.; Esteves, P.F.; Mendes, G.S.; Barbi, N.S.; Menezes, F.S.; Romanos, M.T. Verbascoside isolated from Lepechinia speciosa has inhibitory activity against HSV-1 and HSV-2 in vitro. Nat. Prod. Commun. 2009, 4, 1934578X0900401217. [Google Scholar] [CrossRef] [Green Version]
- Alipieva, K.; Korkina, L.; Orhan, I.E.; Georgiev, M.I. Verbascoside—A review of its occurrence, (bio)synthesis and pharmacological significance. Biotechnol. Adv. 2014, 32, 1065–1076. [Google Scholar] [CrossRef]
- Alvarenga, T.A.; Bertanha, C.S.; de Oliveira, P.F.; Tavares, D.C.; Gimenez, V.M.M.; Silva, M.L.A.; Cunha, W.R.; Januário, A.H.; Pauletti, P.M. Lipoxygenase inhibitory activity of Cuspidaria pulchra and isolated compounds. Nat. Prod. Res. 2015, 29, 1083–1086. [Google Scholar] [CrossRef]
- Brandão, G.C.; Kroon, E.G.; dos Santos, J.R.; Stehmann, J.R.; Lombardi, J.A.; Oliveira, A.B. de Antiviral activities of plants occurring in the state of Minas Gerais, Brazil: Part 2. Screening Bignoniaceae species. Rev. Bras. Farmacogn. 2010, 20, 742–750. [Google Scholar] [CrossRef] [Green Version]
- Le, J.; Lu, W.; Xiong, X.; Wu, Z.; Chen, W. Anti-inflammatory constituents from Bidens frondosa. Molecules 2015, 20, 18496–18510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandão, G.; Kroon, E.; Souza, D.; Filho, J.; Oliveira, A. Chemistry and antiviral activity of Arrabidaea pulchra (Bignoniaceae). Molecules 2013, 18, 9919–9932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDougall, B.; King, P.J.; Wu, B.W.; Hostomsky, Z.; Reinecke, M.G.; Robinson, W.E. Dicaffeoylquinic and Dicaffeoyltartaric acids are selective inhibitors of human immunodeficiency virus type 1 integrase. Antimicrob. Agents Chemother. 1998, 42, 140–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, J.N.; Bolraa, S.; Ji, M.; He, Q.Q.; Ma, C.M. Quantification and antioxidant and anti-HCV activities of the constituents from the inflorescences of Scabiosa comosa and S. tschilliensis. Nat. Prod. Res. 2016, 30, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Kopp, E.; Ghosh, S. Inhibition of NF-kappa B by sodium salicylate and aspirin. Science 1994, 265, 956–959. [Google Scholar] [CrossRef]
- Mitchell, J.A.; Saunders, M.; Barnes, P.J.; Newton, R.; Belvisi, M.G. Sodium Salicylate inhibits Cyclo-Oxygenase-2 activity independently of transcription factor (nuclear factor κB) activation: Role of arachidonic acid. Mol. Pharmacol. 1997, 51, 907–912. [Google Scholar] [CrossRef] [Green Version]
