Dry Powder for Pulmonary Delivery: A Comprehensive Review
Abstract
:1. Introduction
2. Airways System
2.1. Respiratory Tract
2.2. Particles Deposition Pattern
2.3. Particle Clearance Mechanisms
3. Physicochemical Properties of Dry Powder
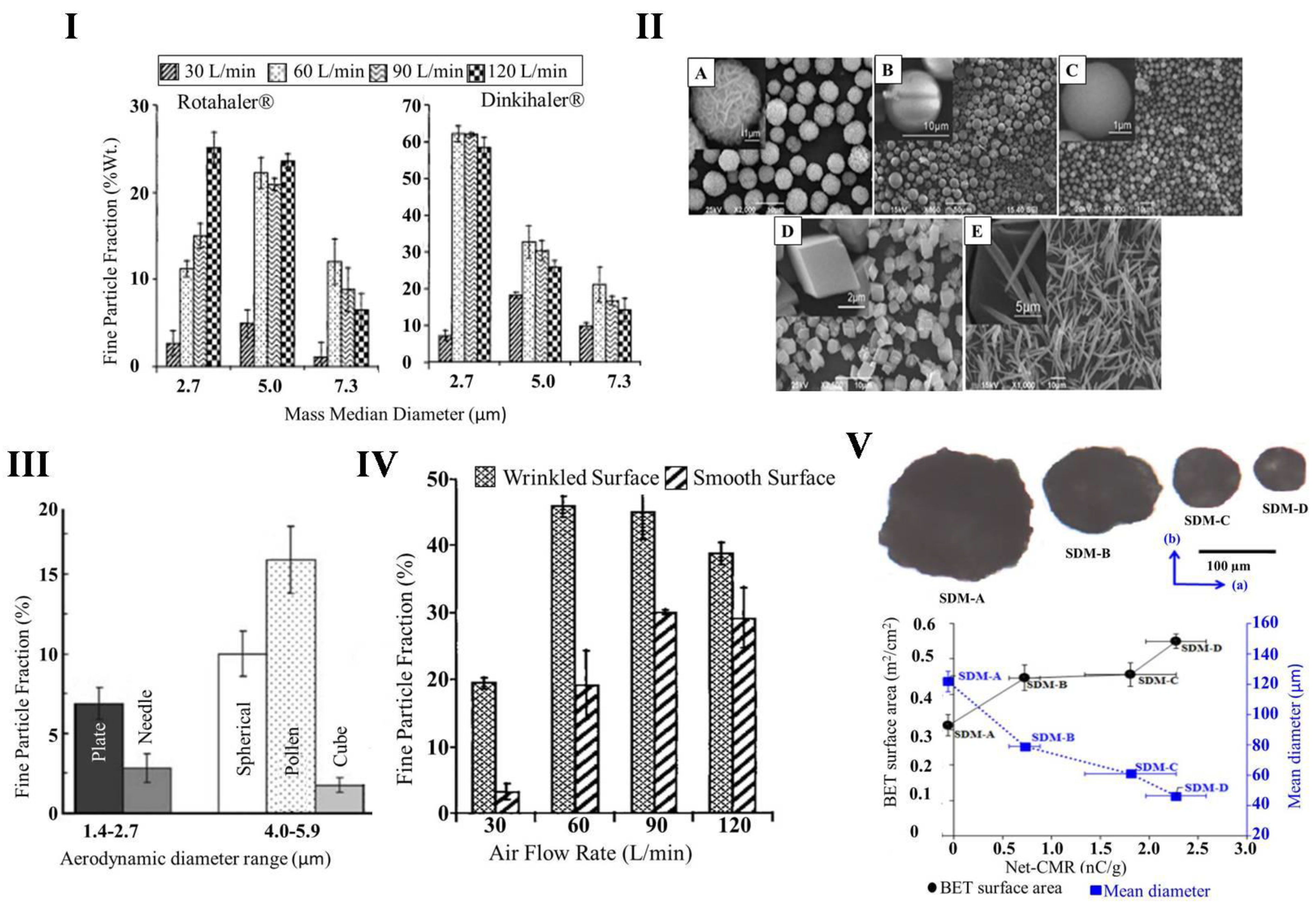
3.1. Size of Particles
3.2. Shape and Surface Morphology of Particles
3.3. Hygroscopicity and Moisture Content
3.4. Surface Electrostatic Charge
4. Techniques for Preparation of Dry Powders
4.1. Milling
4.2. Spray Drying
4.3. Spray-Freeze-Drying
4.4. Supercritical Fluid Drying
4.5. New Emerging Technologies
5. Factors Influencing the Clinical Efficacy and Marketed Formulations of DPIs
6. Challenges and Future Perspectives
7. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Paranjpe, M.; Müller-Goymann, C.C. Nanoparticle-mediated pulmonary drug delivery: A review. Int. J. Mol. Sci. 2014, 15, 5852–5873. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, H.; Danjo, K. Application of supercritical fluid to preparation of powders of high-molecular weight drugs for inhalation. Adv. Drug Deliv. Rev. 2008, 60, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, A.; Discher, D.E. Lung vascular targeting through inhalation delivery: Insight from filamentous viruses and other shapes. IUBMB Life 2011, 63, 607–612. [Google Scholar] [CrossRef]
- Sung, J.C.; Pulliam, B.L.; Edwards, D.A. Nanoparticles for drug delivery to the lungs. Trends Biotechnol. 2007, 25, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Bhavane, R.; Karathanasis, E.; Annapragada, A.V. Agglomerated vesicle technology: A new class of particles for controlled and modulated pulmonary drug delivery. J. Control. Release 2003, 93, 15–28. [Google Scholar] [CrossRef]
- Thorley, A.J.; Tetley, T.D. New perspectives in nanomedicine. Pharmacol. Ther. 2013, 140, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Preedy, E.C.; Prokopovich, P. Novel coatings and biotechnology trends in inhaler devices. In Inhaler Devices; Elsevier: New York, NY, USA, 2013; pp. 37–50. [Google Scholar]
- Alpar, H.; Somavarapu, S.; Atuah, K.N.; Bramwell, V.W. Biodegradable mucoadhesive particulates for nasal and pulmonary antigen and DNA delivery. Adv. Drug Deliv. Rev. 2005, 57, 411–430. [Google Scholar] [CrossRef]
- Hamedinasab, H.; Rezayan, A.H.; Mellat, M.; Mashreghi, M.; Jaafari, M.R. Development of chitosan-coated liposome for pulmonary delivery of N-acetylcysteine. Int. J. Biol. Macromol. 2020, 156, 1455–1463. [Google Scholar] [CrossRef]
- Patton, J.S.; Brain, J.D.; Davies, L.A.; Fiegel, J.; Gumbleton, M.; Kim, K.-J.; Sakagami, M.; Vanbever, R.; Ehrhardt, C. The Particle has Landed—Characterizing the Fate of Inhaled Pharmaceuticals. J. Aerosol Med. Pulm. Drug Deliv. 2010, 23, S-71–S-87. [Google Scholar] [CrossRef] [Green Version]
- Kanig, J.L. Pharmaceutical Aerosols. J. Pharm. Sci. 1963, 52, 513–535. [Google Scholar] [CrossRef]
- Bosquillon, C.; Lombry, C.; Preat, V.; Vanbever, R. Comparison of particle sizing techniques in the case of inhalation dry powders. J. Pharm. Sci. 2001, 90, 2032–2041. [Google Scholar] [CrossRef] [Green Version]
- El-Sherbiny, I.M.; Villanueva, D.G.; Herrera, D.; Smyth, H.D.C. Overcoming Lung Clearance Mechanisms for Controlled Release Drug Delivery. In Controlled Pulmonary Drug Delivery; Springer: New York, NY, USA, 2011; pp. 101–126. [Google Scholar]
- Chew, N.Y.; Chan, H.-K. The Role of Particle Properties in Pharmaceutical Powder Inhalation Formulations. J. Aerosol Med. 2002, 15, 325–330. [Google Scholar] [CrossRef]
- Chen, L.; Okuda, T.; Lu, X.-Y.; Chan, H.-K. Amorphous powders for inhalation drug delivery. Adv. Drug Deliv. Rev. 2016, 100, 102–115. [Google Scholar] [CrossRef] [PubMed]
- Ticehurst, M.D.; Basford, P.A.; Dallman, C.I.; Lukas, T.M.; Marshall, P.V.; Nichols, G.; Smith, D. Characterisation of the influence of micronisation on the crystallinity and physical stability of revatropate hydrobromide. Int. J. Pharm. 2000, 193, 247–259. [Google Scholar] [CrossRef]
- Mackin, L.; Zanon, R.; Park, J.M.; Foster, K.; Opalenik, H.; Demonte, M. Quantification of low levels (<10%) of amorphous content in micronised active batches using dynamic vapour sorption and isothermal microcalorimetry. Int. J. Pharm. 2002, 231, 227–236. [Google Scholar]
- Jain, M.S.; Lohare, G.B.; Bari, M.M.; Chavan, R.B.; Barhate, S.D.; Shah, C.B. Spray Drying in Pharmaceutical Industry: A Review. Res. J. Pharm. Dos. Forms Technol. 2012, 4, 74–79. [Google Scholar]
- Leung, S.S.Y.; Parumasivam, T.; Gao, F.G.; Carrigy, N.B.; Vehring, R.; Finlay, W.H.; Morales, S.; Britton, W.J.; Kutter, E.; Chan, H.-K. Production of Inhalation Phage Powders Using Spray Freeze Drying and Spray Drying Techniques for Treatment of Respiratory Infections. Pharm. Res. 2016, 33, 1486–1496. [Google Scholar] [CrossRef]
- Panão, M.O.; Moreira, A.L.N.; Vicente, J.; Costa, E. Assessment of ultrasonic sprays for spray drying. Assessment 2014, 7, 10. [Google Scholar]
- Joshi, J.T. A review on micronization techniques. J. Pharm. Sci. Technol. 2011, 3, 651–681. [Google Scholar]
- Otake, H.; Okuda, T.; Okamoto, H. Development of Spray-Freeze-Dried Powders for Inhalation with High Inhalation Performance and Antihygroscopic Property. Chem. Pharm. Bull. 2016, 64, 239–245. [Google Scholar] [CrossRef] [Green Version]
- Kondo, M.; Niwa, T.; Okamoto, H.; Danjo, K. Particle characterization of poorly water-soluble drugs using a spray freeze drying technique. Chem. Pharm. Bull. 2009, 57, 657–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishwarya, S.P.; Anandharamakrishnan, C.; Stapley, A.G. Spray-freeze-drying: A novel process for the drying of foods and bioproducts. Trends Food Sci. Technol. 2015, 41, 161–181. [Google Scholar] [CrossRef]
- Ali, M.E.; Lamprecht, A. Spray freeze drying for dry powder inhalation of nanoparticles. Eur. J. Pharm. Biopharm. 2014, 87, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Shoyele, S.A.; Cawthorne, S. Particle engineering techniques for inhaled biopharmaceuticals. Adv. Drug Deliv. Rev. 2006, 58, 1009–1029. [Google Scholar] [CrossRef]
- Davies, O.R.; Lewis, A.L.; Whitaker, M.J.; Tai, H.; Shakesheff, K.M.; Howdle, S.M. Applications of supercritical CO2 in the fabrication of polymer systems for drug delivery and tissue engineering. Adv. Drug Deliv. Rev. 2008, 60, 373–387. [Google Scholar] [CrossRef]
- Hofmann, W. Modelling inhaled particle deposition in the human lung—A review. J. Aerosol Sci. 2011, 42, 693–724. [Google Scholar] [CrossRef]
- Patwa, A.; Shah, A. Anatomy and physiology of respiratory system relevant to anaesthesia. Indian J. Anaesth. 2015, 59, 533–541. [Google Scholar] [CrossRef]
- Hickey, A.J. Pharmaceutical Inhalation Aerosol Technology; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Pilcer, G.; Amighi, K. Formulation strategy and use of excipients in pulmonary drug delivery. Int. J. Pharm. 2010, 392, 1–19. [Google Scholar] [CrossRef]
- Yang, W.; Peters, J.I.; Williams, R.O. Inhaled nanoparticles—A current review. Int. J. Pharm. 2008, 356, 239–247. [Google Scholar] [CrossRef]
- Usmani, O.S.; Biddiscombe, M.F.; Barnes, P.J. Regional Lung Deposition and Bronchodilator Response as a Function of β2-Agonist Particle Size. Am. J. Respir. Crit. Care Med. 2005, 172, 1497–1504. [Google Scholar] [CrossRef]
- O’Donnell, K.P.; Smyth, H.D.C. Macro- and Microstructure of the Airways for Drug Delivery. In Controlled Pulmonary Drug Delivery; Springer: New York, NY, USA, 2011; pp. 1–19. [Google Scholar]
