Photoprotective Activity of Buddleja cordata Cell Culture Methanolic Extract on UVB-irradiated 3T3-Swiss Albino Fibroblasts
Abstract
:1. Introduction
2. Results
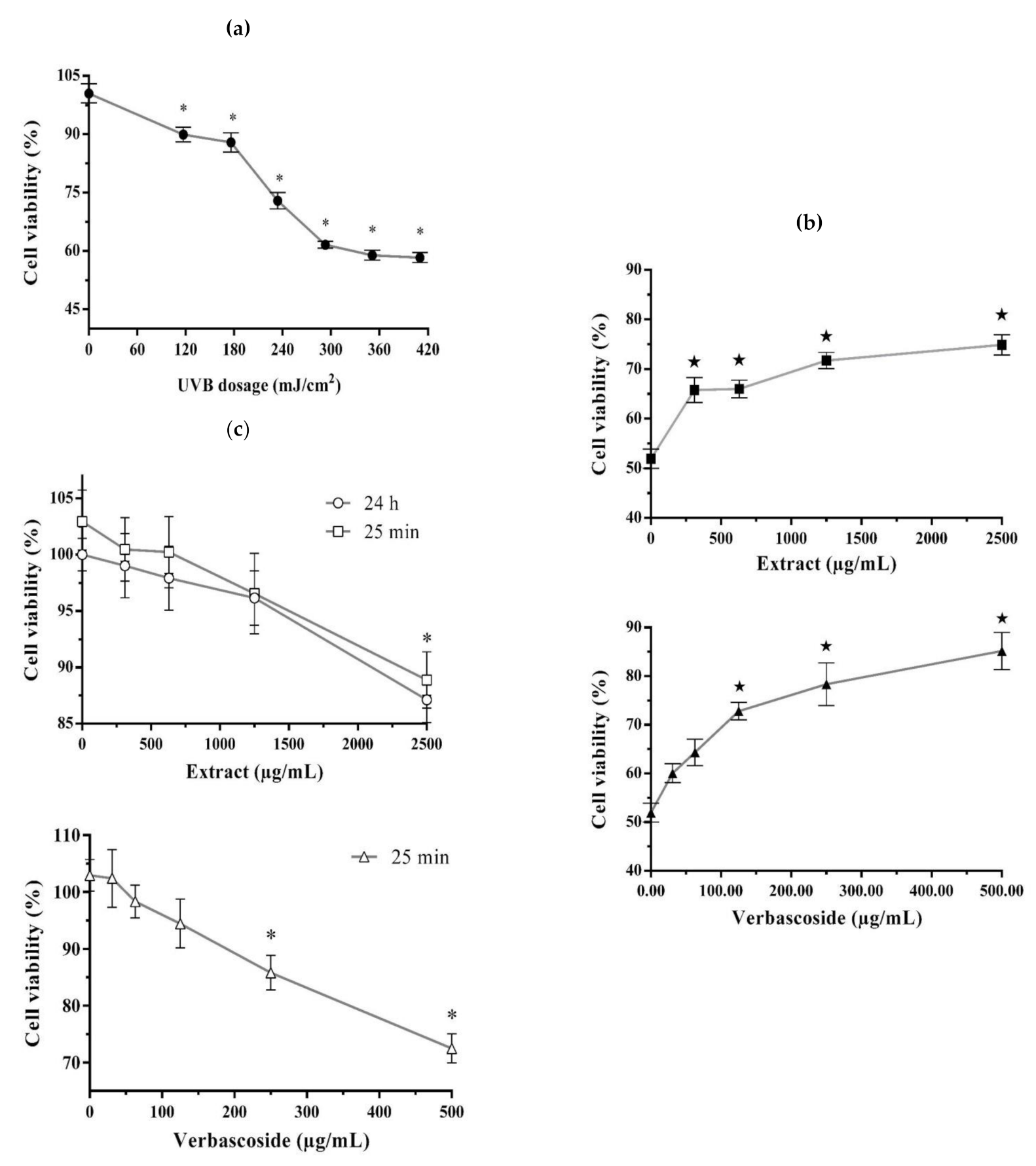
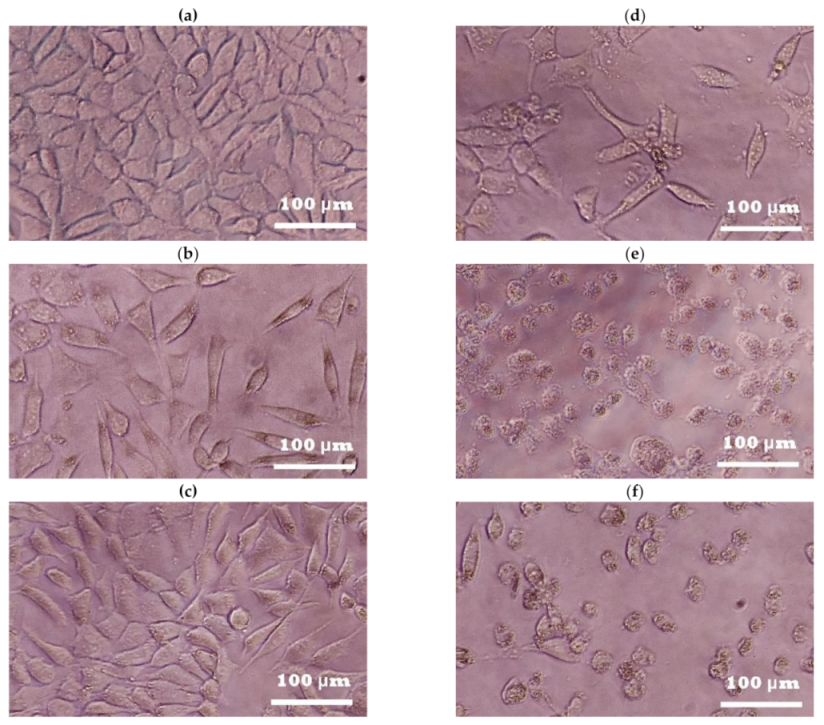
2.1. Photoprotective and Cytotoxic Effects of the B. cordata Cell Culture Methanolic Extract on 3T3-Swiss albino Fibroblasts
2.2. The B. cordata Cell Culture Methanolic Extract Absorbs UV Light, Has a High Total Phenol Content, and Contains Bioactive SMs
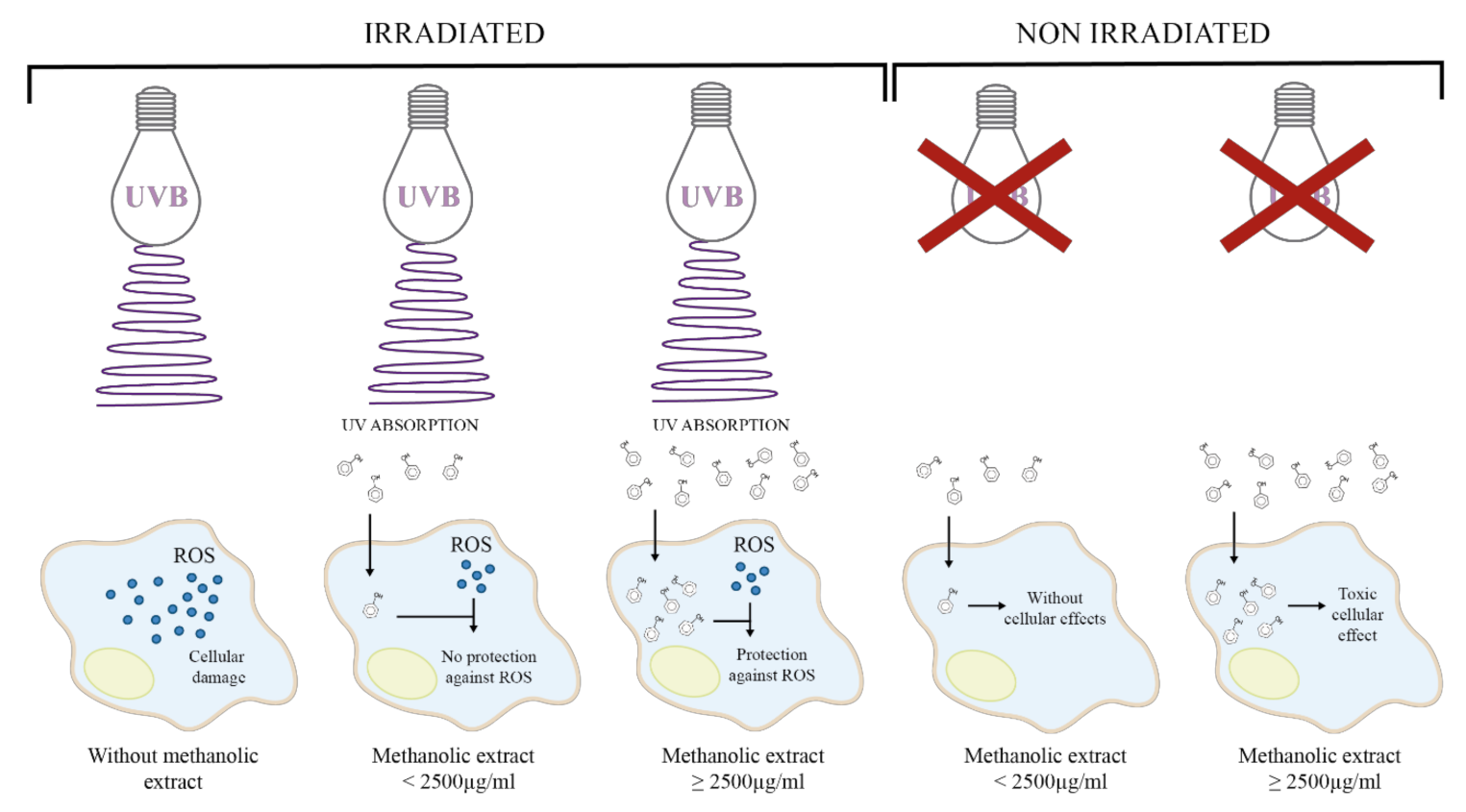
3. Discussion
4. Materials and Methods
4.1. Preparation of the Methanolic Extract and Verbascoside Standard
4.2. In Vitro Bioassays
4.2.1. Conditions of the Cell Culture
4.2.2. Determination of the Photoprotective Activity of the Extract
Irradiation and Selection of the UVB Energy Dose
Photoprotective Bioassay
4.2.3. Determination of the Cytotoxicity of the Extract
4.2.4. Determination of Cell Viability
4.3. Phytochemical Analysis
4.3.1. Measurement of the UV Absorption Spectrum
4.3.2. Quantitative Analysis
4.3.3. Identification of Extract Secondary Metabolites through LC/MS
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scheuer, E.; Warshaw, E. Sunscreen Allergy: A Review of epidemiology, clinical characteristics, and responsible allergens. Dermatitis 2006, 17, 3–11. [Google Scholar] [CrossRef]
- Ruszkiewicz, J.A.; Pinkas, A.; Ferrer, B.; Peres, T.V.; Tsatsakis, A.; Aschner, M. Neurotoxic effect of active ingredients in sunscreen products, a contemporary review. Toxicol. Rep. 2017, 4, 245–259. [Google Scholar] [CrossRef]