- Bitko, V.; Velazquez, A.; Yang, L.; Yang, Y.-C.; Barik, S. Transcriptional induction of multiple Cytokines by human respiratory Syncytial Virus requires activation of NF-κB and is inhibited by Sodium Salicylate and aspirin. Virology 1997, 232, 369–378. [Google Scholar] [CrossRef] [Green Version]
- Speir, E.; Yu, Z.-X.; Ferrans, V.J.; Huang, E.-S.; Epstein, S.E. Aspirin attenuates Cytomegalovirus infectivity and gene expression mediated by Cyclooxygenase-2 in coronary artery smooth muscle cells. Circ. Res. 1998, 83, 210–216. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-J.; Raung, S.-L.; Kuo, M.-D.; Wang, Y.-M. Suppression of Japanese encephalitis virus infection by non-steroidal anti-inflammatory drugs. J. Gen. Virol. 2002, 83, 1897–1905. [Google Scholar] [CrossRef] [Green Version]
- Liao, C.-L.; Lin, Y.-L.; Wu, B.-C.; Tsao, C.-H.; Wang, M.-C.; Liu, C.-I.; Huang, Y.-L.; Chen, J.-H.; Wang, J.-P.; Chen, L.-K. Salicylates inhibit Flavivirus replication independently of blocking nuclear factor Kappa B activation. J. Virol. 2001, 75, 7828–7839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.; Chen, C.; Li, Z.; Guo, W.; Gegner, J.A.; Lin, S.; Han, J. Characterization of the structure and function of a New Mitogen-activated Protein Kinase (p38β). J. Biol. Chem. 1996, 271, 17920–17926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, D. Anti-platelet agents: Past, present and future. ISBT Sci. Ser. 2020, 15, 131–141. [Google Scholar] [CrossRef]
- Alvarenga, T.A.; de Oliveira, P.F.; de Souza, J.M.; Tavares, D.C.; Andrade e Silva, M.L.; Cunha, W.R.; Groppo, M.; Januário, A.H.; Magalhães, L.G.; Pauletti, P.M. Schistosomicidal Activity of Alkyl-phenols from the Cashew Anacardium occidentale against Schistosoma mansoni Adult Worms. J. Agric. Food Chem. 2016, 64, 8821–8827. [Google Scholar] [CrossRef]
- Zhuang, J.-X.; Hu, Y.-H.; Yang, M.-H.; Liu, F.-J.; Qiu, L.; Zhou, X.-W.; Chen, Q.-X. Irreversible competitive inhibitory kinetics of Cardol Triene on mushroom Tyrosinase. J. Agric. Food Chem. 2010, 58, 12993–12998. [Google Scholar] [CrossRef]
- Matutino Bastos, T.; Mannochio Russo, H.; Silvio Moretti, N.; Schenkman, S.; Marcourt, L.; Gupta, M.; Wolfender, J.-L.; Ferreira Queiroz, E.; Botelho Pereira Soares, M. Chemical constituents of Anacardium occidentale as inhibitors of Trypanosoma cruzi Sirtuins. Molecules 2019, 24, 1299. [Google Scholar] [CrossRef] [Green Version]
- Kanyaboon, P.; Saelee, T.; Suroengrit, A.; Hengphasatporn, K.; Rungrotmongkol, T.; Chavasiri, W.; Boonyasuppayakorn, S. Cardol triene inhibits dengue infectivity by targeting kl loops and preventing envelope fusion. Sci. Rep. 2018, 8, 16643. [Google Scholar] [CrossRef] [Green Version]