- Liu, Q.; Guan, J.; Qin, L.; Zhang, X.; Mao, S. Physicochemical properties affecting the fate of nanoparticles in pulmonary drug delivery. Drug Discov. Today 2020, 25, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Taherali, F.; Varum, F.; Basit, A.W. A slippery slope: On the origin, role and physiology of mucus. Adv. Drug Deliv. Rev. 2018, 124, 16–33. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.; Gupta, N.; Ahsan, F. Particle engineering to enhance or lessen particle uptake by alveolar macrophages and to influence the therapeutic outcome. Eur. J. Pharm. Biopharm. 2015, 89, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Mossman, B.T.; Gualtieri, A.F. Lung Cancer: Mechanisms of Carcinogenesis by Asbestos. In Occupational Cancers; Springer: New York, NY, USA, 2020; pp. 239–256. [Google Scholar]
- Pedan, H.; Janosova, V.; Hajtman, A.; Calkovsky, V. Non-Reflex Defense Mechanisms of Upper Airway Mucosa: Possible Clinical Application. Physiol. Res. 2020, 69, S55–S67. [Google Scholar] [CrossRef]
- Thakur, A.K.; Chellappan, D.K.; Dua, K.; Mehta, M.; Satija, S.; Singh, I. Patented therapeutic drug delivery strategies for targeting pulmonary diseases. Expert Opin. Ther. Patents 2020, 30, 375–387. [Google Scholar] [CrossRef]
- Johnson, M.D.; Widdicombe, J.H.; Allen, L.; Barbry, P.; Dobbs, L.G. Alveolar epithelial type I cells contain transport proteins and transport sodium, supporting an active role for type I cells in regulation of lung liquid homeostasis. Proc. Natl. Acad. Sci. USA 2002, 99, 1966–1971. [Google Scholar] [CrossRef] [Green Version]
- Johnson, M.D.; Bao, H.-F.; Helms, M.N.; Chen, X.-J.; Tigue, Z.; Jain, L.; Dobbs, L.G.; Eaton, D.C. Functional ion channels in pulmonary alveolar type I cells support a role for type I cells in lung ion transport. Proc. Natl. Acad. Sci. USA 2006, 103, 4964–4969. [Google Scholar] [CrossRef] [Green Version]
- Wittekindt, O.H. Tight junctions in pulmonary epithelia during lung inflammation. Pflüg. Arch. 2017, 469, 135–147. [Google Scholar] [CrossRef] [Green Version]
- De Boer, A.H.; Gjaltema, D.; Hagedoorn, P.; Frijlink, H.W. Can ‘extrafine’ dry powder aerosols improve lung deposition? Eur. J. Pharm. Biopharm. 2015, 96, 143–151. [Google Scholar] [CrossRef] [Green Version]
- Kaialy, W.; Nokhodchi, A. Freeze-Dried Mannitol for Superior Pulmonary Drug Delivery via Dry Powder Inhaler. Pharm. Res. 2012, 30, 458–477. [Google Scholar] [CrossRef]
- Kaialy, W.; Alhalaweh, A.; Velaga, S.; Nokhodchi, A. Influence of lactose carrier particle size on the aerosol performance of budesonide from a dry powder inhaler. Powder Technol. 2012, 227, 74–85. [Google Scholar] [CrossRef]
- Lin, Y.-W.; Wong, J.; Qu, L.; Chan, H.-K.; Zhou, Q. Powder Production and Particle Engineering for Dry Powder Inhaler Formulations. Curr. Pharm. Des. 2015, 21, 3902–3916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, D.L.; Mitchell, J.P. Measurement of Aerodynamic Particle Size Distribution of Orally Inhaled Products by Cascade Impactor: How to Let the Product Specification Drive the Quality Requirements of the Cascade Impactor. AAPS PharmSciTech 2019, 20, 57. [Google Scholar] [CrossRef]
- El-Gendy, N.; Berkland, C. Combination chemotherapeutic dry powder aerosols via controlled nanoparticle agglomeration. Pharm. Res. 2009, 26, 1752–1763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosquillon, C.; Lombry, C.; Préat, V.; Vanbever, R. Influence of formulation excipients and physical characteristics of inhalation dry powders on their aerosolization performance. J. Control. Release 2001, 70, 329–339. [Google Scholar] [CrossRef]
- Courrier, H.M.; Butz, N.; Vandamme, T.F. Pulmonary drug delivery systems: Recent developments and prospects. Crit. Rev. Ther. Drug Carr. Syst. 2002, 19, 425–498. [Google Scholar] [CrossRef]
- Chew, N.Y. Effect of particle size, air flow and inhaler device on the aerosolisation of disodium cromoglycate powders. Int. J. Pharm. 2000, 206, 75–83. [Google Scholar] [CrossRef]
- Hassan, M.S.; Lau, R. Effect of Particle Shape on Dry Particle Inhalation: Study of Flowability, Aerosolization, and Deposition Properties. AAPS PharmSciTech 2009, 10, 1252–1262. [Google Scholar] [CrossRef] [Green Version]
- Kaialy, W.; Alhalaweh, A.; Velaga, S.P.; Nokhodchi, A. Effect of carrier particle shape on dry powder inhaler performance. Int. J. Pharm. 2011, 421, 12–23. [Google Scholar] [CrossRef]
- Wang, Y.; Hassan, M.S.; Gunawan, P.; Lau, R.; Wang, X.; Xu, R. Polyelectrolyte mediated formation of hydroxyapatite microspheres of controlled size and hierarchical structure. J. Colloid Interface Sci. 2009, 339, 69–77. [Google Scholar] [CrossRef]
- Hassan, M.S.; Lau, R. Pollen Shape Particles for Pulmonary Drug Delivery: In Vitro Study of Flow and Deposition Properties. In Proceedings of the World Congress on Medical Physics and Biomedical Engineering 2006, Seoul, Korea, 27 August–1 September 2009; Volume 23, pp. 1434–1437. [Google Scholar]
- Wang, C.; He, C.; Tong, Z.; Liu, X.; Ren, B.; Zeng, F. Combination of adsorption by porous CaCO3 microparticles and encapsulation by polyelectrolyte multilayer films for sustained drug delivery. Int. J. Pharm. 2006, 308, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Tang, H.; Cheng, B.; Zhao, X. Morphological control of calcium oxalate particles in the presence of poly-(styrene-alt-maleic acid). J. Solid State Chem. 2004, 177, 3368–3374. [Google Scholar] [CrossRef]
- Yu, J.; Lei, M.; Cheng, B.; Zhao, X. Facile preparation of calcium carbonate particles with unusual morphologies by precipitation reaction. J. Cryst. Growth 2004, 261, 566–570. [Google Scholar] [CrossRef]
- Zeng, X.M.; Martin, G.P.; Marriott, C.; Pritchard, J.N. The influence of carrier morphology on drug delivery by dry powder inhalers. Int. J. Pharm. 2000, 200, 93–106. [Google Scholar] [CrossRef]
- French, D.L.; Edwards, D.A.; Niven, R.W. The influence of formulation on emission, deaggregation and deposition of dry powders for inhalation. J. Aerosol Sci. 1996, 27, 769–783. [Google Scholar] [CrossRef]
- Maa, Y.; Nguyen, P.; Andya, J.D.; Dasovich, N.; Sweeney, T.D.; Shire, S.J.; Hsu, C.C. Effect of spray drying and subsequent processing conditions on residual moisture content and physical/biochemical stability of protein inhalation powders. Pharm. Res. 1998, 15, 768–775. [Google Scholar] [CrossRef]
- Van Campen, L.; Amidon, G.L.; Zografi, G. Moisture Sorption Kinetics for Water-Soluble Substances I: Theoretical Considerations of Heat Transport Control. J. Pharm. Sci. 1983, 72, 1381–1388. [Google Scholar] [CrossRef]
- Zhu, K.; Tan, R.B.; Chen, F.; Ong, K.H.; Heng, P.W.S. Influence of particle wall adhesion on particle electrification in mixers. Int. J. Pharm. 2007, 328, 22–34. [Google Scholar] [CrossRef]
- Zhou, Q.; Gengenbach, T.; Denman, J.A.; Yu, H.H.; Li, J.; Chan, H.-K. Synergistic Antibiotic Combination Powders of Colistin and Rifampicin Provide High Aerosolization Efficiency and Moisture Protection. AAPS J. 2013, 16, 37–47. [Google Scholar] [CrossRef] [Green Version]
- Emery, E.; Oliver, J.; Pugsley, T.; Sharma, J.; Zhou, J. Flowability of moist pharmaceutical powders. Powder Technol. 2009, 189, 409–415. [Google Scholar] [CrossRef]
- Kaialy, W. A review of factors affecting electrostatic charging of pharmaceuticals and adhesive mixtures for inhalation. Int. J. Pharm. 2016, 503, 262–276. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Castle, G.S.P.; Inculet, I.I.; Bailey, A.G. Bipolar charging of poly-disperse polymer powders in fluidized beds. IEEE Trans. Ind. Appl. 2003, 39, 612–618. [Google Scholar] [CrossRef]
- Shariare, M.; De Matas, M.; York, P. Effect of crystallisation conditions and feedstock morphology on the aerosolization performance of micronised salbutamol sulphate. Int. J. Pharm. 2011, 415, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Kaialy, W.; Hussain, T.; Alhalaweh, A.; Nokhodchi, A. Towards a More Desirable Dry Powder Inhaler Formulation: Large Spray-Dried Mannitol Microspheres Outperform Small Microspheres. Pharm. Res. 2014, 31, 60–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murtomaa, M.; Mellin, V.; Harjunen, P.; Lankinen, T.; Laine, E.; Lehto, V.-P. Effect of particle morphology on the triboelectrification in dry powder inhalers. Int. J. Pharm. 2004, 282, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, R.K.; Moosmüller, H.; Garro, M.A.; Arnott, W.P.; Slowik, J.G.; Cross, E.S.; Han, J.-H.; Davidovits, P.; Onasch, T.B.; Worsnop, D.R. Morphology based particle segregation by electrostatic charge. J. Aerosol Sci. 2008, 39, 785–792. [Google Scholar] [CrossRef]