- Giraldo, J.C.; Atehortúa, L.; Mejía, M.A. Foto-protección: Mecanismos bioquímicos, punto de partida hacia mejores filtros solares. Dermatología Cosmética Médica Quirúrgica 2014, 12, 272–281. [Google Scholar]
- Gilaberte, Y.; González, S. Novedades en fotoprotección. Actas Dermo-Sifiliogr. 2010, 101, 659–672. [Google Scholar] [CrossRef]
- Latha, M.S.; Martis, J.; Shobha, V.; Shinde, R.S.; Bangera, S.; Krishnankutty, B.; Bellary, S.; Varughese, S.; Rao, P.; Kumar, B.R.N. Sunscreening agents: A review. J. Clin. Aesthet. Dermatol. 2013, 6, 16–26. [Google Scholar] [PubMed]
- Lee, T.; Sigurdson, A.J.; Preston, D.L.; Cachoon, E.K.; Freedman, D.M.; Simon, L.S.; Nelson, K.; Matanoski, G.; Kitahara, C.M.; Liu, J.J.; et al. Occupational ionizing radiation and risk of basal cell carcinoma in US radiologic technologists (1983–2005). Occup. Environ. Med. 2015, 72, 862–869. [Google Scholar] [CrossRef] [PubMed]
- Xiang, F.; Lucas, R.; Hales, S.; Neale, R. Incidence of Nonmelanoma skin cancer in relation to ambient UV radiation in white populations, 1978–2012 Empirical Relationships. JAMA Dermatol. 2014, 105, 1063–1071. [Google Scholar] [CrossRef] [Green Version]
- Moan, J.; Grigalavicius, M.; Baturaite, Z.; Dahlback, A.; Juzeniene, A. The relationship between UV exposure and incidence of skin cancer. Photodermatol. Photoimmunol. Photomed. 2014, 31, 26–35. [Google Scholar] [CrossRef]