- HERBST, K.H. Albothyl as a hemostatic agent in otorhinolaryngology. Z. Laryngol. Rhinol. Otol. 1959, 38, 837. [Google Scholar]
- Silva, L.A.F.; Fioravanti, M.C.S.; Oliveira, K.S.; Atayde, I.B.; Andrade, M.A.; Jayme, V.S.; Rabelo, R.E.; Romani, A.F.; Araújo, E.G. Local utilization of Metacresolsulfonic acid combined with Streptomycin in the treatment of Actinomycosis. Ann. N. Y. Acad. Sci. 2004, 1026, 273–276. [Google Scholar] [CrossRef]
- Ali, A.; Al-sobayil, F.A.; Al-Hawas, A. Evaluating the effectiveness of different treatments of uterine infections in female camels (Camelus dromedarius). Theriogenology 2010, 74, 40–44. [Google Scholar] [CrossRef]
- Tomlinson, S.; Malmstrom, R.; Watowich, S. New approaches to structure-based discovery of Dengue protease inhibitors. Infect. Disord.-Drug Targets 2009, 9, 327–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geiss, B.J.; Stahla, H.; Hannah, A.M.; Gari, H.H.; Keenan, S.M. Focus on flaviviruses: Current and future drug targets. Future Med. Chem. 2009, 1, 327–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, D.; Mao, F.; Ye, Y.; Li, J.; Xu, C.; Luo, X.; Chen, J.; Shen, X. Policresulen, a novel NS2B/NS3 protease inhibitor, effectively inhibits the replication of DENV2 virus in BHK-21 cells. Acta Pharmacol. Sin. 2015, 36, 1126–1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chanprapaph, S.; Saparpakorn, P.; Sangma, C.; Niyomrattanakit, P.; Hannongbua, S.; Angsuthanasombat, C.; Katzenmeier, G. Competitive inhibition of the dengue virus NS3 serine protease by synthetic peptides representing polyprotein cleavage sites. Biochem. Biophys. Res. Commun. 2005, 330, 1237–1246. [Google Scholar] [CrossRef]
- Johnson, K.; Liu, L.; Majdzadeh, N.; Chavez, C.; Chin, P.C.; Morrison, B.; Wang, L.; Park, J.; Chugh, P.; Chen, H.-M.; et al. Inhibition of neuronal apoptosis by the cyclin-dependent kinase inhibitor GW8510: Identification of 3′ substituted indolones as a scaffold for the development of neuroprotective drugs. J. Neurochem. 2005, 93, 538–548. [Google Scholar] [CrossRef]
- Lackey, K.; Cory, M.; Davis, R.; Frye, S.V.; Harris, P.A.; Hunter, R.N.; Jung, D.K.; McDonald, O.B.; McNutt, R.W.; Peel, M.R.; et al. The discovery of potent cRaf1 kinase inhibitors. Bioorg. Med. Chem. Lett. 2000, 10, 223–226. [Google Scholar] [CrossRef]
- Chen, H.-M.; Wang, L.; D’Mello, S.R. Inhibition of ATF-3 expression by B-Raf mediates the neuroprotective action of GW5074. J. Neurochem. 2008, 105, 1300–1312. [Google Scholar] [CrossRef]