- Vladykina, T.; Deryagin, B.; Toporov, Y.P. The effect of surface roughness on triboelectrification of insulators. Phys. Chem. Mech. Surf. 1985, 3, 2817–2821. [Google Scholar]
- Guo, S.; Jańczewski, D.; Zhu, X.; Quintana, R.; He, T.; Neoh, K.G. Surface charge control for zwitterionic polymer brushes: Tailoring surface properties to antifouling applications. J. Colloid Interface Sci. 2015, 452, 43–53. [Google Scholar] [CrossRef]
- Mehrani, P.; Bi, H.T.; Grace, J.R. Electrostatic behavior of different fines added to a Faraday cup fluidized bed. J. Electrost. 2007, 65, 1–10. [Google Scholar] [CrossRef]
- Matsusyama, T.; Yamamoto, H. Impact charging of particulate materials. Chem. Eng. Sci. 2006, 61, 2230–2238. [Google Scholar] [CrossRef]
- Melandri, C.; Tarroni, G.; Prodi, V.; De Zaiacomo, T.; Formignani, M.; Lombardi, C. Deposition of charged particles in the human airways. J. Aerosol Sci. 1983, 14, 657–669. [Google Scholar] [CrossRef]
- Hashish, A.; Bailey, A.; Williams, T. Selective Deposition of Pulsed Charged Aerosols in the Human Lung. J. Aerosol Med. 1994, 7, 167–171. [Google Scholar] [CrossRef]
- Saini, D.; Gunamgari, J.; Zulaloglu, C.; Sims, R.; Mazumder, M. Effect of electrostatic charge and size distributions on respirable aerosol deposition in lung model. In Proceedings of the Conference Record of the 2004 IEEE Industry Applications Conference, 39th IAS Annual Meeting, Seattle, WA, USA, 3–7 October 2004. [Google Scholar]
- Hickey, A.J. Pharmaceutical Inhalation Aerosol Technology. American Association of Pharmaceuticals Scientists, 2nd ed.; Marcel Dekker, Inc.: New York, NY, USA, 2004. [Google Scholar]
- Adjei, A.; Garren, J. Pulmonary Delivery of Peptide Drugs: Effect of Particle Size on Bioavailability of Leuprolide Acetate in Healthy Male Volunteers. Pharm. Res. 1990, 7, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Ibn Yakubu, S.; Assi, K.H.; Chrystyn, H. Aerodynamic dose emission characteristics of dry powder inhalers using an Andersen Cascade Impactor with a mixing inlet: The influence of flow and volume. Int. J. Pharm. 2013, 455, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Beach, S.; Latham, D.; Sidgwick, C.; Hanna, M.; York, P. Control of the Physical Form of Salmeterol Xinafoate. Org. Process. Res. Dev. 1999, 3, 370–376. [Google Scholar] [CrossRef]
- Shekunov, B.Y.; Feeley, J.C.; Chow, A.H.L.; Tong, H.H.Y.; York, P. Aerosolisation behaviour of micronised and supercritically-processed powders. J. Aerosol Sci. 2003, 34, 553–568. [Google Scholar] [CrossRef]
- Mosén, K.; Bäckström, K.; Thalberg, K.; Schaefer, T.; Kristensen, H.G.; Axelsson, P.A. Particle Formation and Capture During Spray Drying of Inhalable Particles. Pharm. Dev. Technol. 2004, 9, 409–417. [Google Scholar] [CrossRef]
- Zhou, Q.; Morton, D.A.; Yu, H.H.; Jacob, J.; Wang, J.; Li, J.; Chan, H.-K. Colistin Powders with High Aerosolisation Efficiency for Respiratory Infection: Preparation and In Vitro Evaluation. J. Pharm. Sci. 2013, 102, 3736–3747. [Google Scholar] [CrossRef]
- Hickey, A. Formulation Challenges of Powders for the Delivery of Small- Molecular-Weight Molecules as Aerosols. In Good Laboratory Practice Regulations, 3rd ed.; Revised and Expanded; Informa UK Limited: Colchester, UK, 2002; Volume 126, pp. 835–848. [Google Scholar]
- Mansour, H.M.; Rhee, Y.-S.; Wu, X. Nanomedicine in pulmonary delivery. Int. J. Nanomed. 2009, 4, 299–319. [Google Scholar] [CrossRef] [Green Version]
- Machiste, E. Characterization of carbamazepine in systems containing a dissolution rate enhancer. Int. J. Pharm. 1995, 126, 65–72. [Google Scholar] [CrossRef]
- Ho, R.; Dilworth, S.E.; Williams, D.R.; Heng, J.Y.Y. Role of Surface Chemistry and Energetics in High Shear Wet Granulation. Ind. Eng. Chem. Res. 2011, 50, 9642–9649. [Google Scholar] [CrossRef]
- Chow, A.H.L.; Tong, H.H.Y.; Chattopadhyay, P.; Shekunov, B.Y. Particle Engineering for Pulmonary Drug Delivery. Pharm. Res. 2007, 24, 411–437. [Google Scholar] [CrossRef] [PubMed]
- Saleem, I.Y.; Smyth, H.D.C. Micronization of a Soft Material: Air-Jet and Micro-Ball Milling. AAPS PharmSciTech 2010, 11, 1642–1649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mackin, L.; Sartnurak, S.; Thomas, I.; Moore, S. The impact of low levels of amorphous material (<5%) on the blending characteristics of a direct compression formulation. Int. J. Pharm. 2002, 231, 21–226. [Google Scholar]
- Steckel, H. In vitro characterization of jet-milled and in-situ-micronized fluticasone-17-propionate. Int. J. Pharm. 2003, 258, 65–75. [Google Scholar] [CrossRef]
- I Ré, M. Microencapsulation by spray drying. Dry. Technol. 1998, 16, 1195–1236. [Google Scholar] [CrossRef]
- Rosenberg, M.; Kopelman, I.J.; Talmon, Y. Factors affecting retention in spray-drying microencapsulation of volatile materials. J. Agric. Food Chem. 1990, 38, 1288–1294. [Google Scholar] [CrossRef]
- Gradon, L.; Sosnowski, T.R. Formation of particles for dry powder inhalers. Adv. Powder Technol. 2014, 25, 43–55. [Google Scholar] [CrossRef]
- Li, X.; Vogt, F.G.; Hayes, D.; Mansour, H.M. Design, Characterization, and Aerosol Dispersion Performance Modeling of Advanced Spray-Dried Microparticulate/Nanoparticulate Mannitol Powders for Targeted Pulmonary Delivery as Dry Powder Inhalers. J. Aerosol Med. Pulm. Drug Deliv. 2014, 27, 81–93. [Google Scholar] [CrossRef]
- Chew, N.Y.K.; Chan, H. Use of Solid Corrugated Particles to Enhance Powder Aerosol Performance. Pharm. Res. 2001, 18, 1570–1577. [Google Scholar] [CrossRef]
- Paudel, A.; Mooter, G.V.D. Influence of Solvent Composition on the Miscibility and Physical Stability of Naproxen/PVP K 25 Solid Dispersions Prepared by Cosolvent Spray-Drying. Pharm. Res. 2011, 29, 251–270. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.P. Spray drying technology: An overview. Indian J. Sci. Technol. 2009, 2, 44–47. [Google Scholar] [CrossRef]
- Patel, B.B.; Patel, J.K.; Chakraborty, S.; Shukla, D. Revealing facts behind spray dried solid dispersion technology used for solubility enhancement. Saudi Pharm. J. 2015, 23, 352–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Obaidi, H.; Ke, P.; Brocchini, S.; Buckton, G. Characterization and stability of ternary solid dispersions with PVP and PHPMA. Int. J. Pharm. 2011, 419, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Harjunen, P.; Lehto, V.-P.; Välisaari, J.; Lankinen, T.; Paronen, P.; Järvinen, K. Effects of Ethanol to Water Ratio in Feed Solution on the Crystallinity of Spray-Dried Lactose. Drug Dev. Ind. Pharm. 2002, 28, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Roncarati, R.; Cortesi, R.; Cervellati, F.; Nastruzzi, C. Production of Eudragit Microparticles by Spray-Drying Technique: Influence of Experimental Parameters on Morphological and Dimensional Characteristics. Pharm. Dev. Technol. 2000, 5, 267–278. [Google Scholar] [CrossRef]
- Rattes, A.L.R.; Oliveira, W. Spray drying conditions and encapsulating composition effects on formation and properties of sodium diclofenac microparticles. Powder Technol. 2007, 171, 7–14. [Google Scholar] [CrossRef]
- Maas, S.G.; Schaldach, G.; Littringer, E.M.; Mescher, A.; Griesser, U.J.; Braun, D.E.; Walzel, P.E.; Urbanetz, N.A. The impact of spray drying outlet temperature on the particle morphology of mannitol. Powder Technol. 2011, 213, 27–35. [Google Scholar] [CrossRef]
- Coppi, G.; Iannuccelli, V.; Bernabei, M.; Cameroni, R. Alginate microparticles for enzyme peroral administration. Int. J. Pharm. 2002, 242, 263–266. [Google Scholar] [CrossRef]