- González-Púmariega, M.; Tamayo, M.V.; Sánchez-Lanar, A. La radiación ultravioleta su efecto dañino y consecuencias para la salud humana. Theoria 2009, 18, 69–80. [Google Scholar]
- Ravanat, J.L.; Douki, T. UV and ionizing radiations induced DNA damage, differences and similarities. Radiat. Phys. Chem. 2016, 128, 92–102. [Google Scholar] [CrossRef]
- Holick, M.F. Biological effects of sunlight, ultraviolet radiation, visible light, infrared radiation and vitamin D for health. Anticancer Res. 2016, 36, 1345–1356. [Google Scholar] [PubMed]
- Young, A.R.; Claveau, J.; Rossi, A.B. Ultraviolet radiation and the skin: Photobiology and sunscreen photoprotection. J. Am. Acad. Dermatolo. 2017, 76, 100–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panich, U.; Tangsupa-a-nan, V.; Onkoksoong, T.; Kongtaphan, K.; Kasetsinsombat, K.; Akarasereenont, P.; Wongkajornslip, A. Inhibition of UVA-mediated melanogenesis by ascorbic acid through modulation of antioxidant defense and nitric oxide system. Arch. Pharm. Res. 2011, 34, 811–820. [Google Scholar] [CrossRef]
- Ortonne, J.P. Photoprotective properties of skin melanin. Br. J. Dermatolo. 2002, 146, 7–10. [Google Scholar] [CrossRef] [PubMed]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV Radiation and the skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef] [Green Version]
- American Academy of Dermatology. Available online: https://www.aad.org/public/spot-skin-cancer/learn-about-skin-cancer/prevent (accessed on 24 August 2020).