- Chin, P.C.; Liu, L.; Morrison, B.E.; Siddiq, A.; Ratan, R.R.; Bottiglieri, T.; D’Mello, S.R. The c-Raf inhibitor GW5074 provides neuroprotection invitro and in an animal model of neurodegeneration through a MEK-ERK and Akt-independent mechanism. J. Neurochem. 2004, 90, 595–608. [Google Scholar] [CrossRef]
- Opoku-Temeng, C.; Onyedibe, K.I.; Aryal, U.K.; Sintim, H.O. Proteomic analysis of bacterial response to a 4-hydroxybenzylidene indolinone compound, which re-sensitizes bacteria to traditional antibiotics. J. Proteom. 2019, 202, 103368. [Google Scholar] [CrossRef]
- Pfleiderer, P.; Sumandea, M.P.; Rybin, V.O.; Wang, C.; Steinberg, S.F. Raf-1: A novel cardiac troponin T kinase. J. Muscle Res. Cell Motil. 2009, 30, 67–72. [Google Scholar] [CrossRef]
- Arita, M.; Wakita, T.; Shimizu, H. Characterization of pharmacologically active compounds that inhibit poliovirus and enterovirus 71 infectivity. J. Gen. Virol. 2008, 89, 2518–2530. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Atkinson, S.; Fraser, J.; Wang, C.; Maher, B.; Roman, N.; Forwood, J.; Wagstaff, K.; Borg, N.; Jans, D. Novel Flavivirus antiviral that targets the host nuclear transport importin α/β1 heterodimer. Cells 2019, 8, 281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.-J.; Lee, Y.M.; Lee, C.-K.; Jung, J.K.; Han, S.B.; Hong, J.T. Therapeutic applications of compounds in the Magnolia family. Pharmacol. Ther. 2011, 130, 157–176. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, X.; Zhu, Y.; Chen, S.; Zhou, D.; Wang, Y. Honokiol inhibits the inflammatory reaction during cerebral ischemia reperfusion by suppressing NF-κB activation and cytokine production of glial cells. Neurosci. Lett. 2013, 534, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Zhang, X.; Wang, Y.; Chen, S. Honokiol inhibits arterial thrombosis through endothelial cell protection and stimulation of prostacyclin. Acta Pharmacol. Sin. 2005, 26, 1063–1068. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.-L.; Man, K.-M.; Huang, P.-H.; Chen, W.-C.; Chen, D.-C.; Cheng, Y.-W.; Liu, P.-L.; Chou, M.-C.; Chen, Y.-H. Honokiol and Magnolol as multifunctional antioxidative molecules for dermatologic disorders. Molecules 2010, 15, 6452–6465. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, P.; Wu, A.-H. Honokiol inhibits carotid artery atherosclerotic plaque formation by suppressing inflammation and oxidative stress. Aging 2020, 12, 8016–8028. [Google Scholar] [CrossRef]
