Present Status, Challenges, and Prospects of Dihydromyricetin in the Battle against Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Antioxidant and Anti-Inflammatory Activities of DHM
3. The Anticancer Potential and Underlying Mechanisms of Dihydromyricetin
3.1. Lung Cancer
3.2. Breast Cancer
3.3. Osteosarcoma
3.4. Reproductive System Cancer
3.5. Hepatocellular Carcinoma
3.6. Gastric Cancer and Cholangiocarcinoma
3.7. Colorectal Cancer
3.8. Melanoma
3.9. Squamous Cell Carcinoma
4. Synergistic Effects of Dihydromyricetin with Anticancer Agents
5. Challenges/Limitations
6. Strategies to Improve the Effects of DHM
6.1. Structural Modification
6.2. Drug Delivery Systems
7. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Kou, X.; Chen, N. Pharmacological potential of ampelopsin in Rattan tea. Food Sci. Hum. Wellness 2012, 1, 14–18. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Zhang, H.; Yan, X. Protective effect of dihydromyricetin revents fatty liver through nuclear factor-κB/p53/B-cell lymphoma 2-associated X protein signaling pathways in a rat model. Mol. Med. Rep. 2019, 19, 1638–1644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Li, Q. Application research and development of dihydromyricetin. Jiangsu Condiment Subsid. Food 2018, 4, 1–5. [Google Scholar] [CrossRef]
- Murakami, T.; Miyakoshi, M.; Araho, D.; Mizutani, K.; Kambara, T.; Ikeda, T.; Chou, W.H.; Inukai, M.; Takenaka, A.; Igarashi, K. Hepatoprotective activity of tocha, the stems and leaves of Ampelopsis grossedentata, and ampelopsin. BioFactors 2004, 21, 175–178. [Google Scholar] [CrossRef]
- Li, H.; Li, Q.; Liu, Z.; Yang, K.; Chen, Z.; Cheng, Q.; Wu, L. The Versatile Effects of Dihydromyricetin in Health. Evid. Based Complement. Altern. Med. 2017, 2017, 1053617. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Mao, Y.; Ding, L.; Zeng, X.A. Dihydromyricetin: A review on identification and quantification methods, biological activities, chemical stability, metabolism and approaches to enhance its bioavailability. Trends Food Sci. Technol. 2019, 91, 586–597. [Google Scholar] [CrossRef]
- Huang, H.; Hu, M.; Zhao, R.; Li, P.; Li, M. Dihydromyricetin suppresses the proliferation of hepatocellular carcinoma cells by inducing G2/M arrest through the Chk1/Chk2/Cdc25C pathway. Oncol. Rep. 2013, 30, 2467–2475. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.F.; Zhai, J.; Liu, Z.R.; Chao, L.; Zhao, Y.F.; Wu, Y.J.; Cui, M.X. Ampelopsin sodium induces mitochondrial-mediated apoptosis in human lung adenocarcinoma SPC-A-1 cell line. Die Pharm. 2016, 71, 455–459. [Google Scholar] [CrossRef]
- Huang, C.; Huang, Y.L.; Wang, C.C.; Pan, Y.L.; Lai, Y.H.; Huang, H.C. Ampelopsins A and C Induce Apoptosis and Metastasis through Downregulating AxL, TYRO3, and FYN Expressions in MDA-MB-231 Breast Cancer Cells. J. Agric. Food Chem. 2019, 67, 2818–2830. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gill, J.G.; Piskounova, E.; Morrison, S.J. Cancer, Oxidative Stress, and Metastasis. Cold Spring Harb. Symp. Quant. Biol. 2016, 81, 163–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanigawa, S.; Fujii, M.; Hou, D.X. Action of Nrf2 and Keap1 in ARE-mediated NQO1 expression by quercetin. Free Radic. Biol. Med. 2007, 42, 1690–1703. [Google Scholar] [CrossRef] [PubMed]
- Tebay, L.E.; Robertson, H.; Durant, S.T.; Vitale, S.R.; Penning, T.M.; Dinkova-Kostova, A.T.; Hayes, J.D. Mechanisms of activation of the transcription factor Nrf2 by redox stressors, nutrient cues, and energy status and the pathways through which it attenuates degenerative disease. Free Radic. Biol. Med. 2015, 88, 108–146. [Google Scholar] [CrossRef] [Green Version]
- Xie, K.; He, X.; Chen, K.; Chen, J.; Sakao, K.; Hou, D.X. Antioxidant Properties of a Traditional Vine Tea, Ampelopsis grossedentata. Antioxidants 2019, 8, 295. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Lu, S.; Dong, X.; Xu, L.; Sun, G.; Sun, X. Dihydromyricetin protects human umbilical vein endothelial cells from injury through ERK and Akt mediated Nrf2/HO-1 signaling pathway. Apoptosis Int. J. Program. Cell Death 2017, 22, 1013–1024. [Google Scholar] [CrossRef]
- Li, H.; Yu, F.; Sun, X.; Xu, L.; Miu, J.; Xiao, P. Dihydromyricetin ameliorates memory impairment induced by acute sleep deprivation. Eur. J. Pharmacol. 2019, 853, 220–228. [Google Scholar] [CrossRef]
- Wu, F.; Li, Y.; Song, H.; Zhang, Y.; Zhang, Y.; Jiang, M.; Wang, F.; Mu, Q.; Zhang, W.; Li, L.; et al. Preventive Effect of Dihydromyricetin against Cisplatin-Induced Nephrotoxicity In Vitro and In Vivo. Evid. Based Complement. Altern. Med. 2016, 2016, 7937385. [Google Scholar] [CrossRef] [Green Version]