- Rai, R.; Shanmuga, S.C.; Srinivas, C.R. Update on photoprotection. Indian J. Dermatol. 2012, 57, 335–342. [Google Scholar] [CrossRef]
- Skin Cancer Foundation. Available online: https://www.skincancer.org/prevention/sun-protection/sunscreen (accessed on 24 August 2020).
- Ulrich, C.; Jürgensen, J.S.; Degen, A.; Hackethal, M.; Ulrich, M.; Patel, M.J.; Eberle, J.; Terhorst, D.; Sterry, W.; Stcokfleth, E. Prevention of non-melanoma skin cancer in organ transplant patients by regular use of a sunscreen: A 24 months, prospective, case-control study. Br. J. Dermatol. 2009, 16, 78–84. [Google Scholar] [CrossRef]
- Ghiasvand, R.; Weiderpass, E.; Green, A.C.; Lund, E.; Veirod, B. Sunscreen use and subsequent melanoma risk: A population-based cohort study. J. Clin. Oncol. 2016, 33, 3976–3983. [Google Scholar] [CrossRef] [Green Version]
- Green, A.C.; Williams, M.G.; Logan, V.; Strutton, G.M. Reduced melanoma after regular sunscreen use: Randomized trial follow-up. J. Clin. Oncol. 2011, 29, 257–263. [Google Scholar] [CrossRef] [Green Version]
- Belščak-Cvitanović, A.; Durgo, K.; Hudek, A.; Bačun-Družina, V.; Komes, D. Overview of polyphenols and their properties. In Polyphenols: Properties, Recovery, and Applications, 1st ed.; Ganalakis, C.M., Ed.; Woodhead Publishing: Vienna, Austria, 2018; pp. 3–44. [Google Scholar]
- Solovchenko, A.E.; Merzlyak, M.N. Screening of visible and UV radiation as a photoprotective mechanism in plants. Russ. J. Plant Physiol. 2008, 55, 719–737. [Google Scholar] [CrossRef]
- Oliveira, M.M.; Daré, R.G.; Barizão, É.O.; Visentainer, J.V.; Romagnol, M.B.; Nakamura, C.V.; Truiti, M.D.C.T. Photodamage attenuating potential of Nectandra hihua against UVB-induced oxidative stress in L292 fibroblasts. J. Photochem. Photobiol. B 2018, 181, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, F.M.; Volpato, H.; Lazarin-Bidóia, D.; Desoti, V.C.; de Souza, R.O.; Fonseca, M.J.V.; Ueda-Nakamura, T.; Nakamura, C.V.; Silva, S.O. The extended production of UV-induced reactive oxygen species in L929 fibroblast is attenuated by posttreatment with Arrabidaea chica through scavenging mechanisms. J. Photochem. Photobiol. B 2018, 178, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Stockert, J.C.; Blázquez-Castro, A.; Cañate, M.; Horobin, R.W.; Villanueva, A. MTT assay for cell viability: Intracellular localization of the formazan product is in lipid droplets. Acta Histochem. 2012, 144, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Zúñiga, M.E.; Cruz-Sosa, F.; Rodríguez-Monroy, M.; Verde-Calvo, J.R.; Vernon-Carter, E.J. Phenylpropanoid production in callus and cell suspension cultures of Buddleja cordata Kunth. Plant Cell Tissue Organ Cult. 2009, 97, 39–47. [Google Scholar] [CrossRef]
- Khan, S.; Ullah, H.; Zhang, L. Review: Bioactive constituents from Buddleja species. Pak. J. Pharm. Sci. 2019, 32, 721–741. [Google Scholar]
- Norman, E.M. Flora Neotropica Monograph 81, 1st ed.; The Organization for Flora Neotropica: New York, NY, USA, 2000; pp. 2–61. [Google Scholar]
- Tatli, I.I.; Kahraman, C.; Akdemin, Z.S. Therapeutic activities of selected Scrophulariaceae and Buddlejaceae species and their secondary metabolites against neurodegenerative diseases. In Bioactive Nutraceuticals and Dietary Supplements in Neurological and Brain Disease. Prevention and Therapy, 1st ed.; Ross, R.W., Preedy, V.R., Eds.; Academic Press: London, UK, 2015; pp. 95–111. [Google Scholar] [CrossRef]
- Houghton, P.J.; Mensah, A.Y.; Lessa, N.; Hong, L.Y. Terpenoids in Buddleja: Relevance to chemosystematics, chemical ecology and biological activity. Phytochemistry 2003, 64, 385–393. [Google Scholar] [CrossRef]