- Talarek, S.; Listos, J.; Barreca, D.; Tellone, E.; Sureda, A.; Nabavi, S.F.; Braidy, N.; Nabavi, S.M. Neuroprotective effects of honokiol: From chemistry to medicine. BioFactors 2017, 43, 760–769. [Google Scholar] [CrossRef]
- Hahm, E.-R.; Arlotti, J.A.; Marynowski, S.W.; Singh, S.V. Honokiol, a constituent of oriental medicinal herb Magnolia officinalis, inhibits growth of PC-3 Xenografts in vivo in association with Apoptosis induction. Clin. Cancer Res. 2008, 14, 1248–1257. [Google Scholar] [CrossRef] [Green Version]
- Bai, X.; Cerimele, F.; Ushio-Fukai, M.; Waqas, M.; Campbell, P.M.; Govindarajan, B.; Der, C.J.; Battle, T.; Frank, D.A.; Ye, K.; et al. Honokiol, a small molecular weight natural product, inhibits Angiogenesis in vitro and tumor growth in Vivo. J. Biol. Chem. 2003, 278, 35501–35507. [Google Scholar] [CrossRef] [Green Version]
- Fried, L.E.; Arbiser, J.L. Honokiol, a multifunctional antiangiogenic and antitumor agent. Antioxid. Redox Signal. 2009, 11, 1139–1148. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kim, J.; Jeong, S.-I.; Jahng, K.Y.; Yu, K.-Y. Antimicrobial effects and resistant regulation of Magnolol and Honokiol on Methicillin-resistant Staphylococcus aureus. Biomed Res. Int. 2015, 2015, 283630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Li, L.; Tan, L.; Liang, X. Inhibition of herpes simplex Virus-1 replication by natural compound Honokiol. Virol. Sin. 2019, 34, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Lan, K.-H.; Wang, Y.-W.; Lee, W.-P.; Lan, K.-L.; Tseng, S.-H.; Hung, L.-R.; Yen, S.-H.; Lin, H.-C.; Lee, S.-D. Multiple effects of honokiol on the life cycle of hepatitis C virus. Liver Int. 2012, 32, 989–997. [Google Scholar] [CrossRef]
- Fang, C.-Y.; Chen, S.-J.; Wu, H.-N.; Ping, Y.-H.; Lin, C.-Y.; Shiuan, D.; Chen, C.-L.; Lee, Y.-R.; Huang, K.-J. Honokiol, a lignan Biphenol derived from the Magnolia tree, inhibits Dengue virus type 2 infection. Viruses 2015, 7, 4894–4910. [Google Scholar] [CrossRef] [Green Version]

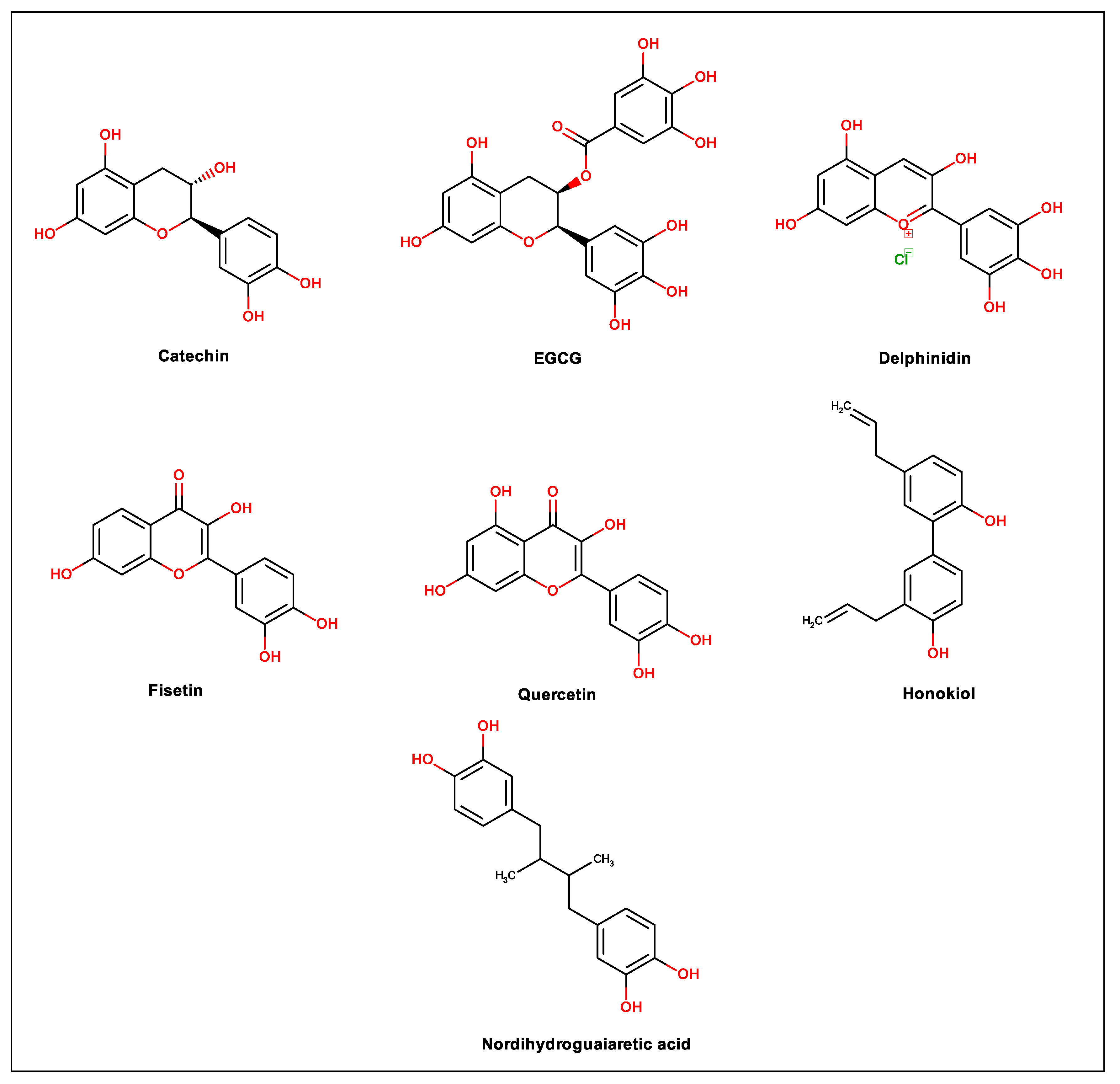
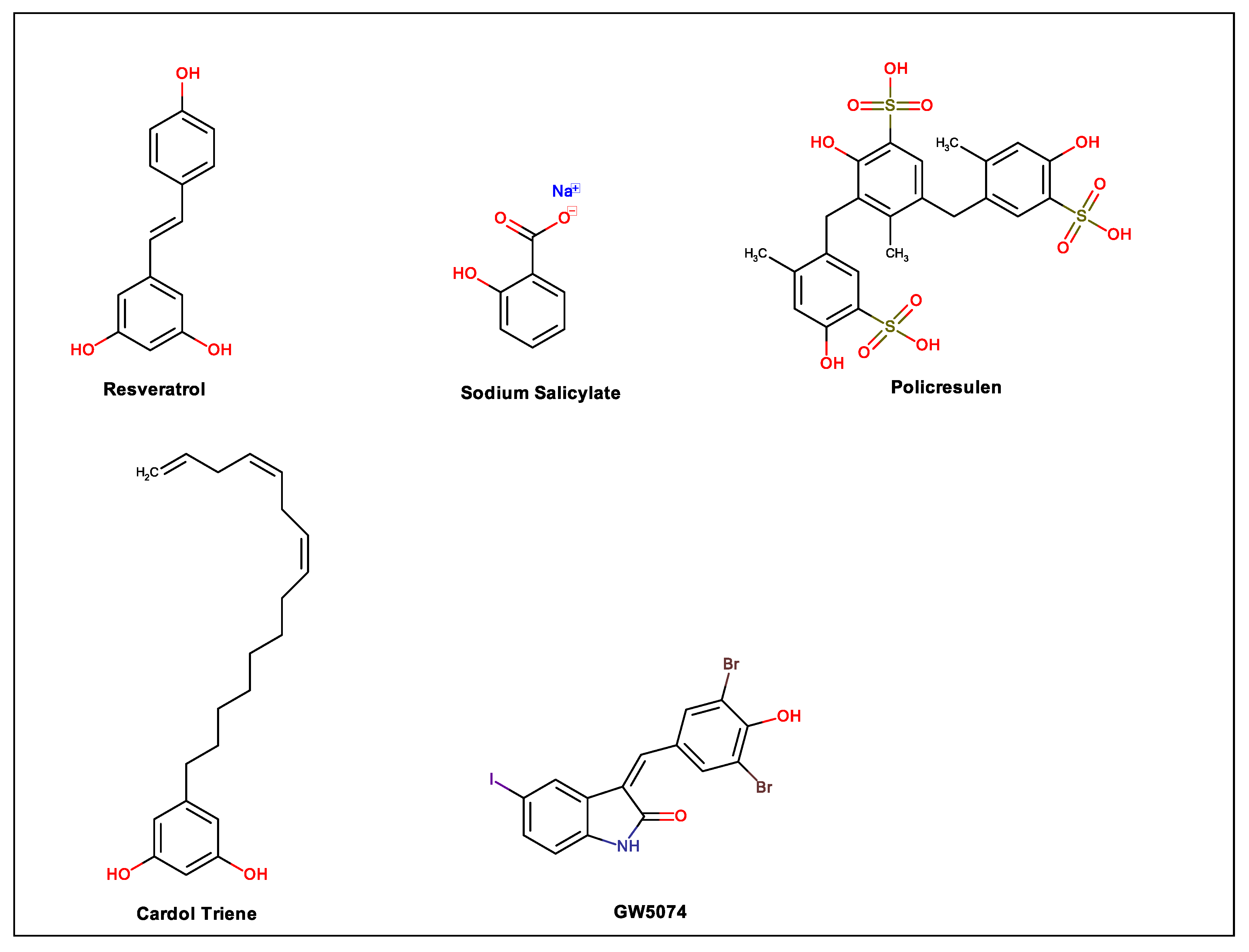

| Compound and Structure | IUPAC Name | Experimental Model Used | IC50 | Mechanism of Action | Reference |
|---|---|---|---|---|---|
| Geraniin | [(1R,7R,8S,26R,28S,29R,38R)- 1,13,14,15,18,19,20,34,35,39,39-undecahydroxy-2,5,10,23,31-pentaoxo-6,9,24,27,30,40 exaoxaoctacyclo[34.3.1.04,38.07,26.08,29.011,16.017,22.032,37]tetraconta-3,11,13,15,17,19,21,32,34,36-decaen-28-yl] 3,4,5-trihydroxybenzoate | VERO cells | 8.91 µM | Possible effect on viral particle Effect on cellular proteins involved in viral replication cycle and cellular metabolisms | [44] |