- Canli, Ö.; Nicolas, A.M.; Gupta, J.; Finkelmeier, F.; Goncharova, O.; Pesic, M.; Neumann, T.; Horst, D.; Löwer, M.; Sahin, U.; et al. Myeloid Cell-Derived Reactive Oxygen Species Induce Epithelial Mutagenesis. Cancer Cell 2017, 32, 869–883.e5. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Zhao, F.T.; Fan, K.J.; Zhang, J.; Xu, B.X.; Wang, Q.S.; Tang, T.T.; Wang, T.Y. Dihydromyricetin Inhibits Inflammation of Fibroblast-Like Synoviocytes through Regulation of Nuclear Factor-κB Signaling in Rats with Collagen-Induced Arthritis. J. Pharmacol. Exp. Ther. 2019, 368, 218–228. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Lu, W.; Lin, N.; Lin, H.; Zhang, J.; Ni, T.; Meng, L.; Zhang, C.; Guo, H. Dihydromyricetin alleviates doxorubicin-induced cardiotoxicity by inhibiting NLRP3 inflammasome through activation of SIRT1. Biochem. Pharmacol. 2020, 175, 113888. [Google Scholar] [CrossRef] [PubMed]
- Qiu, P.; Dong, Y.; Li, B.; Kang, X.J.; Gu, C.; Zhu, T.; Luo, Y.Y.; Pang, M.X.; Du, W.F.; Ge, W.H. Dihydromyricetin modulates p62 and autophagy crosstalk with the Keap-1/Nrf2 pathway to alleviate ethanol-induced hepatic injury. Toxicol. Lett. 2017, 274, 31–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Zhao, X.; Wan, J.; Ran, L.; Qin, Y.; Wang, X.; Gao, Y.; Shu, F.; Zhang, Y.; Liu, P.; et al. Dihydromyricetin improves glucose and lipid metabolism and exerts anti-inflammatory effects in nonalcoholic fatty liver disease: A randomized controlled trial. Pharmacol. Res. 2015, 99, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhang, J.; Beeraka, N.M.; Tang, C.; Babayeva, Y.V.; Sinelnikov, M.Y.; Zhang, X.; Zhang, J.; Liu, J.; Reshetov, I.V.; et al. Advances in the Prevention and Treatment of Obesity-Driven Effects in Breast Cancers. Front. Oncol. 2022, 12, 820968. [Google Scholar] [CrossRef]
- Chen, K.; Lu, P.; Beeraka, N.M.; Sukocheva, O.A.; Madhunapantula, S.V.; Liu, J.; Sinelnikov, M.Y.; Nikolenko, V.N.; Bulygin, K.V.; Mikhaleva, L.M.; et al. Mitochondrial mutations and mitoepigenetics: Focus on regulation of oxidative stress-induced responses in breast cancers. Semin. Cancer Biol. 2022, 83, 556–569. [Google Scholar] [CrossRef]
- Garo, L.P.; Ajay, A.K.; Fujiwara, M.; Gabriely, G.; Raheja, R.; Kuhn, C.; Kenyon, B.; Skillin, N.; Kadowaki-Saga, R.; Saxena, S.; et al. MicroRNA-146a limits tumorigenic inflammation in colorectal cancer. Nat. Commun. 2021, 12, 2419. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Bareschino, M.A.; Schettino, C.; Rossi, A.; Maione, P.; Sacco, P.C.; Zeppa, R.; Gridelli, C. Treatment of advanced non small cell lung cancer. J. Thorac. Dis. 2011, 3, 122–133. [Google Scholar] [CrossRef]
- Kao, S.J.; Lee, W.J.; Chang, J.H.; Chow, J.M.; Chung, C.L.; Hung, W.Y.; Chien, M.H. Suppression of reactive oxygen species-mediated ERK and JNK activation sensitizes dihydromyricetin-induced mitochondrial apoptosis in human non-small cell lung cancer. Environ. Toxicol. 2017, 32, 1426–1438. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Heldin, C.H.; Westermark, B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol. Rev. 1999, 79, 1283–1316. [Google Scholar] [CrossRef]
- Fan, K.J.; Yang, B.; Liu, Y.; Tian, X.D.; Wang, B. Inhibition of human lung cancer proliferation through targeting stromal fibroblasts by dihydromyricetin. Mol. Med. Rep. 2017, 16, 9758–9762. [Google Scholar] [CrossRef] [Green Version]
- Correa, R.L.; Bruckner, F.P.; de Souza Cascardo, R.; Alfenas-Zerbini, P. The Role of F-Box Proteins during Viral Infection. Int. J. Mol. Sci. 2013, 14, 4030–4049. [Google Scholar] [CrossRef] [Green Version]
- Ji, S.; Qin, Y.; Shi, S.; Liu, X.; Hu, H.; Zhou, H.; Gao, J.; Zhang, B.; Xu, W.; Liu, J.; et al. ERK kinase phosphorylates and destabilizes the tumor suppressor FBW7 in pancreatic cancer. Cell Res. 2015, 25, 561–573. [Google Scholar] [CrossRef]
- Chen, X.M.; Xie, X.B.; Zhao, Q.; Wang, F.; Bai, Y.; Yin, J.Q.; Jiang, H.; Xie, X.L.; Jia, Q.; Huang, G. Ampelopsin induces apoptosis by regulating multiple c-Myc/S-phase kinase-associated protein 2/F-box and WD repeat-containing protein 7/histone deacetylase 2 pathways in human lung adenocarcinoma cells. Mol. Med. Rep. 2015, 11, 105–112. [Google Scholar] [CrossRef] [Green Version]