- García-Bores, A.M.; Hernández, T.; Arcienegas, A.R.; Benítez, J.C.; González, M.R.; López, M.; Vivar, A.R.; Avila, J.G. Photoprotective activity of some Mexican plant. In Natural Antioxidants and Biocides from Wild Medicinal Plants, 1st ed.; Césped, C.L., Sampierto, D.A., Seigler, D.L., Rai, M., Eds.; CAB International: Wallingford, UK, 2013; pp. 254–266. [Google Scholar]
- Ávila Acevedo, J.G.; Castañeda, C.M.; Benítez, F.J.; Durán, D.A.; Barroso, V.R.; Martínez, C.G.; Muñoz, L.J.; Martínez, C.A.; Romo de Vivar, A. Photoprotective activity of Buddleja scordioides. Fitoterapia 2005, 76, 301–309. [Google Scholar] [CrossRef]
- Acevedo, J.G.A.; González, A.M.E.; Campos, D.M.D.M.; Flores, J.D.C.B.; Delgado, T.H.; Maya, S.F.; Contreras, J.C.; López, J.L.M.; Bores, A.M.G. Photoprotection of Buddleja cordata extract against UVB-induced skin damage in SKH-1 hairless mice. BMC Complement. Altern. Med. 2014, 14, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Espinoza-González, A.M.; García-Bores, A.M.; Benítez-Flores, J.C.; Sandoval-Pérez, E.; González-Valle, M.R.L.; Céspedes, C.; Avila-Acevedo, J.G. Photoprotective effect of verbascoside from Buddleja cordata in SKH-1 mice exposed to acute and chronic UV-B radiation. Boletín Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas 2016, 15, 288–300. [Google Scholar]
- Alipieva, K.; Korkina, L.; Orhan, I.E.; Georgiev, M.I. Verbascoside-a review of its occurrence, (bio) synthesis and pharmacological significance. Biotechnol. Adv. 2014, 32, 1065–1075. [Google Scholar] [CrossRef]
- Pesce, M.; Franceschelli, S.; Ferrone, A.; De LutIis, M.A.; Patruno, A.; Grilli, A.; Felaco, M.; Speranza, L. Verbascoside down-regulates some pro-inflammatory signal transduction pathways by increasing the activity of tyrosine phosphatase, SHP-1 in the U937 cell line. J. Cell. Mol. Med. 2015, 19, 1548–1556. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Wu, Y.C.; Meng, M.; Sun, Q.S.; Gao, S.M.; Sun, H. Linarin prevents LPS-induced acute lung injury by suppressing oxidative stress and inflammation via inhibition of TXNIP/NLRP3 and NF-κB pathways. Int. J. Mol. Med. 2018, 42, 1460–1472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estrada-Zúñiga, M.E.; Gutiérrez-Rebolledo, G.A.; Nieto-Trujillo, A.; Bernabé-Antonio, A.; Cruz Sosa, F. Buddleja species distributed in Mexico against inflammatory diseases, their therapeutic activities, secondary metabolites and biotechnology. In Recent Advances in Biological Research, 1st ed.; Yanik, T., Ed.; Book Publisher International: West Bengal, India, 2019; Volume 5, pp. 78–98. [Google Scholar]
- Álvarez, M.A. Plant secondary metabolism. In Plant Biotechnology for Health, 1st ed.; Álvarez, M.A., Ed.; Springer: Cham, Switzerland, 2014; pp. 15–31. [Google Scholar]
- Vázquez-Marquez, A.M.; Zepeda-Gómez, C.; Burrola-Aguilar, C.; Bernabé-Antonio, A.; Nieto-Trujillo, A.; Cruz-Sosa, F.; Rodríguez-Monroy, M.; Estrada-Zúñiga, M.E. Effect of stirring speed on the production of phenolic secondary metabolites and growth of Buddleja cordata cells cultured in mechanically agitated bioreactor. Plant Cell Tissue Organ Cult 2019, 139, 155–166. [Google Scholar] [CrossRef]
- Arano-Varela, H.; Cruz-Sosa, F.; Estrada-Zúñiga, M.E.; Fernández, F.J. Effects of phenylalanine and methyl jasmonate on verbascoside production in Buddleja cordata Kunth cell suspension cultures. S. Afr. J. Bot. 2020, 135, 41–49. [Google Scholar] [CrossRef]