| VERO cells Molecular docking | 1.75 μM | Dose-dependent virucidal effect Inhibition of adhesion of viral particle Possible inhibition of early steps of virus replication cycle Interference with cell receptor interaction by binding to the E-DIII protein | [45] | ||
| BALB/c mice | 1.78 μM | Viremia reduction Prevention of liver damage | [47] | ||
| Chebulagic Acid | 2-[(4R,5S,7R,25S,26R,29S,30S,31S)-13,14,15,18,19,20,31,35,36-nonahydroxy-2,10,23,28,32-pentaoxo-5-(3,4,5-trihydroxybenzoyl)oxy-3,6,9,24,27,33-hexaoxaheptacyclo[28.7.1.04,25.07,26.011,16.017,22.034,38]octatriaconta-1(37),11,13,15,17,19,21,34(38),35-nonaen-29-yl]acetic acid | HELA, VERO, A549 and HEp-2 cells. | 13.11 μM | Inhibition of viral particle adhesion and fusion to cell membrane steps Possible GAG-competitor | [58] |
| Punicalagin | (1R,35R,38R,55S)-6,7,8,11,12,23,24,27,28,29,37,43,44,45,48,49,50-heptadecahydroxy-2,14,21,33,36,39,54-heptaoxaundecacyclo[33.20.0.04,9.010,19.013,18.016,25.017,22.026,31.038,55.041,46.047,52]pentapentaconta-4,6,8,10,12,16,18,22,24,26,28,30,41,43,45,47,49,51-octadecaene-3,15,20,32,40,53-hexone | HELA, VERO, A549 and HEp-2 cells. | 7.86 μM | Inhibition of viral particle adhesion and fusion to cell membrane steps Possible GAG-competitor | [58] |
| Quercetin | 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxychromen-4-one | U937-DC-SIGN cells | 24.5 µM | Downregulation of TNF-α | [85] |
| Molecular docking | Unreported Unreported | In silico interaction with E, NS1, NS3 and NS5 proteins | [89,90] | ||
| VERO cells | 19.2 μg/mL | Inhibition in pre and posttreatment strategies but mechanism not completely elucidated | [87] | ||
| BHK-21 cells | 125 μg/mL | Possible virucide effect | [73] | ||
| Molecular docking and enzymatic reaction | 35.2 µM a 22.7 µM b | Enzymatic inhibition of DENV-2 a and DENV-3 b NS2B-NS3 protease and in silico interaction with DENV-3 protease | [91] | ||
| In silico | Unreported | Protease binding | [92] | ||
| In silico; BHK-21 cells | Unreported | Protease binding; inhibition adsorption of viral particles | [88] | ||
| Fisetin | 2-(3,4-dihydroxyphenyl)-3,7-dihydroxychromen-4-one | U937-DC-SIGN cells | 7.3 µM | Downregulation of TNF-α | [85] |
| Molecular docking | Unreported Unreported | In silico interaction with E, NS1, NS2B-NS3 and NS5 proteins | [89,90] | ||
| VERO cells | 43.12 µg/mL c 55 µg/mL d 50 µg/mL e | Inhibition in pre c and posttreatment d strategies, and genome inhibition e but mechanism not completely elucidated | [95] | ||