- Broadhead, J.; Rouan, S.K.E.; Hau, I.; Rhodes, C.T. The Effect of Process and Formulation Variables on the Properties of Spray-dried β-Galactosidase. J. Pharm. Pharmacol. 1994, 46, 458–467. [Google Scholar] [CrossRef]
- Mizoe, T.; Beppu, S.; Ozeki, T.; Okada, H. One-step preparation of drug-containing microparticles to enhance the dissolution and absorption of poorly water-soluble drugs using a 4-fluid nozzle spray drier. J. Control. Release 2007, 120, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Cheow, W.S.; Ng, M.L.L.; Kho, K.; Hadinoto, K. Spray-freeze-drying production of thermally sensitive polymeric nanoparticle aggregates for inhaled drug delivery: Effect of freeze-drying adjuvants. Int. J. Pharm. 2011, 404, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Maa, Y.-F.; Prestrelski, S.J. Biopharmaceutical Powders Particle Formation and Formulation Considerations. Curr. Pharm. Biotechnol. 2000, 1, 283–302. [Google Scholar] [CrossRef] [PubMed]
- Leuenberger, H. Spray Freeze-drying–The Process of Choice for Low Water Soluble Drugs? J. Nanoparticle Res. 2002, 4, 111–119. [Google Scholar] [CrossRef]
- Rogers, T.L.; Nelsen, A.C.; Sarkari, M.; Young, T.J.; Johnston, K.P.; Williams, R.O. Enhanced Aqueous Dissolution of a Poorly Water Soluble Drug by Novel Particle Engineering Technology: Spray-Freezing into Liquid with Atmospheric Freeze-Drying. Pharm. Res. 2003, 20, 485–493. [Google Scholar] [CrossRef]
- Costantino, H.R.; Firouzabadian, L.; Hogeland, K.; Wu, C.; Beganski, C.; Carrasquillo, K.G.; Córdova, M.; Griebenow, K.; Zale, S.E.; Tracy, M.A. Protein spray-freeze drying. Effect of atomization conditions on particle size and stability. Pharm. Res. 2000, 17, 1374–1382. [Google Scholar] [CrossRef]
- D’Addio, S.M.; Chan, J.G.Y.; Kwok, P.; Prud’Homme, R.K.; Chan, H.-K. Constant size, variable density aerosol particles by ultrasonic spray freeze drying. Int. J. Pharm. 2012, 427, 185–191. [Google Scholar] [CrossRef]
- Maa, Y.; Nguyen, P.; Sweeney, T.; Shire, S.J.; Hsu, C.C. Protein Inhalation Powders: Spray Drying vs Spray Freeze Drying. Pharm. Res. 1999, 16, 249–254. [Google Scholar] [CrossRef]
- Maa, Y.-F.; Nguyen, P.-A. Method of Spray Freeze Drying Proteins for Pharmaceutical Administration. U.S. Patent 6284282B1, 4 September 2001. [Google Scholar]
- Wanning, S.; Süverkrüp, R.; Lamprecht, A. Pharmaceutical spray freeze drying. Int. J. Pharm. 2015, 488, 136–153. [Google Scholar] [CrossRef]
- Costantino, H.R.; Jaworowicz, W.E.; Tracy, M.A.; Beganski, C.P. Method of Producing Sub-Micron Particles of Biologically Active Agents and Uses Thereof. U.S. Patent 6,284,283, 2002. [Google Scholar]
- Kuo, J.-H.S.; Hwang, R. Preparation of DNA dry powder for non-viral gene delivery by spray-freeze drying: Effect of protective agents (polyethyleneimine and sugars) on the stability of DNA. J. Pharm. Pharmacol. 2004, 56, 27–33. [Google Scholar] [CrossRef]
- Shekunov, B.Y.; Chattopadhyay, B.; Gibson, A.; Lehmkuhl, C. Influence of spray-freezing parameters on particle size and morphology of insulin. In Proceedings of the Conference on Respiratory Drug Delivery, Boca Raton, FL, USA, 24–27 April 2006. [Google Scholar]
- Costantino, H.R.; Johnson, O.L.; Zale, S.E. Relationship between encapsulated drug particle size and initial release of recombinant human growth hormone from biodegradable microspheres. J. Pharm. Sci. 2004, 93, 2624–2634. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, X.C.; Herberger, J.D.; Burke, P.A. Protein Powders for Encapsulation: A Comparison of Spray-Freeze Drying and Spray Drying of Darbepoetin Alfa. Pharm. Res. 2004, 21, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chua, K.M.; Wang, C.-H. Stabilization and encapsulation of human immunoglobulin G into biodegradable microspheres. J. Colloid Interface Sci. 2004, 271, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Sonner, C.; Maa, Y.; Lee, G. Spray-freeze-drying for protein powder preparation: Particle characterization and a case study with trypsinogen stability. J. Pharm. Sci. 2002, 91, 2122–2139. [Google Scholar] [CrossRef]
- Lam, X.M.; Duenas, E.T.; Cleland, J.L. Encapsulation and stabilization of nerve growth factor into poly(lactic-co-glycolic) acid microspheres. J. Pharm. Sci. 2001, 90, 1356–1365. [Google Scholar] [CrossRef] [PubMed]
- Shekunov, B.; Chattopadhyay, B.; Seitzinger, J. Production of respirable particles using spray-freeze-drying with compressed CO2. In Proceedings of the Conference on Respiratory Drug Delivery, Palm Springs, CA, USA, 7–10 May 2004. [Google Scholar]
- Shekunov, B.Y.; Chattopadhyay, P.; Seitzinger, J.S. Lyophilization Method and Apparatus for Producing Particles. U.S. Patent 6,931,888, 23 August 2005. [Google Scholar]
- Costantino, H.R.; Firouzabadian, L.; Wu, C.; Carrasquillo, K.G.; Griebenow, K.; Zale, S.E.; Tracy, M.A. Protein spray freeze drying. 2. Effect of formulation variables on particle size and stability. J. Pharm. Sci. 2002, 91, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Ogienko, A.A.; Bogdanova, E.; Trofimov, N.; Myz, S.; Kolesov, B.; Yunoshev, A.; Zubikov, N.; Manakov, A.; Boldyrev, V.; Boldyreva, E.V. Large porous particles for respiratory drug delivery. Glycine-based formulations. Eur. J. Pharm. Sci. 2017, 110, 148–156. [Google Scholar] [CrossRef]
- Esfandiari, N. Production of micro and nano particles of pharmaceutical by supercritical carbon dioxide. J. Supercrit. Fluids 2015, 100, 129–141. [Google Scholar] [CrossRef]
- Grenha, A.; Seijo, B.; Remuñán-López, C. Microencapsulated chitosan nanoparticles for lung protein delivery. Eur. J. Pharm. Sci. 2005, 25, 427–437. [Google Scholar] [CrossRef]
- Telko, M.J.; Hickey, A.J. Dry powder inhaler formulation. Respir. Care 2005, 50, 1209–1227. [Google Scholar]
- Velaga, S.; Berger, R.; Carlfors, J. Supercritical fluids crystallization of budesonide and flunisolide. Pharm. Res. 2002, 19, 1564–1571. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, M.; Ranjbarian, S. Production of micro- and nano-composite particles by supercritical carbon dioxide. J. Supercrit. Fluids 2007, 40, 263–283. [Google Scholar] [CrossRef]
- Shariati, A.; Peters, C.J. Recent developments in particle design using supercritical fluids. Curr. Opin. Solid State Mater. Sci. 2003, 7, 371–383. [Google Scholar] [CrossRef]
- Rehman, M.; Shekunov, B.Y.; York, P.; Lechuga-Ballesteros, D.; Miller, D.P.; Tan, T.; Colthorpe, P. Optimisation of powders for pulmonary delivery using supercritical fluid technology. Eur. J. Pharm. Sci. 2004, 22, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Steckel, H.; Müller, B.W. Metered-dose inhaler formulation of fluticasone-17-propionate micronized with supercritical carbon dioxide using the alternative propellant HFA-227. Int. J. Pharm. 1998, 173, 25–33. [Google Scholar] [CrossRef]
- Tong, H.H.Y.; Shekunov, B.Y.; York, P.; Chow, A.H.L. Characterization of Two Polymorphs of Salmeterol Xinafoate Crystallized From Supercritical Fluids. Pharm. Res. 2001, 18, 852–858. [Google Scholar] [CrossRef]
- Cabral, R.; Sousa, A.; Silva, A.; Paninho, A.; Temtem, M.; Costa, E.; Casimiro, T.; Aguiar-Ricardo, A. Design of experiments approach on the preparation of dry inhaler chitosan composite formulations by supercritical CO2-assisted spray-drying. J. Supercrit. Fluids 2016, 116, 26–35. [Google Scholar] [CrossRef]
- Thiering, R.; Dehghani, F.; Dillow, A.; Foster, N.R. Solvent effects on the controlled dense gas precipitation of model proteins. J. Chem. Technol. Biotechnol. 2000, 75, 42–53. [Google Scholar] [CrossRef]
- Nesta, D.P.; JElliott, S.; Warr, J.P. Supercritical fluid precipitation of recombinant human immunoglobulin from aqueous solutions. Biotechnol. Bioeng. 2000, 67, 457–464. [Google Scholar] [CrossRef]