- Arano-Varela, H.; Fernández, F.J.; Estrada-Zúñiga, M.E.; Cruz-Sosa, F. Verbascoside production in long-term Buddleja cordata Kunth cell suspension cultures. 3 Biotech 2020, 10. [Google Scholar] [CrossRef]
- Gutiérrez-Rebolledo, G.A.; Estrada-Zúñiga, M.E.; Nieto-Trujillo, A.; Cruz-Sosa, F.; Jiménez-Arellanes, M.A. In vivo anti-inflamatory activity and acute toxicity of methanolic extracts from wild plant leaves and cell suspension cultures of Buddleja cordata Kunth (Buddlejaceae). Rev. Mex. Ing. Quím. 2018, 17, 317–330. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-Rebolledo, G.A.; Estrada-Zúñiga, M.E.; Garduño-Siciliano, L.; García-Gutiérrez, G.E.; Reséndiz Mora, C.A.; Calderón-Amador, J.; Cruz-Sosa, F. In vivo anti-arthritic effect and repeated dose toxicity of standardized methanolic extracts of Buddleja cordata Kunth (Scrophulariaceae) wild plant leaves and cell culture. J. Ethnopharmacol. 2019, 240, 1–15. [Google Scholar] [CrossRef]
- Phol, J.; Christophers, E. Growth characteristics of skin fibroblast and 3T3 cells entrapped by polymerizing fibrin. In Vitro 1979, 15, 624–630. [Google Scholar] [CrossRef]
- Menon, G.K. Chapter 2 Skin basics; structure and function. In Lipids and Skin Health, 1st ed.; Pappas, A., Ed.; Springer: Cham, Switzerland; New York, NY, USA, 2015; pp. 9–23. [Google Scholar]
- Zeng, Q.; Zhou, F.; Lei, L.; Chen, J.; Lu, J.; Zhou, J.; Cao, K.; Xia, F.; Ding, S.; Huang, L.; et al. Ganodena lucidum polysaccharides protect fibroblast against UVB-induce photoaging. Mol. Med. Rep. 2017, 15, 111–116. [Google Scholar] [CrossRef] [Green Version]
- Chung, Y.H.; Jeong, S.A.; Choi, H.S.; Ro, S.; Lee, J.S.; Park, J.K. Protective effects of ginsenoside Rg2 and astaxanthin mixture against UVB-induce DNA damage. Anim. Cells Syst. 2018, 6, 400–406. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Otkur, W.; Zhang, Y.; Li, Q.; Ye, Y.; Zang, L.; He, H.; Hayashi, T.; Tashiro, S.; Onodera, S.; et al. Silibinin protects murine fibroblast L929 cells from UVB-induced apoptosis through the simultaneous inhibition of ATM-p53 pathway and autophagy. FEBS J. 2013, 280, 4572–4584. [Google Scholar] [CrossRef]
- Petrucci, R.; Astolfi, P.; Greci, L.; Firuzi, O.; Saso, L.; Marrosu, G. A spectroelectrochemical and chemical study on oxidation of hydroxycinnamic acids in aprotic medium. Electrochim. Acta. 2007, 52, 2461–2470. [Google Scholar] [CrossRef]
- Narayanan, D.L.; Saladin, R.N.; Fox, J.L. Ultraviolet radiation and skin cancer. Int. J. Dermatol. 2010, 49, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Khare, S.; Singh, N.B.; Singh, A.; Hussain, I.; Niharika, K.; Yadav, V.; Bano, C.; Yadav, R.K.; Amist, N. Plant secondary metabolites synthesis and their regulations under biotic and abiotic constraints. J. Plant Biol. 2020, 63, 203–216. [Google Scholar] [CrossRef]
- Cardinali, A.; Pati, S.; Minervini, F.; D’Antuono, I.; Linsalata, V.; Lattanzio, V. Verbascoside, isoverbascoside, and their derivatives recovered from olive mill wastewater as possible food antioxidants. J. Agric. Food Chem. 2012, 60, 1822–1829. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gan, L.; Li, G.Q.; Deng, L.; Zhang, X.; Deng, Y. Pharmacokinetics of plantamajoside and acteoside from Plantago asiatica in rats by liquid chromatography-mass spectrometry. J. Pharm. Biomed. Anal. 2014, 89, 251–256. [Google Scholar] [CrossRef]
- Fraga, C.G.; Galleano, M.; Verstraeten, S.V.; Oteiza, P.I. Basic biochemical mechanisms behind the health benefits of polyphenols. Mol. Aspects. Med. 2010, 31, 435–445. [Google Scholar] [CrossRef]
- Ried, A.M.; Juvonen, R.; Huuskonen, P.; Lehtonen, M.; Pasanen, M.; Lall, M. In vitro human metabolism and inhibitory potency of verbascoside for CYP enzymes. Molecules 2019, 24, 2191. [Google Scholar] [CrossRef] [Green Version]