| Naringin | ((2S)-7-[(2S,3R,4S,5S,6R)-4,5-dihydroxy-6-(hydroxymethyl)-3-[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxyoxan-2-yl]oxy-5-hydroxy-2-(4-hydroxyphenyl)-2,3-dihydrochromen-4-one) | VERO cells | 47.9 μg/mL | Inhibition in posttreatment strategy but mechanism not completely elucidated | [87] |
| VERO cells | 168.2 μg mL | Anti-adsorption activity with reduction in RNA production | [96] | ||
| Catechin | (2R,3S)-2-(3,4-dihydroxyphenyl)-3,4-ddihydro-2H-chromene-3,5,7-triol | VERO cells | 33.7 μg/mL | Inhibition in pre and posttreatment strategies but mechanism not completely elucidated | [87] |
| VERO cells | Unreported | Mechanism not completely elucidated | [97] | ||
| Delphinidin | 2-(3,4,5-trihydroxyphenyl)chromenylium-3,5,7-triol;chloride | VERO cells | Unreported | Mechanism not completely elucidated | [97] |
| EGCG | [(2R,3R)-5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)-3,4-dihydro-2H-chromen-3-yl] 3,4,5-trihydroxybenzoate | VERO cells | 18.0 µM | Inhibition in pretreatment strategy but mechanism not completely elucidated | [97] |
| VERO cells | Unreported | Directed to viral particle | [98] | ||
| Resveratrol | 5-[(E)-2-(4-hydroxyphenyl)ethenyl]benzene-1,3-diol | HEK293T/17 cells | 24.37 μM | Dose-dependent inhibition in stages after viral entry but mechanism not completely elucidated | [109] |
| Huh7 cells | Unreported | Induction of HMGB1 protein accumulation Induction of interferon stimulated genes (ISG) | [110] | ||
| Huh7 cells | 8.12 nM f 7.22 nM g | Inhibition of viral genome not affecting the viral polymerase (resveratrol analogs PNR-4-44 f and PNR-5-02 g) | [112] | ||
| Nordihydroguaiaretic acid | 4-[4-(3,4-dihydroxyphenyl)-2,3-dimethylbutyl]benzene-1,2-diol | Huh-7, U937 and VERO cells | Unreported | Reduction in the amount of lipid droplets; Reduction in the production of NS1; Prevention of the correct assembly of the DENV viral particle | [123] |
| Curcumin | (1E,6E)-1,7-bis(4-hydroxy-3-methoxyphenyl)hepta-1,6-diene-3,5-dione | BHK-21 or VERO cells | 11.51 µM | Intracellular accumulation of viral proteins and ubiquitin-conjugated proteins but mechanism not completely elucidated | [141] |
| VERO cells | Unreported | Could affect cell-membrane and viral envelope structure | [142] | ||
| Salidroside | (2R,3S,4S,5R,6R)-2-(hydroxymethyl)-6-[2-(4-hydroxyphenyl)ethoxy]oxane-3,4,5-triol | hPBMC, VERO and THP-1 cells | Unreported | Activation of type 1 interferons via IRF-3 | [155] |
| Verbascoside | ([(2R,3R,4R,5R,6R)-6-[2-(3,4-dihydroxyphenyl)ethoxy]-5-hydroxy-2-(hydroxymethyl)-4-[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxyoxan-3-yl] (E)-3-(3,4-dihydroxyphenyl)prop-2-enoate) | VERO and LLCMK2 cells | 3.4 μg/mL | Mechanism not completely elucidated | [165] |