- Velaga, S.; Carlfors, J. Supercritical Fluids Processing of Recombinant Human Growth Hormone. Drug Dev. Ind. Pharm. 2005, 31, 135–149. [Google Scholar] [CrossRef]
- Tservistas, M.; Levy, M.; Lo-Yim, M.; O’Kennedy, R.; York, P.; Humphrey, G.; Hoare, M. The formation of plasmid DNA loaded pharmaceutical powders using supercritical fluid technology. Biotechnol. Bioeng. 2000, 72, 12–18. [Google Scholar] [CrossRef]
- Okamoto, H.; Nishida, S.; Todo, H.; Sakakura, Y.; Iida, K.; Danjo, K. Pulmonary Gene Delivery by Chitosan–pDNA Complex Powder Prepared by a Supercritical Carbon Dioxide Process. J. Pharm. Sci. 2003, 92, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Sievers, R.E.; Cape, S.P.; Kisich, K.O.; Bennett, D.J.; Braun, C.S.; Burger, J.L.; Searles, J.A.; Best, D.H.; McAdams, N.A.; Wolters, N.A.; et al. Challenges of developing a stable dry powder live viral vaccine. In Proceedings of the Respiratory Drug Delivery, Scottsdale, AZ, USA, 11–15 May 2008; Volume 1, pp. 281–290. [Google Scholar]
- Burger, J.L.; Cape, S.P.; Braun, C.S.; McAdams, D.H.; Best, J.A.; Bhagwat, P.; Pathak, P.; Rebits, L.G.; Sievers, R.E. Stabilizing Formulations for Inhalable Powders of Live-Attenuated Measles Virus Vaccine. J. Aerosol Med. Pulm. Drug Deliv. 2008, 21, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Sievers, R.E.; Bennett, D.J.; Cape, S.P.; Braun, C.S.; Best, J.A.; Morin, A.L.; Bhagwat, P.A.; Quinn, B.P.; Pathak, P.; Searles, J.A.; et al. Micronization of measles vaccine and siRNA by CAN-BD for aerosol delivery by air expansion of powders with a PuffHaler™. In Proceedings of the RDD Europe, Lisbon, Portugal, 29 January 2007; pp. 17–20. [Google Scholar]
- Garcia, A.; Mack, P.; Williams, S.; Fromen, C.; Shen, T.; Tully, J.; Pillai, J.; Kuehl, P.; Napier, M.; DeSimone, J.M.; et al. Microfabricated Engineered Particle Systems for Respiratory Drug Delivery and Other Pharmaceutical Applications. J. Drug Deliv. 2012, 2012, 1–10. [Google Scholar] [CrossRef]
- Scoutaris, N.; Ross, S.; Douroumis, D. Current Trends on Medical and Pharmaceutical Applications of Inkjet Printing Technology. Pharm. Res. 2016, 33, 1799–1816. [Google Scholar] [CrossRef]
- López-Iglesias, C.; Casielles, A.M.; Altay, A.; Bettini, R.; Alvarez-Lorenzo, C.; García-González, C.A. From the printer to the lungs: Inkjet-printed aerogel particles for pulmonary delivery. Chem. Eng. J. 2019, 357, 559–566. [Google Scholar] [CrossRef]
- Moon, C.; Watts, A.B.; Lu, X.; Su, Y.; Williams, R.O. Enhanced Aerosolization of High Potency Nanoaggregates of Voriconazole by Dry Powder Inhalation. Mol. Pharm. 2019, 16, 1799–1812. [Google Scholar] [CrossRef]
- Sahakijpijarn, S.; Moon, C.; Ma, X.; Su, Y.; Koleng, J.J.; Dolocan, A.; Williams, R.O. Using thin film freezing to minimize excipients in inhalable tacrolimus dry powder formulations. Int. J. Pharm. 2020, 586, 119490. [Google Scholar] [CrossRef]
- Lin, L.; Quanb, G.; Peng, T.; Huang, Z.; Singh, V.; Lu, M.; Wuab, C. Development of fine solid-crystal suspension with enhanced solubility, stability, and aerosolization performance for dry powder inhalation. Int. J. Pharm. 2017, 533, 84–92. [Google Scholar] [CrossRef]
- Gibbons, A.; McElvaney, N.G.; Cryan, S.-A. A Dry Powder Formulation of Liposome-Encapsulated Recombinant Secretory Leukocyte Protease Inhibitor (rSLPI) for Inhalation: Preparation and Characterisation. AAPS PharmSciTech 2010, 11, 1411–1421. [Google Scholar] [CrossRef] [Green Version]
- Lu, D.; Hickey, A.J. Liposomal dry powders as aerosols for pulmonary delivery of proteins. AAPS PharmSciTech 2005, 6, E641–E648. [Google Scholar] [CrossRef] [PubMed]
- Luinstra, M.; Grasmeijer, F.; Hagedoorn, P.; Moes, J.R.; Frijlink, H.W.; De Boer, A.H. A levodopa dry powder inhaler for the treatment of Parkinson’s disease patients in off periods. Eur. J. Pharm. Biopharm. 2015, 97, 22–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giry, K.; Pean, J.; Giraud, L.; Marsas, S.; Rolland, H.; Wüthrich, P. Drug/lactose co-micronization by jet milling to improve aerosolization properties of a powder for inhalation. Int. J. Pharm. 2006, 321, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Yazdi, A.K.; Smyth, H.D.C. Hollow crystalline straws of diclofenac for high-dose and carrier-free dry powder inhaler formulations. Int. J. Pharm. 2016, 502, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Tulbah, A.S.; Ong, H.X.; Morgan, L.; Colombo, P.; Young, P.M.; Traini, D. Dry powder formulation of simvastatin. Expert Opin. Drug Deliv. 2014, 12, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Duret, C.; Wauthoz, N.; Sebti, T.; Vanderbist, F.; Amighi, K. New inhalation-optimized itraconazole nanoparticle-based dry powders for the treatment of invasive pulmonary aspergillosis. Int. J. Nanomed. 2012, 7, 5475–5489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laaksonen, T.; Liu, P.; Rahikkala, A.; Peltonen, L.; Kauppinen, E.I.; Hirvonen, J.T.; Järvinen, K.; Raula, J. Intact Nanoparticulate Indomethacin in Fast-Dissolving Carrier Particles by Combined Wet Milling and Aerosol Flow Reactor Methods. Pharm. Res. 2011, 28, 2403–2411. [Google Scholar] [CrossRef]
- Endo, K.; Amikawa, S.; Matsumoto, A.; Sahashi, N.; Onoue, S. Erythritol-based dry powder of glucagon for pulmonary administration. Int. J. Pharm. 2005, 290, 63–71. [Google Scholar] [CrossRef]
- Onoue, S.; Yamamoto, K.; Kawabata, Y.; Hirose, M.; Mizumoto, T.; Yamada, S. Novel dry powder inhaler formulation of glucagon with addition of citric acid for enhanced pulmonary delivery. Int. J. Pharm. 2009, 382, 144–150. [Google Scholar] [CrossRef]
- Ling, J.; Mangal, S.; Park, H.; Wang, S.; Cavallaro, A.; Zhou, Q. Simultaneous Particle Size Reduction and Homogeneous Mixing to Produce Combinational Powder Formulations for Inhalation by the Single-Step Co-Jet Milling. J. Pharm. Sci. 2019, 108, 3146–3151. [Google Scholar] [CrossRef] [Green Version]
- Brodka-Pfeiffer, K.; Langguth, P.; Graβ, P.; Häusler, H. Influence of mechanical activation on the physical stability of salbutamol sulphate. Eur. J. Pharm. Biopharm. 2003, 56, 393–400. [Google Scholar] [CrossRef]
- Ourique, A.F.; Chaves, P.D.S.; Souto, G.D.; Pohlmann, A.R.; Guterres, S.S.; Beck, R.C.R. Redispersible liposomal-N-acetylcysteine powder for pulmonary administration: Development, in vitro characterization and antioxidant activity. Eur. J. Pharm. Sci. 2014, 65, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Chougule, M.; Padhi, B.; Misra, A. Development of Spray Dried Liposomal Dry Powder Inhaler of Dapsone. AAPS PharmSciTech 2008, 9, 47–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manca, M.L.; Valenti, D.; Sales, O.D.; Nacher, A.; Fadda, A.M.; Manconi, M. Fabrication of polyelectrolyte multilayered vesicles as inhalable dry powder for lung administration of rifampicin. Int. J. Pharm. 2014, 472, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.G.Y.; Duke, C.C.; Ong, H.X.; Chan, J.C.Y.; Tyne, A.S.; Chan, H.-K.; Britton, W.J.; Young, P.M.; Traini, D. A Novel Inhalable Form of Rifapentine. J. Pharm. Sci. 2014, 103, 1411–1421. [Google Scholar] [CrossRef]
- Rojanarat, W.; Changsan, N.; Tawithong, E.; Pinsuwan, S.; Chow, S.F.; Srichana, T. Isoniazid Proliposome Powders for Inhalation—Preparation, Characterization and Cell Culture Studies. Int. J. Mol. Sci. 2011, 12, 4414–4434. [Google Scholar] [CrossRef] [Green Version]
- Khatib, I.; Khanal, D.; Ruan, J.; Cipolla, D.; Dayton, F.; Blanchard, J.D.; Chan, H.-K. Ciprofloxacin nanocrystals liposomal powders for controlled drug release via inhalation. Int. J. Pharm. 2019, 566, 641–651. [Google Scholar] [CrossRef]
- Chougule, M.; Padhi, B.; Misra, A. Nano-liposomal dry powder inhaler of tacrolimus: Preparation, characterization, and pulmonary pharmacokinetics. Int. J. Nanomed. 2007, 2, 675–688. [Google Scholar]