- Kapepula, P.M.; Mbombo Munditshi, P.; Frank, T.; Mouithys-Mickalad, A.; Mumba Ngoyi, D.; Kalenda, P.D.T.; Kabamba Ngombe, N.; Serteyn, D.; Tits, M.; Frédérich, M.; et al. Antioxidant potentiality of three herbal teas consumed in Bandundu rural areas of Congo. Nat. Prod. Res. 2017, 31, 1940–1943. [Google Scholar] [CrossRef]
- Zhao, C.; Dodin, G.; Yuan, C.; Chen, H.; Zheng, R.; Jia, Z.; Fan, B.T. “In vitro” protection of DNA from fenton reaction by plant polyphenol verbascoside. Biochim. Biophy. Acta 2005, 1723, 114–123. [Google Scholar] [CrossRef]
- Rui-Chuan, C.; Jin-Hua, S.; Shan-Min, Y.; Ji, L.; Tian-Jiao, W.; Hong, Z. Effect of isoverbascoside, a phenylpropanoid glycoside antioxidant, on proliferation and differentiation of human gastric cancer cell. Acta Pharmacol. Sin. 2002, 23, 997–1001. [Google Scholar]
- Crivellari, I.; Vertuani, S.; Lim, Y.; Cervellati, F.; Baldisserotto, A.; Mandredini, S.; Valacchi, G. ES2 as a novel verbascoside-derived compound in the treatment of cutaneous wound healing. Cosmetics 2018, 5, 65. [Google Scholar] [CrossRef] [Green Version]
- Henn, J.G.; Steffens, L.; de Moura Sperotto, N.D.; de Souza Ponce, B.; Veríssimo, R.M.; Boaretto, F.B.M.; Hassemer, G.; Péres, V.F.; Schirme, H.; Picada, J.N.; et al. Toxicological evaluation of a standardized hydroethanolic extract from leaves of Plantago australis and its major compound, verbascoside. J. Ethnopharmacol. 2019, 229, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, T.; Xiao, W.; Yasudo, H.; Kawakami, Y. Regulation of proliferation, survival, differentiation, and activation by the signaling platform for SHP-1 phosphatase. Adv. Enzym. Regul. 2012, 57, 7–15. [Google Scholar] [CrossRef]
- Poole, A.W.; Jones, M.L. A SHPing tale: Perspectives on the regulation of SHP-1 and SHP-2 tyrosine phosphatases by the C-terminal tail. Cell Signal 2005, 17, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Kostyuk, V.; Potapovich, A.; Suhan, T.; De Luca, C.; Pressi, G.; Dal Toso, R.; Korkina, L. Plant polyphenols against UV-C-induced cellular death. Planta Med. 2008, 74, 509–514. [Google Scholar] [CrossRef]
- Pastore, S.; Potapovich, A.; Kostyuk, V.; Mariani, V.; Lulli, D.; De Luca, C.; Korkina, L. Plant polyphenols effectively protect HaCaT cells from ultraviolet C-Triggered necrosis and suppress inflammatory chemokine expression. Ann. N. Y. Acad. Sci. 2009, 1171, 305–3013. [Google Scholar] [CrossRef]
- Speranza, L.; Fransceschelli, S.; Pesce, M.; Reale, M.; Menghini, L.; Vinciguerra, I.; De LuitiIss, M.A.; Felaco, M.; Grilli, A. Antiinflamatory effects in THP-1 cells treated with verbascoside. Phytother. Res. 2010, 24, 1398–1404. [Google Scholar] [CrossRef]
- De Moura Sperotto, N.D.; Steffens, L.; Veríssimo, R.M.; Henn, J.G.; Péres, V.F.; Vianna, P.; Chies, J.A.B.; Roehe, A.; Saffi, J.; Moura, D.M. Wound healing and anti-inflammatory activities induced by Plantango australis hydroethanolic extract standardized in verbascoside. J. Ethnopharmacol. 2018, 225, 178–188. [Google Scholar] [CrossRef]
- Liu, M.J.; Li, J.X.; Guo, H.Z.; Lee, K.M.; Qin, L.; Chan, K.M. The effects of verbascoside on plasma lipid peroxidation level and erythrocyte membrane fluidity immobilization in rabbits: A time course study. Life Sci. 2003, 73, 883–892. [Google Scholar] [CrossRef]