- George, A.L.; Rajoria, S.; Suriano, R.; Mittleman, A.; Tiwari, R.K. Hypoxia and estrogen are functionally equivalent in breast cancer-endothelial cell interdependence. Mol. Cancer 2012, 11, 80. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Shu, F.; Liang, X.; Chang, H.; Shi, L.; Peng, X.; Zhu, J.; Mi, M. Ampelopsin induces cell growth inhibition and apoptosis in breast cancer cells through ROS generation and endoplasmic reticulum stress pathway. PLoS ONE 2014, 9, e89021. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Y.; Wang, M.; Lin, X.; Zhang, Y.; Laurent, I.; Zhong, Y.; Li, J. Ampelopsin Inhibits Breast Cancer Cell Growth through Mitochondrial Apoptosis Pathway. Biol. Pharm. Bull. 2021, 44, 1738–1745. [Google Scholar] [CrossRef]
- Li, X.; He, S.; Ma, B. Autophagy and autophagy-related proteins in cancer. Mol. Cancer 2020, 19, 12. [Google Scholar] [CrossRef]
- Liu, W.; Meng, Y.; Zong, C.; Zhang, S.; Wei, L. Autophagy and Tumorigenesis. Adv. Exp. Med. Biol. 2020, 1207, 275–299. [Google Scholar] [CrossRef]
- Guo, J.Y.; Xia, B.; White, E. Autophagy-mediated tumor promotion. Cell 2013, 155, 1216–1219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Liang, X.; Chang, H.; Shu, F.; Wu, Y.; Zhang, T.; Fu, Y.; Zhang, Q.; Zhu, J.D.; Mi, M. Ampelopsin-induced autophagy protects breast cancer cells from apoptosis through Akt-mTOR pathway via endoplasmic reticulum stress. Cancer Sci. 2014, 105, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Biermann, J.S.; Adkins, D.R.; Agulnik, M.; Benjamin, R.S.; Brigman, B.; Butrynski, J.E.; Cheong, D.; Chow, W.; Curry, W.T.; Frassica, D.A.; et al. Bone cancer. J. Natl. Compr. Cancer Netw. 2013, 11, 688–723. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, W.; Qiu, E. Protection of oxidative stress induced apoptosis in osteosarcoma cells by dihydromyricetin through down-regulation of caspase activation and up-regulation of BcL-2. Saudi J. Biol. Sci. 2017, 24, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Pavón, M.A.; Arroyo-Solera, I.; Céspedes, M.V.; Casanova, I.; León, X.; Mangues, R. uPA/uPAR and SERPINE1 in head and neck cancer: Role in tumor resistance, metastasis, prognosis and therapy. Oncotarget 2016, 7, 57351–57366. [Google Scholar] [CrossRef] [Green Version]
- Chou, C.H.; Lu, K.H.; Yang, J.S.; Hsieh, Y.H.; Lin, C.W.; Yang, S.F. Dihydromyricetin suppresses cell metastasis in human osteosarcoma through SP-1- and NF-κB-modulated urokinase plasminogen activator inhibition. Phytomed. Int. J. Phytother. Phytopharm. 2021, 90, 153642. [Google Scholar] [CrossRef]
- Gialeli, C.; Theocharis, A.D.; Karamanos, N.K. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J. 2011, 278, 16–27. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, P.; Yang, Y.; Xu, X.; Wang, L.; Li, B. Ampelopsin suppresses TNF-α-induced migration and invasion of U2OS osteosarcoma cells. Mol. Med. Rep. 2016, 13, 4729–4736. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Yin, J.Q.; Wu, M.S.; Song, G.; Xie, X.B.; Zou, C.; Tang, Q.; Wu, Y.; Lu, J.; Wang, Y.; et al. Dihydromyricetin activates AMP-activated protein kinase and P38(MAPK) exerting antitumor potential in osteosarcoma. Cancer Prev. Res. 2014, 7, 927–938. [Google Scholar] [CrossRef] [Green Version]
- Yokoi, A.; Matsuzaki, J.; Yamamoto, Y.; Yoneoka, Y.; Takahashi, K.; Shimizu, H.; Uehara, T.; Ishikawa, M.; Ikeda, S.I.; Sonoda, T.; et al. Integrated extracellular microRNA profiling for ovarian cancer screening. Nat. Commun. 2018, 9, 4319. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, S.; Chan, H.F.; Lu, H.; Lin, Z.; He, C.; Chen, M. Dihydromyricetin Induces Apoptosis and Reverses Drug Resistance in Ovarian Cancer Cells by p53-mediated Downregulation of Survivin. Sci. Rep. 2017, 7, 46060. [Google Scholar] [CrossRef] [Green Version]
- Nisticò, P.; Bissell, M.J.; Radisky, D.C. Epithelial-mesenchymal transition: General principles and pathological relevance with special emphasis on the role of matrix metalloproteinases. Cold Spring Harb. Perspect. Biol. 2012, 4, a011908. [Google Scholar] [CrossRef]
- Parikh, A.; Lee, C.; Joseph, P.; Marchini, S.; Baccarini, A.; Kolev, V.; Romualdi, C.; Fruscio, R.; Shah, H.; Wang, F.; et al. microRNA-181a has a critical role in ovarian cancer progression through the regulation of the epithelial-mesenchymal transition. Nat. Commun. 2014, 5, 2977. [Google Scholar] [CrossRef]
- Liu, T.; Liu, P.; Ding, F.; Yu, N.; Li, S.; Wang, S.; Zhang, X.; Sun, X.; Chen, Y.; Wang, F.; et al. Ampelopsin reduces the migration and invasion of ovarian cancer cells via inhibition of epithelial-to-mesenchymal transition. Oncol. Rep. 2015, 33, 861–867. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Chen, X.; Yuan, D.; Yi, Y.; Luo, Y. Golgi reassembly and stacking protein 65 downregulation is required for the anti-cancer effect of dihydromyricetin on human ovarian cancer cells. PLoS ONE 2019, 14, e0225450. [Google Scholar] [CrossRef] [Green Version]