| Caffeoylcalleryanin | [3-hydroxy-4-[[(2S,3R,4R,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]methyl]phenyl]methyl (E)-3-(3,4-dihydroxyphenyl)prop-2-enoate | VERO and LLCMK2 cells | 2.8 μg/mL | Mechanism not completely elucidated | [165] |
| Sodium salicylate | Sodium 2-hydroxybenzoate | BHK-21 and N18 cells | Unreported | Dose-dependent inhibition posttreatment but mechanism not completely elucidated | [173] |
| Cardol triene | 5-[(8Z,11Z)-pentadeca-8,11,14-trienyl]benzene-1,3-diol | VERO cells | 7.13 µM | Inhibition of cell membrane fusion with the viral envelope protein | [179] |
| Policresulen | 2-hydroxy-3,5-bis[(4-hydroxy-2-methyl-5-sulfophenyl)methyl]-4-methylbenzenesulfonic acid | BHK-21 cells transfected with Rlu-DENV-Rep | 4.99 μg/mL | Inhibition of DENV2 NS2B/NS3 protease | [185] |
| GW5074 | (3Z)-3-[(3,5-dibromo-4-hydroxyphenyl)methylidene]-5-iodo-1H-indol-2-one | VERO cells | 5.4 µM h 0.5 µM i | Inhibition of NS5–IMPα/β1 interaction in vitro h as well as NS5 nuclear localization in infected cells; posttreatment activity i | [194] |
| Honokiol | 2-(4-hydroxy-3-prop-2-enylphenyl)-4-prop-2-enylphenol | BHK and Huh7 cells | 10.6 µM | Inhibit early steps of DENV infection, suppressing the upregulation of early endosomes Reduce viral protein expression (NS1 and NS3) and double-stranded RNA | [207] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loaiza-Cano, V.; Monsalve-Escudero, L.M.; Filho, C.d.S.M.B.; Martinez-Gutierrez, M.; Sousa, D.P.d. Antiviral Role of Phenolic Compounds against Dengue Virus: A Review. Biomolecules 2021, 11, 11. https://doi.org/10.3390/biom11010011
Loaiza-Cano V, Monsalve-Escudero LM, Filho CdSMB, Martinez-Gutierrez M, Sousa DPd. Antiviral Role of Phenolic Compounds against Dengue Virus: A Review. Biomolecules. 2021; 11(1):11. https://doi.org/10.3390/biom11010011
Chicago/Turabian StyleLoaiza-Cano, Vanessa, Laura Milena Monsalve-Escudero, Carlos da Silva Maia Bezerra Filho, Marlen Martinez-Gutierrez, and Damião Pergentino de Sousa. 2021. "Antiviral Role of Phenolic Compounds against Dengue Virus: A Review" Biomolecules 11, no. 1: 11. https://doi.org/10.3390/biom11010011
APA StyleLoaiza-Cano, V., Monsalve-Escudero, L. M., Filho, C. d. S. M. B., Martinez-Gutierrez, M., & Sousa, D. P. d. (2021). Antiviral Role of Phenolic Compounds against Dengue Virus: A Review. Biomolecules, 11(1), 11. https://doi.org/10.3390/biom11010011