- Zhu, X.; Kong, Y.; Liu, Q.; Lu, Y.; Xing, H.; Lu, X.; Yang, Y.; Xu, J.; Li, N.; Zhao, D.; et al. Inhalable dry powder prepared from folic acid-conjugated docetaxel liposomes alters pharmacodynamic and pharmacokinetic properties relevant to lung cancer chemotherapy. Pulm. Pharmacol. Ther. 2019, 55, 50–61. [Google Scholar] [CrossRef]
- Chougule, M.B.; Padhi, B.K.; Misra, A. Nano-Liposomal Dry Powder Inhaler of Amiloride Hydrochloride. J. Nanosci. Nanotechnol. 2006, 6, 3001–3009. [Google Scholar] [CrossRef]
- Hamed, A.; Osman, R.; Al-Jamal, K.T.; Holayel, S.M.; Geneidi, A.-S. Enhanced antitubercular activity, alveolar deposition and macrophages uptake of mannosylated stable nanoliposomes. J. Drug Deliv. Sci. Technol. 2019, 51, 513–523. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Zhang, H.; Lu, X.; Jiang, L.; Xi, X.; Liu, J.; Zhu, J. Development and evaluation of a dry powder formulation of liposome-encapsulated oseltamivir phosphate for inhalation. Drug Deliv. 2015, 22, 608–618. [Google Scholar] [CrossRef]
- Kulvanich, P.; Sinsuebpol, C.; Chatchawalsaisin, J. Preparation and in vivo absorption evaluation of spray dried powders containing salmon calcitonin loaded chitosan nanoparticles for pulmonary delivery. Drug Des. Dev. Ther. 2013, 7, 861–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Vogt, F.G.; Hayes, D.; Mansour, H.M. Physicochemical characterization and aerosol dispersion performance of organic solution advanced spray-dried microparticulate/nanoparticulate antibiotic dry powders of tobramycin and azithromycin for pulmonary inhalation aerosol delivery. Eur. J. Pharm. Sci. 2014, 52, 191–205. [Google Scholar] [CrossRef]
- Meenach, S.A.; Anderson, K.W.; Hilt, J.Z.; McGarry, R.C.; Mansour, H.M. High-Performing Dry Powder Inhalers of Paclitaxel DPPC/DPPG Lung Surfactant-Mimic Multifunctional Particles in Lung Cancer: Physicochemical Characterization, In Vitro Aerosol Dispersion, and Cellular Studies. AAPS PharmSciTech 2014, 15, 1574–1587. [Google Scholar] [CrossRef] [Green Version]
- Ungaro, F.; D’Angelo, I.; Coletta, C.; Bianca, R.D.D.V.; Sorrentino, R.; Perfetto, B.; Tufano, M.A.; Miro, A.; La Rotonda, M.I.; Quaglia, F. Dry powders based on PLGA nanoparticles for pulmonary delivery of antibiotics: Modulation of encapsulation efficiency, release rate and lung deposition pattern by hydrophilic polymers. J. Control. Release 2012, 157, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Geller, D.E.; Weers, J.; Heuerding, S. Development of an Inhaled Dry-Powder Formulation of Tobramycin Using PulmoSphere™ Technology. J. Aerosol Med. Pulm. Drug Deliv. 2011, 24, 175–182. [Google Scholar] [CrossRef]
- Cai, X.; Yang, Y.; Xie, X.; Yu, F.; Yang, Y.; Yang, Z.; Zhang, T.; Mei, X. Preparation, characterization and pulmonary pharmacokinetics of a new inhalable zanamivir dry powder. Drug Deliv. 2015, 23, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Bi, R.; Shao, W.; Wang, Q.; Zhang, N. Spray-freeze-dried dry powder inhalation of insulin-loaded liposomes for enhanced pulmonary delivery. J. Drug Target. 2008, 16, 639–648. [Google Scholar] [CrossRef]
- Tanaka, R.; Hattori, Y.; Otsuka, M.; Ashizawa, K. Application of spray freeze drying to theophylline-oxalic acid cocrystal engineering for inhaled dry powder technology. Drug Dev. Ind. Pharm. 2020, 46, 179–187. [Google Scholar] [CrossRef]
- Sweeney, L.G.; Wang, Z.; Loebenberg, R.; Wong, J.P.; Lange, C.F.; Finlay, W.H. Spray-freeze-dried liposomal ciprofloxacin powder for inhaled aerosol drug delivery. Int. J. Pharm. 2005, 305, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Kho, K.; Hadinoto, K. Optimizing aerosolization efficiency of dry-powder aggregates of thermally-sensitive polymeric nanoparticles produced by spray-freeze-drying. Powder Technol. 2011, 214, 169–176. [Google Scholar] [CrossRef]
- Wang, Y.; Kho, K.; Cheow, W.S.; Hadinoto, K. A comparison between spray drying and spray freeze drying for dry powder inhaler formulation of drug-loaded lipid–polymer hybrid nanoparticles. Int. J. Pharm. 2012, 424, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Chan, A.Y.; Chow, M.Y.; Lo, F.F.; Qiu, Y.; Kwok, P.C.; Lam, J.K.W. Spray freeze drying of small nucleic acids as inhaled powder for pulmonary delivery. Asian J. Pharm. Sci. 2018, 13, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.; Yip, L.; Chow, M.Y.; Chow, S.F.; Chan, H.-K.; Kwok, P.; Lam, J.K.W. Porous and highly dispersible voriconazole dry powders produced by spray freeze drying for pulmonary delivery with efficient lung deposition. Int. J. Pharm. 2019, 560, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Hou, A.; Li, L.; Huang, Y.; Singh, V.; Zhu, C.; Pan, X.; Quanb, G.; Wu, C. Fragmented particles containing octreotide acetate prepared by spray drying technique for dry powder inhalation. Drug Deliv. Transl. Res. 2018, 8, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Pouya, M.A.; Daneshmand, B.; Aghababaie, S.; Faghihi, H.; Vatanara, A. Spray-Freeze Drying: A Suitable Method for Aerosol Delivery of Antibodies in the Presence of Trehalose and Cyclodextrins. AAPS PharmSciTech 2018, 19, 2247–2254. [Google Scholar] [CrossRef]
- Okuda, T.; Suzuki, Y.; Kobayashi, Y.; Ishii, T.; Uchida, S.; Itaka, K.; Kataoka, K.; Okamoto, H. Development of Biodegradable Polycation-Based Inhalable Dry Gene Powders by Spray Freeze Drying. Pharmaceutics 2015, 7, 233–254. [Google Scholar] [CrossRef] [Green Version]
- Maa, Y.; Ameri, M.; Shu, C.; Payne, L.G.; Chen, D. Influenza Vaccine Powder Formulation Development: Spray-Freeze-Drying and Stability Evaluation. J. Pharm. Sci. 2004, 93, 1912–1923. [Google Scholar] [CrossRef] [PubMed]
- Amorij, J.-P.; Saluja, V.; Petersen, A.; Hinrichs, W.; Huckriede, A.; Frijlink, H.W. Pulmonary delivery of an inulin-stabilized influenza subunit vaccine prepared by spray-freeze drying induces systemic, mucosal humoral as well as cell-mediated immune responses in BALB/c mice. Vaccine 2007, 25, 8707–8717. [Google Scholar] [CrossRef] [PubMed]
- Van Drooge, D.-J.; Hinrichs, W.L.; Dickhoff, B.H.; Elli, M.; Visser, M.R.; Zijlstra, G.S.; Frijlink, H.W. Spray freeze drying to produce a stable Δ9-tetrahydrocannabinol containing inulin-based solid dispersion powder suitable for inhalation. Eur. J. Pharm. Sci. 2005, 26, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Sioutas, C.; Fine, P.; Shing, K.S. Effect of albumin on physical characteristics of drug particles produced by supercritical fluid technology. Powder Technol. 2008, 182, 354–363. [Google Scholar] [CrossRef]
- Chunhachaichana, C.; Sritharadol, R.; Sawatdee, S.; Heng, P.W.S.; Srichana, T. Development of nanodispersion-based sildenafil metered-dose inhalers stabilized by poloxamer 188: A potential candidate for the treatment of pulmonary arterial hypertension. Pharm. Dev. Technol. 2019, 24, 1218–1228. [Google Scholar] [CrossRef] [PubMed]
- Reverchon, E.; Della Porta, G.; Pallado, P. Supercritical antisolvent precipitation of salbutamol microparticles. Powder Technol. 2001, 114, 17–22. [Google Scholar] [CrossRef]
- Schiavone, H.; Palakodaty, S.; Clark, A.; York, P.; Tzannis, S.T. Evaluation of SCF-engineered particle-based lactose blends in passive dry powder inhalers. Int. J. Pharm. 2004, 281, 55–66. [Google Scholar] [CrossRef]
- Bakhbakhi, Y.; Charpentier, P.A.; Rohani, S. Experimental study of the GAS process for producing microparticles of beclomethasone-17,21-dipropionate suitable for pulmonary delivery. Int. J. Pharm. 2006, 309, 71–80. [Google Scholar] [CrossRef]
- Kunastitchai, S.; Pichert, L.; Sarisuta, N.; Müller, B.W. Application of aerosol solvent extraction system (ASES) process for preparation of liposomes in a dry and reconstitutable form. Int. J. Pharm. 2006, 316, 93–101. [Google Scholar] [CrossRef]
- Cho, W.; Kim, M.-S.; Jung, M.-S.; Park, J.; Cha, K.-H.; Park, J.; Park, H.J.; Alhalaweh, A.; Velaga, S.; Hwang, S.-J. Design of salmon calcitonin particles for nasal delivery using spray-drying and novel supercritical fluid-assisted spray-drying processes. Int. J. Pharm. 2015, 478, 288–296. [Google Scholar] [CrossRef]