- Pires, L.V.; Yi, Y.; Cheng, J.C.; Pizzolato, L.S.; Cordero, E.; Leung, P.C.K.; Brum, I.S. Lapatinib Inhibits Amphiregulin-induced BeWo Choriocarcinoma Cell Proliferation by Reducing ERK1/2 and AKT Signaling Pathways. Anticancer Res. 2019, 39, 2377–2383. [Google Scholar] [CrossRef]
- Pearce, H.; Edwards, D.C.; Levy, J.A.; McGreen, B.H.; Mackovick, L.; Brennan, M.; McHugh, M.; Mapow, B.; Schanne, F.J.; Belkoff, L. Acute pulmonary hemorrhage associated with metastatic testicular choriocarcinoma in a 46-year-old incarcerated male. Urol. Ann. 2019, 11, 109–112. [Google Scholar] [CrossRef]
- Zuo, Y.; Lu, Y.; Xu, Q.; Sun, D.; Liang, X.; Li, X.; Li, Y. Inhibitory effect of dihydromyricetin on the proliferation of JAR cells and its mechanism of action. Oncol. Lett. 2020, 20, 357–363. [Google Scholar] [CrossRef] [Green Version]
- Zuo, Y.; Xu, Q.; Lu, Y.; Sun, D.; Wang, K.; Lei, Y.; Liang, X.; Li, Y. Dihydromyricetin induces apoptosis in a human choriocarcinoma cell line. Oncol. Lett. 2018, 16, 4229–4234. [Google Scholar] [CrossRef] [Green Version]
- Ni, F.; Gong, Y.; Li, L.; Abdolmaleky, H.M.; Zhou, J.R. Flavonoid ampelopsin inhibits the growth and metastasis of prostate cancer in vitro and in mice. PLoS ONE 2012, 7, e38802. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Zhang, H.; Chen, S.; Xu, Y.; Yao, A.; Liao, Q.; Han, L.; Zou, Z.; Zhang, X. Dihydromyricetin induces mitochondria-mediated apoptosis in HepG2 cells through down-regulation of the Akt/Bad pathway. Nutr. Res. 2017, 38, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shu, Y.; Zhang, Q.; Liu, B.; Xia, J.; Qiu, M.; Miao, H.; Li, M.; Zhu, R. Dihydromyricetin induces apoptosis and inhibits proliferation in hepatocellular carcinoma cells. Oncol. Lett. 2014, 8, 1645–1651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Liu, J.; Liu, B.; Xia, J.; Chen, N.; Chen, X.; Cao, Y.; Zhang, C.; Lu, C.; Li, M.; et al. Dihydromyricetin promotes hepatocellular carcinoma regression via a p53 activation-dependent mechanism. Sci. Rep. 2014, 4, 4628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, S.; Kou, X.; Lv, J.; Qi, Z.; Yan, L. Ampelopsin induces apoptosis in HepG2 human hepatoma cell line through extrinsic and intrinsic pathways: Involvement of P38 and ERK. Environ. Toxicol. Pharmacol. 2015, 40, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Matsuno, K.; Diederich, R.J.; Go, M.J.; Blaumueller, C.M.; Artavanis-Tsakonas, S. Deltex acts as a positive regulator of Notch signaling through interactions with the Notch ankyrin repeats. Development 1995, 121, 2633–2644. [Google Scholar] [CrossRef]
- Artavanis-Tsakonas, S.; Rand, M.D.; Lake, R.J. Notch signaling: Cell fate control and signal integration in development. Science 1999, 284, 770–776. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.J.; He, Y.F.; Yuan, W.Z.; Xiang, L.J.; Zhang, J.; Liang, Y.R.; Duan, J.; He, Y.H.; Li, M.Y. Dihydromyricetin-mediated inhibition of the Notch1 pathway induces apoptosis in QGY7701 and HepG2 hepatoma cells. World J. Gastroenterol. 2017, 23, 6242–6251. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Li, R.; Zeng, G.F.; Liu, B.; Liu, J.; Shu, Y.; Liu, Z.K.; Qiu, Z.D.; Wang, D.J.; Miao, H.L.; et al. Dihydromyricetin inhibits migration and invasion of hepatoma cells through regulation of MMP-9 expression. World J. Gastroenterol. 2014, 20, 10082–10093. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef]
- Liu, B.; Zhou, W.; Chen, X.; Xu, F.; Chen, Y.; Liu, J.; Zhang, Q.; Bao, S.; Chen, N.; Li, M.; et al. Dihydromyricetin induces mouse hepatoma Hepal-6 cell apoptosis via the transforming growth factor-β pathway. Mol. Med. Rep. 2015, 11, 1609–1614. [Google Scholar] [CrossRef] [Green Version]
- Xia, J.; Guo, S.; Fang, T.; Feng, D.; Zhang, X.; Zhang, Q.; Liu, J.; Liu, B.; Li, M.; Zhu, R. Dihydromyricetin induces autophagy in HepG2 cells involved in inhibition of mTOR and regulating its upstream pathways. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2014, 66, 7–13. [Google Scholar] [CrossRef]
- Wang, F.H.; Shen, L.; Li, J.; Zhou, Z.W.; Liang, H.; Zhang, X.T.; Tang, L.; Xin, Y.; Jin, J.; Zhang, Y.J.; et al. The Chinese Society of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cancer. Cancer Commun. 2019, 39, 10. [Google Scholar] [CrossRef] [Green Version]
- Wei, L.; Sun, J.; Zhang, N.; Zheng, Y.; Wang, X.; Lv, L.; Liu, J.; Xu, Y.; Shen, Y.; Yang, M. Noncoding RNAs in gastric cancer: Implications for drug resistance. Mol. Cancer 2020, 19, 62. [Google Scholar] [CrossRef] [Green Version]
- Ji, F.J.; Tian, X.F.; Liu, X.W.; Fu, L.B.; Wu, Y.Y.; Fang, X.D.; Jin, H.Y. Dihydromyricetin induces cell apoptosis via a p53-related pathway in AGS human gastric cancer cells. Genet. Mol. Res. 2015, 14, 15564–15571. [Google Scholar] [CrossRef]