- Singh, M.; Devi, S.; Rana, V.S.; Kumar, J.; Ahluwalia, V. Delivery of phytochemicals by liposome cargos: Recent progress, challenges and opportunities. J. Microencapsul. 2019, 36, 215–235. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, R.; Miranda, A.; Vergara, L. Modulation of endogenous antioxidant system by wine polyphenols in huma disease. Clin.Chim. Acta 2011, 412, 410–424. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Xu, T.; Zhao, Y.; Song, H.; Zhang, L.; Wu, X.; Lu, B. Chitosan-coated liposomes as delivery systems for improving the stability and oral bioavailability of acteoside. Food Hydrocol. 2018, 83, 17–24. [Google Scholar] [CrossRef]
- Isacchi, B.; Bergonzi, M.C.; Iacopi, R.; Ghelardini, C.; Galeotti, N.; Bilia, A.R. Liposomal formulation to increase stability and prolong antineuropathic activity of verbascoside. Planta Med. 2017, 83, 412–419. [Google Scholar] [CrossRef]
- Sinico, C.; Caddeo, C.; Valenti, D.; Fadda, A.M.; Bila, A.R.; Vincieri, F.F. Liposomes as carriers for verbascoside: Stability and skin permeation studies. J. Liposome Res. 2008, 18, 83–90. [Google Scholar] [CrossRef]
- Ambrosone, L.; Guerra, G.; Cinelli, M.; Filippelli, M.; Mosca, M.; Vizzarri, F.; Giorgio, D.; Costagliola, C. Corneal epitelial wound healing promoted by verbascoside-based liposomal eyedrops. BioMed Res. Int. 2014, 2014, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Schuch, A.P.; Moreno, N.C.; Schuch, N.J.; Menk, C.F.M.; Garcia, C.C.M. Sunlight damage to cellular DNA: Focus on oxidatively generated lesions. Free Radic. Biol. Med. 2017, 107, 110–124. [Google Scholar] [CrossRef]
- Chen, A.C.; Halliday, G.M.; Damian, D.L. Non-melanoma skin cancer: Carcinogenesis and chemoprevention. Pathology 2013, 45, 331–341. [Google Scholar] [CrossRef]
- Canul, M.E.A.; Rocher, C.C.; Zapata, H.R.; Trujillo, H.P. Cáncer de piel en Yucatán: Un estudio epidemiológico de 10 años. Dermatología Cosmética Médica Quirúrgica 2015, 13, 7–12. [Google Scholar]
- Pinedo-Vega, J.L.; Castañeda-López, R.; Dávila-Rangel, J.I.; Mireles-García, F.; Ríos-Martínez, C.; López Saucedo, A. Incidencia de cáncer de piel en Zacatecas. Rev. Med. Inst. Mex. Seguro Soc. 2014, 52, 282–289. [Google Scholar]
- Alfaro-Sánchez, A.; García-Hidalgo, L.; Casados-Vergara, R.; Rodríguez-Cabral, R.; Piña-Osuna, A.K.; Sánchez-Ramos, A. Cáncer de piel. Epidemiología y variedades histológicas, estudio de cinco años en el noreste de México. Dermatol. Rev. Mex. 2016, 60, 106–113. [Google Scholar]
- Antonio-Gutiérrez, O.T.; López Mano, A.; Paulo, E.; Ramírez-Corona, N. Métodos para la determinación de la dosis de radiación ultravioleta de onda corta (UVC) en alimentos. TSIA 2015, 9, 34–40. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Hernández, M.A.; Flores-Merino, M.V.; Sánchez-Flores, J.E.; Burrola-Aguilar, C.; Zepeda-Gómez, C.; Nieto-Trujillo, A.; Estrada-Zúñiga, M.E. Photoprotective Activity of Buddleja cordata Cell Culture Methanolic Extract on UVB-irradiated 3T3-Swiss Albino Fibroblasts. Plants 2021, 10, 266. https://doi.org/10.3390/plants10020266
Gómez-Hernández MA, Flores-Merino MV, Sánchez-Flores JE, Burrola-Aguilar C, Zepeda-Gómez C, Nieto-Trujillo A, Estrada-Zúñiga ME. Photoprotective Activity of Buddleja cordata Cell Culture Methanolic Extract on UVB-irradiated 3T3-Swiss Albino Fibroblasts. Plants. 2021; 10(2):266. https://doi.org/10.3390/plants10020266
Chicago/Turabian StyleGómez-Hernández, Milton Abraham, Miriam V. Flores-Merino, Jesús Enrique Sánchez-Flores, Cristina Burrola-Aguilar, Carmen Zepeda-Gómez, Aurelio Nieto-Trujillo, and María Elena Estrada-Zúñiga. 2021. "Photoprotective Activity of Buddleja cordata Cell Culture Methanolic Extract on UVB-irradiated 3T3-Swiss Albino Fibroblasts" Plants 10, no. 2: 266. https://doi.org/10.3390/plants10020266