- Patomchaiviwat, V.; Paeratakul, O.; Kulvanich, P. Formation of Inhalable Rifampicin–Poly(l-lactide) Microparticles by Supercritical Anti-solvent Process. AAPS PharmSciTech 2008, 9, 1119–1129. [Google Scholar] [CrossRef] [Green Version]
- Reverchon, E.; De Marco, I.; Caputo, G.; Della Porta, G. Pilot scale micronization of amoxicillin by supercritical antisolvent precipitation. J. Supercrit. Fluids 2003, 26, 1–7. [Google Scholar] [CrossRef]
- Van Hees, T.; Piel, G.; Evrard, B.; Otte, X.; Thunus, L.; Delattre, L. Application of Supercritical Carbon Dioxide for the Preparation of a Piroxicam-β-Cyclodextrin Inclusion Compound. Pharm. Res. 1999, 16, 1864–1870. [Google Scholar] [CrossRef] [PubMed]
- Snavely, W.K.; Subramaniam, B.; Rajewski, R.A.; DeFelippis, M.R. Micronization of insulin from halogenated alcohol solution using supercritical carbon dioxide as an antisolvent. J. Pharm. Sci. 2002, 91, 2026–2039. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, H.; Sakakura, Y.; Shiraki, K.; Oka, K.; Nishida, S.; Todo, H.; Iida, K.; Danjo, K. Stability of chitosan–pDNA complex powder prepared by supercritical carbon dioxide process. Int. J. Pharm. 2005, 290, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Mayo, A.S.; Ambati, B.K.; Kompella, U.B. Gene delivery nanoparticles fabricated by supercritical fluid extraction of emulsions. Int. J. Pharm. 2010, 387, 278–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okuda, T.; Kito, D.; Oiwa, A.; Fukushima, M.; Hira, D.; Okamoto, H. Gene Silencing in a Mouse Lung Metastasis Model by an Inhalable Dry Small Interfering RNA Powder Prepared Using the Supercritical Carbon Dioxide Technique. Biol. Pharm. Bull. 2013, 36, 1183–1191. [Google Scholar] [CrossRef] [Green Version]
- Kalantarian, P.; Najafabadi, A.R.; Haririan, I.; Vatanara, A.; Yamini, Y.; Darabi, M.; Gilani, K. Preparation of 5-fluorouracil nanoparticles by supercritical antisolvents for pulmonary delivery. Int. J. Nanomed. 2010, 5, 763–770. [Google Scholar] [CrossRef]
- Kurniawansyah, F.; Duong, H.T.T.; Luu, T.D.; Mammucari, R.; Vittorio, O.; Boyer, C.; Foster, N.R. Inhalable curcumin formulations: Micronization and bioassay. Chem. Eng. J. 2015, 279, 799–808. [Google Scholar] [CrossRef]
- Adami, R.; Reverchon, E.; Järvenpää, E.; Huopalahti, R. Supercritical AntiSolvent micronization of nalmefene HCl on laboratory and pilot scale. Powder Technol. 2008, 182, 105–112. [Google Scholar] [CrossRef]
- Tandya, A.; Dehghani, F.; Foster, N.R. Micronization of cyclosporine using dense gas techniques. J. Supercrit. Fluids 2006, 37, 272–278. [Google Scholar] [CrossRef]
- Laube, B.L.; Janssens, H.M.; De Jongh, F.H.C.; Devadason, S.G.; Dhand, R.; Diot, P.; Everard, M.L.; Horvath, I.; Navalesi, P.; Voshaar, T.; et al. What the pulmonary specialist should know about the new inhalation therapies. Eur. Respir. J. 2011, 37, 1308–1417. [Google Scholar] [CrossRef] [Green Version]
- Janson, C.; Lööf, T.; Telg, G.; Stratelis, G. Impact of Inhalation Flow, Inhalation Volume and Critical Handling Errors on Delivered Budesonide/Formoterol Dose in Different Inhalers: An In Vitro Study. Pulm. Ther. 2017, 3, 243–253. [Google Scholar] [CrossRef]
- Novartis Pharmaceuticals UK Ltd. Ultibro Breezhaler. 2019. Available online: https://www.medicines.org.uk/emc/medicine/29533#gref (accessed on 25 October 2020).
- Boehringer Ingelheim Limited. Spiriva 18 Microgram Inhalation Powder, Hard Capsule. 2019. Available online: https://www.medicines.org.uk/emc/product/1693/smpc#gref (accessed on 25 October 2020).
- Seheult, J.N.; Costello, S.; Tee, K.C.; Bholah, T.; Al Bannai, H.; Sulaiman, I.; Costello, R.W. Investigating the relationship between peak inspiratory flow rate and volume of inhalation from a Diskus™ Inhaler and baseline spirometric parameters: A cross-sectional study. SpringerPlus 2014, 3, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Lavorini, F. Inhaled Drug Delivery in the Hands of the Patient. J. Aerosol Med. Pulm. Drug Deliv. 2014, 27, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Laba, T.-L.; Jan, S.; Zwar, N.A.; Roughead, E.; Marks, G.B.; Flynn, A.W.; Goldman, M.D.; Heaney, A.; Lembke, K.A.; Reddel, H.K. Cost-Related Underuse of Medicines for Asthma—Opportunities for Improving Adherence. J. Allergy Clin. Immunol. Pract. 2019, 7, 2298–2306.e12. [Google Scholar] [CrossRef] [PubMed]
- Melani, A.S.; Bonavia, M.; Cilenti, V.; Cinti, C.; Lodi, M.; Martucci, P.; Serra, M.; Scichilone, N.; Sestini, P.; Aliani, M.; et al. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir. Med. 2011, 105, 930–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westerik, J.A.M.; Carter, V.; Chrystyn, H.; Burden, A.; Thompson, S.L.; Ryan, D.; Gruffydd-Jones, K.; Haughney, J.; Roche, N.; Lavorini, F.; et al. Characteristics of patients making serious inhaler errors with a dry powder inhaler and association with asthma-related events in a primary care setting. J. Asthma 2016, 53, 321–329. [Google Scholar] [CrossRef]
- Wauthoz, N.; Rosière, R.; Amighi, K. Inhaled cytotoxic chemotherapy: Clinical challenges, recent developments, and future prospects. Expert Opin. Drug Deliv. 2020, 1–22. [Google Scholar] [CrossRef]
- Rogueda, P.; Traini, D. The future of inhalers: How can we improve drug delivery in asthma and COPD? Expert Rev. Respir. Med. 2016, 10, 1041–1044. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.; Tang, P.; Leung, S.S.Y.; Chan, J.G.Y.; Chan, H.-K. Emerging inhalation aerosol devices and strategies: Where are we headed? Adv. Drug Deliv. Rev. 2014, 75, 3–17. [Google Scholar] [CrossRef]
- Williams, R.; Rankin, N.; Smith, T.; Galler, D.; Seakins, P. Relationship between the humidity and temperature of inspired gas and the function of the airway mucosa. Crit. Care Med. 1996, 24, 1920–1929. [Google Scholar] [CrossRef]
- Stanke, F. The contribution of the airway epithelial cell to host defense. Mediat. Inflamm. 2015, 2015, 463016. [Google Scholar] [CrossRef] [PubMed]

| Techniques | Control Parameters | Advantages | Disadvantages |
|---|---|---|---|
| Milling |
|
|
|
| Spray-drying |
|
|
|
| Spray-freeze-drying |
|
|
|
| Supercritical fluid drying |
|
|
|
| Drug/Payload | Additives | Median Size | Ref. |
|---|---|---|---|
| A. Milling | |||
| 1. Recombinant secretory leukocyte protease inhibitor | 1,2-Dioleoyl-sn-glycero 3[phosphor-Lserine], Cholesterol | 2.44 µm | [156] |
| 2. β-Glucuronidase | Dimyristoylphosphatyl-Choline, Cholesterol | 6.4 µm | [157] |
| 3. Beclomethasone dipropionate | Not mentioned | ~5 µm | [94] |
| 4. Levodopa | L-Leucine | <5 µm | [158] |
| 5. Fluticasone-17-propionate | HPMC | ~2 µm | [94] |
| 6. Fusafungine | Lactose | ~5 µm | [159] |
| 7. Diclofenac | Not mentioned | 2.36 µm | [160] |
| 8. Simvastatin | Not mentioned | 2.2 µm | [161] |
| 9. Itraconazole | Mannitol and Sodium taurocholate | 5.91 µm | [162] |
| 10. Indomethacin | Mannitol and L-leucine | 0.96 µm | [163] |
| 11. Glucagon | Pharmatose, Erythritol | ~2.5 µm | [164] |
| 12. Glucagon | Citric acid, Lactose | 4.7–52.1 µm | [165] |
| 13. Ciprofloxacin HCl and Colistin sulfate | Not mentioned | <5.4 µm | [166] |
| 14. Salbutamol sulphate | Not mentioned | ~10 µm | [167] |
| B. Spray drying | |||
| 1. N-acetylcysteine | Soya phosphatidylcholine, Cholesterol, Polysorbate 80 | 2.72 µm | [168] |
| 2. Dapsone | Dipalmitoylphosphatidylcholine, Cholesterol, Polysorbate 80 | 2.2 µm | [169] |
| 3. Rifampicin | Soya phosphatidylcholine, Cholesterol, Hydrogenated soybean phosphatidylcholine | ~2 µm | [170] |
| 4. Rifapentine | Not mentioned | 1.92 µm | [171] |
| 5. Isoniazide | L-α-soybean phosphatidylcholine, Cholesterol, Mannitol | 4.92 µm | [172] |
| 6. Ciprofloxacine | Hydrogenated soybean phosphatidylcholine, Cholestrol, Sucrose | ~1 µm | [173] |