- Wang, F.J.; Zong, X.Y.; Du, J.L.; Wang, W.S.; Yuan, D.P.; Chen, X.B. Effects of dihydromyricetin on the migration and invasion of human gastric cancer MKN45 cells and its mechanism. Zhongguo Ying Yong Sheng Li Xue Za Zhi = Zhongguo Yingyong Shenglixue Zazhi = Chin. J. Appl. Physiol. 2019, 35, 428–432. [Google Scholar] [CrossRef]
- Ohmori, H.; Luo, Y.; Kuniyasu, H. Non-histone nuclear factor HMGB1 as a therapeutic target in colorectal cancer. Expert Opin. Ther. Targets 2011, 15, 183–193. [Google Scholar] [CrossRef]
- Wang, S.; Ge, F.; Cai, T.; Qi, S.; Qi, Z. Dihydromyricetin inhibits proliferation and migration of gastric cancer cells through regulating Akt/STAT3 signaling pathways and HMGB1 expression. Nan Fang Yi Ke Da Xue Xue Bao = J. South. Med. Univ. 2021, 41, 87–92. [Google Scholar] [CrossRef]
- Calin, G.A.; Croce, C.M. MicroRNA signatures in human cancers. Nat. Rev. Cancer 2006, 6, 857–866. [Google Scholar] [CrossRef]
- Lu, J.; Getz, G.; Miska, E.A.; Alvarez-Saavedra, E.; Lamb, J.; Peck, D.; Sweet-Cordero, A.; Ebert, B.L.; Mak, R.H.; Ferrando, A.A.; et al. MicroRNA expression profiles classify human cancers. Nature 2005, 435, 834–838. [Google Scholar] [CrossRef]
- Zhan, T.; Zhu, Q.; Han, Z.; Tan, J.; Liu, M.; Liu, W.; Chen, W.; Chen, X.; Chen, X.; Deng, J.; et al. miR-455-3p Functions as a Tumor Suppressor by Restraining Wnt/β-Catenin Signaling via TAZ in Pancreatic Cancer. Cancer Manag. Res. 2020, 12, 1483–1492. [Google Scholar] [CrossRef] [Green Version]
- Yunqi, H.; Fangrui, Y.; Yongyan, Y.; Yunjian, J.; Wenhui, Z.; Kun, C.; Min, L.; Xianfeng, L.; Caixia, B. miR-455 Functions as a Tumor Suppressor Through Targeting GATA6 in Colorectal Cancer. Oncol. Res. 2019, 27, 311–316. [Google Scholar] [CrossRef]
- Li, X.; Yang, Z.S.; Cai, W.W.; Deng, Y.; Chen, L.; Tan, S.L. Dihydromyricetin Inhibits Tumor Growth and Epithelial-Mesenchymal Transition through regulating miR-455-3p in Cholangiocarcinoma. J. Cancer 2021, 12, 6058–6070. [Google Scholar] [CrossRef]
- Chen, L.; Yang, Z.S.; Zhou, Y.Z.; Deng, Y.; Jiang, P.; Tan, S.L. Dihydromyricetin inhibits cell proliferation, migration, invasion and promotes apoptosis via regulating miR-21 in Human Cholangiocarcinoma Cells. J. Cancer 2020, 11, 5689–5699. [Google Scholar] [CrossRef]
- Akil, H.; Perraud, A.; Jauberteau, M.-O.; Mathonnet, M. Tropomyosin-related kinase B/brain derived-neurotrophic factor signaling pathway as a potential therapeutic target for colorectal cancer. World J. Gastroenterol. 2016, 22, 490–500. [Google Scholar] [CrossRef]
- Zhu, X.H.; Lang, H.D.; Wang, X.L.; Hui, S.C.; Zhou, M.; Kang, C.; Yi, L.; Mi, M.T.; Zhang, Y. Synergy between dihydromyricetin intervention and irinotecan chemotherapy delays the progression of colon cancer in mouse models. Food Funct. 2019, 10, 2040–2049. [Google Scholar] [CrossRef]
- Grifantini, R.; Taranta, M.; Gherardini, L.; Naldi, I.; Parri, M.; Grandi, A.; Giannetti, A.; Tombelli, S.; Lucarini, G.; Ricotti, L.; et al. Magnetically driven drug delivery systems improving targeted immunotherapy for colon-rectal cancer. J. Control. Release Off. J. Control. Release Soc. 2018, 280, 76–86. [Google Scholar] [CrossRef]
- Park, G.B.; Jeong, J.Y.; Kim, D. Ampelopsin-induced reactive oxygen species enhance the apoptosis of colon cancer cells by activating endoplasmic reticulum stress-mediated AMPK/MAPK/XAF1 signaling. Oncol. Lett. 2017, 14, 7947–7956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izano, M.; Wei, E.K.; Tai, C.; Swede, H.; Gregorich, S.; Harris, T.B.; Klepin, H.; Satterfield, S.; Murphy, R.; Newman, A.B.; et al. Chronic inflammation and risk of colorectal and other obesity-related cancers: The health, aging and body composition study. Int. J. Cancer 2016, 138, 1118–1128. [Google Scholar] [CrossRef] [Green Version]
- Bopanna, S.; Ananthakrishnan, A.N.; Kedia, S.; Yajnik, V.; Ahuja, V. Risk of colorectal cancer in Asian patients with ulcerative colitis: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2017, 2, 269–276. [Google Scholar] [CrossRef] [Green Version]
- Clarke, W.T.; Feuerstein, J.D. Updates in colorectal cancer screening in inflammatory bowel disease. Curr. Opin. Gastroenterol. 2018, 34, 208–216. [Google Scholar] [CrossRef]
- Zhang, Y.; Kang, C.; Wang, X.L.; Zhou, M.; Chen, M.T.; Zhu, X.H.; Liu, K.; Wang, B.; Zhang, Q.Y.; Zhu, J.D.; et al. Dietary Factors Modulate Colonic Tumorigenesis Through the Interaction of Gut Microbiota and Host Chloride Channels. Mol. Nutr. Food Res. 2018, 62, 201700554. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hou, Y.; Ma, L.; Sun, C.; Pan, J.; Yang, Y.; Zhou, H.; Zhang, J. Regulation of semaphorin 4D expression and cell proliferation of ovarian cancer by ERalpha and ERbeta. Braz. J. Med. Biol. Res. = Rev. Bras. Pesqui. Med. E Biol. 2017, 50, e6057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, X.; Qiu, L.; Zhang, L.; Xi, J.; Li, D.; Huang, X.; Zhao, Y.; Wang, X.; Sun, Q. The role of semaphorin 4D as a potential biomarker for antiangiogenic therapy in colorectal cancer. OncoTargets Ther. 2016, 9, 1189–1204. [Google Scholar] [CrossRef] [Green Version]
- Liang, J.; Wu, J.; Wang, F.; Zhang, P.; Zhang, X. Semaphoring 4D is required for the induction of antioxidant stress and anti-inflammatory effects of dihydromyricetin in colon cancer. Int. Immunopharmacol. 2019, 67, 220–230. [Google Scholar] [CrossRef]
- Pietrobono, S.; Anichini, G.; Sala, C.; Manetti, F.; Almada, L.L.; Pepe, S.; Carr, R.M.; Paradise, B.D.; Sarkaria, J.N.; Davila, J.I.; et al. ST3GAL1 is a target of the SOX2-GLI1 transcriptional complex and promotes melanoma metastasis through AXL. Nat. Commun. 2020, 11, 5865. [Google Scholar] [CrossRef]
- Zeng, G.; Liu, J.; Chen, H.; Liu, B.; Zhang, Q.; Li, M.; Zhu, R. Dihydromyricetin induces cell cycle arrest and apoptosis in melanoma SK-MEL-28 cells. Oncol. Rep. 2014, 31, 2713–2719. [Google Scholar] [CrossRef] [Green Version]
- Zhou, D.Z.; Sun, H.Y.; Yue, J.Q.; Peng, Y.; Chen, Y.M.; Zhong, Z.J. Dihydromyricetin induces apoptosis and cytoprotective autophagy through ROS-NF-κB signalling in human melanoma cells. Free Radic. Res. 2017, 51, 517–528. [Google Scholar] [CrossRef]
- Zheng, H.Q.; Liu, D.Y. Anti-invasive and anti-metastatic effect of ampelopsin on melanoma. Ai Zheng = Aizheng = Chin. J. Cancer 2003, 22, 363–367. [Google Scholar]
- Mosca, L.; Minopoli, M.; Pagano, M.; Vitiello, F.; Carriero, M.V.; Cacciapuoti, G.; Porcelli, M. Effects of S-adenosyl-L-methionine on the invasion and migration of head and neck squamous cancer cells and analysis of the underlying mechanisms. Int. J. Oncol. 2020, 56, 1212–1224. [Google Scholar] [CrossRef] [Green Version]
- Fan, T.F.; Wu, T.F.; Bu, L.L.; Ma, S.R.; Li, Y.C.; Mao, L.; Sun, Z.J.; Zhang, W.F. Dihydromyricetin promotes autophagy and apoptosis through ROS-STAT3 signaling in head and neck squamous cell carcinoma. Oncotarget 2016, 7, 59691–59703. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Sun, P.; Zhou, Q.Y.; Gao, X.; Han, Q. Long noncoding RNA MALAT1 promotes uveal melanoma cell growth and invasion by silencing of miR-140. Am. J. Transl. Res. 2016, 8, 3939–3946. [Google Scholar]
- Liu, J.; Peng, W.X.; Mo, Y.Y.; Luo, D. MALAT1-mediated tumorigenesis. Front. Biosci. 2017, 22, 66–80. [Google Scholar] [CrossRef]
- Tan, M.; Jiang, B.; Wang, H.; Ouyang, W.; Chen, X.; Wang, T.; Dong, D.; Yi, S.; Yi, J.; Huang, Y.; et al. Dihydromyricetin induced lncRNA MALAT1-TFEB-dependent autophagic cell death in cutaneous squamous cell carcinoma. J. Cancer 2019, 10, 4245–4255. [Google Scholar] [CrossRef] [Green Version]
- Abdullah, B.; Alias, A.; Hassan, S. Challenges in the management of nasopharyngeal carcinoma: A review. Malays. J. Med. Sci. 2009, 16, 50–54. [Google Scholar]
- Li, C.H.; Ding, H.; Shi, J.L.; Huang, B.; Ding, H.; Lin, H.G.; Zeng, J.C.; Zhao, Y.; Luo, G.Q. Dihydromyricetin promotes apoptosis, suppresses proliferation and tumor necrosis factor-α-mediated nuclear factor kappa-B activation in nasopharyngeal carcinoma CNE-2 cell. J. Tradit. Chin. Med. = Chung I Tsa Chih Ying Wen Pan 2021, 41, 367–375. [Google Scholar] [CrossRef]
- Huang, C.C.; Su, C.W.; Wang, P.H.; Lu, Y.T.; Ho, Y.T.; Yang, S.F.; Hsin, C.H.; Lin, C.W. Dihydromyricetin inhibits cancer cell migration and matrix metalloproteinases-2 expression in human nasopharyngeal carcinoma through extracellular signal-regulated kinase signaling pathway. Environ. Toxicol. 2022, 37, 1244–1253. [Google Scholar] [CrossRef]
- Chen, Z.; Shi, T.; Zhang, L.; Zhu, P.; Deng, M.; Huang, C.; Hu, T.; Jiang, L.; Li, J. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: A review of the past decade. Cancer Lett. 2016, 370, 153–164. [Google Scholar] [CrossRef]
- Yan, L.H.; Wei, W.Y.; Cao, W.L.; Zhang, X.S.; Xie, Y.B.; Xiao, Q. Overexpression of CDX2 in gastric cancer cells promotes the development of multidrug resistance. Am. J. Cancer Res. 2015, 5, 321–332. [Google Scholar]
- Theodoulou, M.; Hudis, C. Cardiac profiles of liposomal anthracyclines: Greater cardiac safety versus conventional doxorubicin? Cancer 2004, 100, 2052–2063. [Google Scholar] [CrossRef]