| 7. Tacrolimus | Hydrogenated soybean phosphatidylcholine, Cholesterol, Trehalose | 2.2 µm | [174] |
| 8. Docetaxel | Phosphatidylcholine, Cholesterol, Mannitol, Leucine | 3.1 µm | [175] |
| 9. Amiloride HCl | Hydrogenated soy phosphatidycholine, Cholesterol, Mannitol | 2.3 µm | [176] |
| 10. Moxifloxacin | Phosphatidylcholine, Cholesterol, Dextran | <5 µm | [177] |
| 11. Oseltamivir phosphate | Ovelecithin, Cholesterol, Leucine | ~3.5 µm | [178] |
| 12. Salmon calcitonin | Sodium tripolyphosphate, Chitosan, Mannitol | 4.7 µm | [179] |
| 13. Azethromycin | Not mentioned | 1.6 µm | [180] |
| 14. Paclitaxel | Dipalmitoylphosphatidylcholine, dipalmitoylphosphatidylglycerol | 2.3 µm | [181] |
| 15. Tobramycin | Poly(lactic-co-glycolic acid), Poly(vinyl alcohol) | 3.3 µm | [182] |
| 16. Tobramycin (PulmoSphere™) | Distearoylphosphatidlcholin, perflurooctyl bromide | ~5 µm | [183] |
| 17. Zanamivir (Relenaza®) | Mannitol, L-leucine, Poloxamer 188 | 2.3 µm | [184] |
| C. Spray-freeze drying | |||
| 1. Insulin | Soya lecithin, Cholesterol, Cholate, Mannitol | 3.9 µm | [185] |
| 2. Theophylline anhydrate and oxalic acid | Not mentioned | 3.0 µm | [186] |
| 3. Ciprofloxacin | Dimyristoylphosphatidylglycerol, lactose | 2.8 µm | [187] |
| 4. Levofloxacin | Polycaprolactone, L-leucine, Mannitol | ~4–5 µm | [188] |
| 5. Levofloxacin | Soybean lecithin, D-mannitol, L-leucine | 5.6 µm | [189] |
| 6. Small interfering RNA | Mannitol | 10–14.9 µm | [190] |
| 7. Voriconazole | Mannitol | 3.8 µm | [191] |
| 8. Octreotide acetate | Mannitol, ammonium carbonate | 2.6 µm | [192] |
| 9. Human IgG | Hydroxypropyl β-cyclodextrin, trehalose | ~5.32 µm | [193] |
| 10. Humanized anti-IgE monoclonal antibody | Carbohydrate excipients | ~3 µm | [117] |
| 11. PlasmidDNA-Luc | Β-benzyl-L-aspartate N-carboxy-anhydride | 7.6 µm | [194] |
| 12. Viral protein (hemagglutinin) | Dextran, Mannitol, Poloxamer 188, Polysorbate 20, Trehalose | 30–60 µm | [195] |
| 13. Monovalent influenza subunit hemagglutinin | Inulin | 11.05 µm | [196] |
| 14. Δ9-Tetrahydro-cannabinol | Inulin | 84.1 µm | [197] |
| D. Supercritical fluid drying | |||
| 1. Terbutaline sulphate | α-Lactose monohydrate | 2.85–3.43 µm | [138] |
| 2. Ipratropium bromide | Bovine serum albumin | 1–5 µm | [198] |
| 3. Fluticasone-17-propionte | Poloxamer 188 | ~1.69 µm | [199] |
| 4. Salmeterol xinafoate | Not mentioned | Not mentioned | [140] |
| 5. Salbutamol sulphate | N-methyl 2-pyrrolidone | 1–3 µm | [200] |
| 6. Albuterol sulfate | α-lactose monohydrate | 2.4 µm | [201] |
| 7. Beclomethasone-17,21-dipropionate | Not mentioned | 7.9 µm | [202] |
| 8. Miconazole | Phosphatidylcholine, Cholesterol, Poloxamer 407 | 3.6–9.4 µm | [203] |
| 9. Salmon calcitonin | Inulin, Trehalose, Chitosan, Sodium taurocholate, β-cyclodextrin | 2.2–2.9 µm | [204] |
| 10. Rifampicin | Poly(L-lactide) | <5 µm | [205] |
| 11. Amoxicillin trihydrate | Not mentioned | Not mentioned | [206] |
| 12. Piroxicam | β-Cyclodextrin | Not mentioned | [207] |
| 13. Ibuprofen | Chitosan | 2.1–2.7 µm | [141] |
| 14. Insulin | Not mentioned | 2–3 µm | [208] |
| 15. Plasmid pSVβ | Mannitol | Not mentioned | [145] |
| 16. Plasmid pCMV-Luc | Chitosan, trehalose | <10 µm | [209] |
| 17. Plasmid DNA | Poly(D,L-lactic-co-glycolic) acid | Not mentioned | [210] |
| 18. siRNA | Chitosan | <10 µm | [211] |
| 19. 5-fluorouracil | α-lactose monohydrate | Not mentioned | [212] |
| 20. Curcumin | Hydroxypropyl-β-cyclodextrin | ~5.8 µm | [213] |
| 21. Nalmefene hydrochloride | Not mentioned | 0.5–2 µm | [214] |
| 22. Cyclosporine A | Not mentioned | <2.5 µm | [215] |
| Drug | Additives | Product | Manufacturer | Indications |
|---|---|---|---|---|
| Albuterol sulfate | Lactose monohydrate | ProAir Respiclick | Teva | Asthma and COPD |
| Salbutamol sulfate | Lactose monohydrate | Pulvinal Salbutamol | Chiesi | Asthma and COPD |
| Salbutamol sulfate | Lactose monohydrate | Easyhaler Salbutamol Sulfate | Orion | Asthma and COPD |
| Terbutaline sulfate | N/A | BricanylTurbohaler | AstraZeneca | Asthma and COPD |
| Salmeterol xinafoate | Lactose monohydrate | Serevent Diskus | GlaxoSmithKline | Asthma and COPD |
| Formoterol fumarate | Lactose monohydrate | ForadilAerolizer | Novartis | Asthma and COPD |
| Formoterol fumarate | Lactose monohydrate, Magnesium stearate | ForadilCertihaler | Novartis | Asthma and COPD |
| Formoterol fumarate | Lactose monohydrate | OxisTurbohaler | AstraZeneca | Asthma and COPD |
| Formoterol fumarate | Lactose monohydrate | Easyhaler Formoterol | Orion | Asthma and COPD |
| Indacaterol maleate | Lactose monohydrate | ArcaptaNeohaler | Novartis | Asthma and COPD |
| Tritropium bromide | Lactose monohydrate | Spiriva Handihaler | Boehringer Ingelheim | Asthma and COPD |
| Aclidinium bromide | Lactose monohydrate | TudorzaPressair | Forest | Asthma and COPD |
| Glycopyrronium bromide | Lactose monohydrate, Magnesium stearate | SeebriBreezhaler | Novartis | Asthma and COPD |
| Umeclidinium | Lactose monohydrate, Magnesium stearate | Incruse Ellipta | GlaxoSmithKline | Asthma and COPD |
| Budesonide | Lactose monohydrate | Easyhaler Budesonide | Orion | Asthma and COPD |
| Budesonide | Lactose monohydrate | Pulmicort Flexhaler | AstraZeneca | Asthma and COPD |
| Mometasone furoate | Lactose anhydrate | Asmanex Twisthaler | Merck | Asthma and COPD |
| Beclomethasone dipropionate | Lactose monohydrate, Magnesium stearate | PulvinalBeclometasone dipropionate | Chiesi | Asthma and COPD |
| Beclomethasone dipropionate | Lactose monohydrate | EasyhalerBeclometasone | Orion | Asthma and COPD |
| Fluticasone propionate | Lactose monohydrate | Flovent Diskus | GlaxoSmithKline | Asthma and COPD |
| Fluticasone furoate | Lactose monohydrate | Arnuity Ellipta | GlaxoSmithKline | Asthma and COPD |
| Beclomethasone dipropionate + Formoterol fumarte | Lactose monohydrate, Magnesium stearate | FostairNexthaler | Chiesi | Asthma and COPD |
| Beclomethasone dipropionate + Formoterol fumarte | Lactose monohydrate | Symbicort Turbohaler | AstraZeneca | Asthma and COPD |
| Beclomethasone dipropionate + Formoterol fumarte | Lactose monohydrate | DuoRespSpiromax | Teva | Asthma and COPD |
| Fluticasone furoate + Vilanterol | Lactose monohydrate, Magnesium stearate | Breo Ellipta | GlaxoSMithKline | Asthma and COPD |
| Fluticasone furoate + Salmeterol | Lactose monohydrate | Advair Diskus | GlaxoSMithKline | Asthma and COPD |
| Umeclidinium + Vilanterol | Lactose monohydrate, Magnesium stearate | Anoro Ellipta | GlaxoSMithKline | Asthma and COPD |
| Tobramycin | 1,2-distearoyl-sn-glycero-3-phosphocholine, Calcium chloride | TOBI Podhaler | Novartis | Cystic Fibrosis infection |
| Zanamivir | Lactose | RelenazaDiskhaler | GlaxoSmithKline | Influenza |
| Insulin Human | Fumaryl diketopiperazine, Polysorbate 80 | Afrezza | Sanofi Aventis | Diabetes |
| Loxapine | N/A | Adasuve | Teva | Schizopherina/Bipolar disorder |
| Ciprofloxacin | 1,2-distearoyl-sn-glycero-3-phosphocholine, Calcium chloride | Ciprofloxacin PulmoSphere | Novartis | Cystic fibrosis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaurasiya, B.; Zhao, Y.-Y. Dry Powder for Pulmonary Delivery: A Comprehensive Review. Pharmaceutics 2021, 13, 31. https://doi.org/10.3390/pharmaceutics13010031
Chaurasiya B, Zhao Y-Y. Dry Powder for Pulmonary Delivery: A Comprehensive Review. Pharmaceutics. 2021; 13(1):31. https://doi.org/10.3390/pharmaceutics13010031
Chicago/Turabian StyleChaurasiya, Birendra, and You-Yang Zhao. 2021. "Dry Powder for Pulmonary Delivery: A Comprehensive Review" Pharmaceutics 13, no. 1: 31. https://doi.org/10.3390/pharmaceutics13010031
APA StyleChaurasiya, B., & Zhao, Y.-Y. (2021). Dry Powder for Pulmonary Delivery: A Comprehensive Review. Pharmaceutics, 13(1), 31. https://doi.org/10.3390/pharmaceutics13010031