- Zhu, H.; Luo, P.; Fu, Y.; Wang, J.; Dai, J.; Shao, J.; Yang, X.; Chang, L.; Weng, Q.; Yang, B.; et al. Dihydromyricetin prevents cardiotoxicity and enhances anticancer activity induced by adriamycin. Oncotarget 2015, 6, 3254–3267. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Liu, W.; Wang, C.; Meng, Q.; Liu, Z.; Huo, X.; Yang, X.; Sun, P.; Sun, H.; Ma, X.; et al. Combination of dihydromyricetin and ondansetron strengthens antiproliferative efficiency of adriamycin in K562/ADR through downregulation of SORCIN: A new strategy of inhibiting P-glycoprotein. J. Cell. Physiol. 2019, 234, 3685–3696. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Zheng, Y.; Liu, D. Reversal effect and its mechanism of ampelopsin on multidrug resistance in K562/ADR cells. Zhongguo Zhong Yao Za Zhi = Zhongguo Zhongyao Zazhi = China J. Chin. Mater. Med. 2009, 34, 761–765. [Google Scholar]
- Wu, M.; Jiang, M.; Dong, T.; Xu, L.; Lv, J.; Xue, M.; Huang, M. Reversal Effect of Dihydromyricetin on Multiple Drug Resistance in SGC7901/5-FU Cells. Asian Pac. J. Cancer Prev. 2020, 21, 1269–1274. [Google Scholar] [CrossRef] [PubMed]
- Altieri, D.C. Validating survivin as a cancer therapeutic target. Nat. Rev. Cancer 2003, 3, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.W.; Park, N.S.; Noh, M.H.; Shim, J.A.; Ahn, B.N.; Kim, Y.S.; Kim, D.; Lee, H.K.; Hur, D.Y. Combination treatment with erlotinib and ampelopsin overcomes erlotinib resistance in NSCLC cells via the Nox2-ROS-Bim pathway. Lung Cancer 2017, 106, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zhang, Q.; Ren, H.; Ma, S.; Lu, C.; Liu, B.; Liu, J.; Liang, J.; Li, M.; Zhu, R. Dihydromyricetin Enhances the Chemo-Sensitivity of Nedaplatin via Regulation of the p53/Bcl-2 Pathway in Hepatocellular Carcinoma Cells. PLoS ONE 2015, 10, e0124994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Sun, X.; Feng, Y.; Liu, X.; Zhou, L.; Sui, H.; Ji, Q.; Chen, J.; Wu, L.; Li, Q. Dihydromyricetin reverses MRP2-mediated MDR and enhances anticancer activity induced by oxaliplatin in colorectal cancer cells. Anti-Cancer Drugs 2017, 28, 281–288. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, X.; Feng, Y.; Wang, Y.; Zhang, L.; Wang, Y.; Fang, Z.; Azami, N.L.B.; Sun, M.; Li, Q. Dihydromyricetin reverses MRP2-induced multidrug resistance by preventing NF-κB-Nrf2 signaling in colorectal cancer cell. Phytomed. Int. J. Phytother. Phytopharm. 2021, 82, 153414. [Google Scholar] [CrossRef]
- Ruan, L.P.; Yu, B.Y.; Fu, G.M.; Zhu, D.N. Improving the solubility of ampelopsin by solid dispersions and inclusion complexes. J. Pharm. Biomed. Anal. 2005, 38, 457–464. [Google Scholar] [CrossRef]
- Chen, L.; Shi, M.; Lv, C.; Song, Y.; Wu, Y.; Liu, S.; Zheng, Z.; Lu, X.; Qin, S. Dihydromyricetin Acts as a Potential Redox Balance Mediator in Cancer Chemoprevention. Mediat. Inflamm. 2021, 2021, 6692579. [Google Scholar] [CrossRef]
- Xiang, D.; Wang, C.G.; Wang, W.Q.; Shi, C.Y.; Xiong, W.; Wang, M.D.; Fang, J.G. Gastrointestinal stability of dihydromyricetin, myricetin, and myricitrin: An in vitro investigation. Int. J. Food Sci. Nutr. 2017, 68, 704–711. [Google Scholar] [CrossRef]
- Abuhelwa, A.Y.; Foster, D.J.R.; Upton, R.N. A Quantitative Review and Meta-Models of the Variability and Factors Affecting Oral Drug Absorption-Part I: Gastrointestinal pH. AAPS J. 2016, 18, 1309–1321. [Google Scholar] [CrossRef]
- Tong, Q.; Hou, X.; Fang, J.; Wang, W.; Xiong, W.; Liu, X.; Xie, X.; Shi, C. Determination of dihydromyricetin in rat plasma by LC-MS/MS and its application to a pharmacokinetic study. J. Pharm. Biomed. Anal. 2015, 114, 455–461. [Google Scholar] [CrossRef]
- Xiang, D.; Fan, L.; Hou, X.L.; Xiong, W.; Shi, C.Y.; Wang, W.Q.; Fang, J.G. Uptake and Transport Mechanism of Dihydromyricetin Across Human Intestinal Caco-2 Cells. J. Food Sci. 2018, 83, 1941–1947. [Google Scholar] [CrossRef]
- Yun, C.W.; Lee, S.H. The Roles of Autophagy in Cancer. Int. J. Mol. Sci. 2018, 19, 3466. [Google Scholar] [CrossRef] [Green Version]
- Kenific, C.M.; Debnath, J. Cellular and metabolic functions for autophagy in cancer cells. Trends Cell Biol. 2015, 25, 37–45. [Google Scholar] [CrossRef] [Green Version]
- Dikic, I.; Elazar, Z. Mechanism and medical implications of mammalian autophagy. Nat. Rev. Mol. Cell Biol. 2018, 19, 349–364. [Google Scholar] [CrossRef]
- Chebil, L.; Humeau, C.; Falcimaigne, A.; Engasser, J.-M.; Ghoul, M. Enzymatic acylation of flavonoids. Process Biochem. 2006, 41, 2237–2251. [Google Scholar] [CrossRef]
- Li, W.; Wu, H.; Liu, B.; Hou, X.; Wan, D.; Lou, W.; Zhao, J. Highly efficient and regioselective synthesis of dihydromyricetin esters by immobilized lipase. J. Biotechnol. 2015, 199, 31–37. [Google Scholar] [CrossRef]
- Cao, S.L.; Deng, X.; Xu, P.; Huang, Z.X.; Zhou, J.; Li, X.H.; Zong, M.H.; Lou, W.Y. Highly Efficient Enzymatic Acylation of Dihydromyricetin by the Immobilized Lipase with Deep Eutectic Solvents as Cosolvent. J. Agric. Food Chem. 2017, 65, 2084–2088. [Google Scholar] [CrossRef]
- Guo, Q.Q.; Zeng, J.H.; Lu, Y.; Shu, X.G. Effects of Solubility, Thermal Stability and Antioxidant Properties of Acylating Dihydromyricetin. Adv. Mater. Res. 2013, 791–793, 101–105. [Google Scholar] [CrossRef]
- Plaza, M.; Pozzo, T.; Liu, J.; Gulshan Ara, K.Z.; Turner, C.; Nordberg Karlsson, E. Substituent effects on in vitro antioxidizing properties, stability, and solubility in flavonoids. J. Agric. Food Chem. 2014, 62, 3321–3333. [Google Scholar] [CrossRef]
- Lee, Y.S.; Woo, J.B.; Ryu, S.I.; Moon, S.K.; Han, N.S.; Lee, S.B. Glucosylation of flavonol and flavanones by Bacillus cyclodextrin glucosyltransferase to enhance their solubility and stability. Food Chem. 2017, 229, 75–83. [Google Scholar] [CrossRef]
- Woo, H.J.; Kang, H.K.; Nguyen, T.T.; Kim, G.E.; Kim, Y.M.; Park, J.S.; Kim, D.; Cha, J.; Moon, Y.H.; Nam, S.H.; et al. Synthesis and characterization of ampelopsin glucosides using dextransucrase from Leuconostoc mesenteroides B-1299CB4: Glucosylation enhancing physicochemical properties. Enzym. Microb. Technol. 2012, 51, 311–318. [Google Scholar] [CrossRef]
- Zhang, J.; Brodbelt, J.S.; Wang, J. Threshold dissociation and molecular modeling of transition metal complexes of flavonoids. J. Am. Soc. Mass Spectrom. 2005, 16, 139–151. [Google Scholar] [CrossRef] [Green Version]
- Samsonowicz, M.; Regulska, E. Spectroscopic study of molecular structure, antioxidant activity and biological effects of metal hydroxyflavonol complexes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 173, 757–771. [Google Scholar] [CrossRef]
- Guo, Q.; Yuan, J.; Zeng, J. Binding of dihydromyricetin and its metal ion complexes with bovine serum albumin. Biotechnol. Biotechnol. Equip. 2014, 28, 333–341. [Google Scholar] [CrossRef]
- Bilia, A.R.; Piazzini, V.; Guccione, C.; Risaliti, L.; Asprea, M.; Capecchi, G.; Bergonzi, M.C. Improving on Nature: The Role of Nanomedicine in the Development of Clinical Natural Drugs. Planta Med. 2017, 83, 366–381. [Google Scholar] [CrossRef] [Green Version]
- Ye, J.; Bao, S.; Zhao, S.; Zhu, Y.; Ren, Q.; Li, R.; Xu, X.; Zhang, Q. Self-Assembled Micelles Improve the Oral Bioavailability of Dihydromyricetin and Anti-Acute Alcoholism Activity. AAPS PharmSciTech 2021, 22, 111. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Zeng, D.; Chen, R.; Zafar, A.; Weng, L.; Wang, W.; Tian, Y.; Hasan, M.; Shu, X. PEGylated dihydromyricetin-loaded nanoliposomes coated with tea saponin inhibit bacterial oxidative respiration and energy metabolism. Food Funct. 2021, 12, 9007–9017. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.C.; Su, H.; Zheng, G.D.; Wang, W.J.; Yuan, E.; Zhang, Q.F. Fabrication and characterization of dihydromyricetin encapsulated zein-caseinate nanoparticles and its bioavailability in rat. Food Chem. 2020, 330, 127245. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Hu, Y.; Tiwari, J.K.; Velikov, K.P. Synthesis and characterisation of zein-curcumin colloidal particles. Soft Matter 2010, 6, 6192–6199. [Google Scholar] [CrossRef]
- Gascon, N.; Almansa, C.; Merlos, M.; Miguel Vela, J.; Encina, G.; Morte, A.; Smith, K.; Plata-Salamán, C. Co-crystal of tramadol-celecoxib: Preclinical and clinical evaluation of a novel analgesic. Expert Opin. Investig. Drugs 2019, 28, 399–409. [Google Scholar] [CrossRef]
- Shinozaki, T.; Ono, M.; Higashi, K.; Moribe, K. A Novel Drug-Drug Cocrystal of Levofloxacin and Metacetamol: Reduced Hygroscopicity and Improved Photostability of Levofloxacin. J. Pharm. Sci. 2019, 108, 2383–2390. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Zhang, M.; Zhang, Y.; Lou, B. A Drug-Drug Cocrystal of Dihydromyricetin and Pentoxifylline. J. Pharm. Sci. 2022, 111, 82–87. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, W.; Hu, Q.; Zhao, L.; Wei, Y.; Pi, C.; Yang, Y.; Yang, X.; Yuan, H.; Zhang, Y.; et al. Gastric floating sustained-release tablet for dihydromyricetin: Development, characterization, and pharmacokinetics study. Saudi Pharm. J. 2019, 27, 1000–1008. [Google Scholar] [CrossRef]
- Liu, B.; Tan, X.; Liang, J.; Wu, S.; Liu, J.; Zhang, Q.; Zhu, R. A reduction in reactive oxygen species contributes to dihydromyricetin-induced apoptosis in human hepatocellular carcinoma cells. Sci. Rep. 2014, 4, 7041. [Google Scholar] [CrossRef] [Green Version]
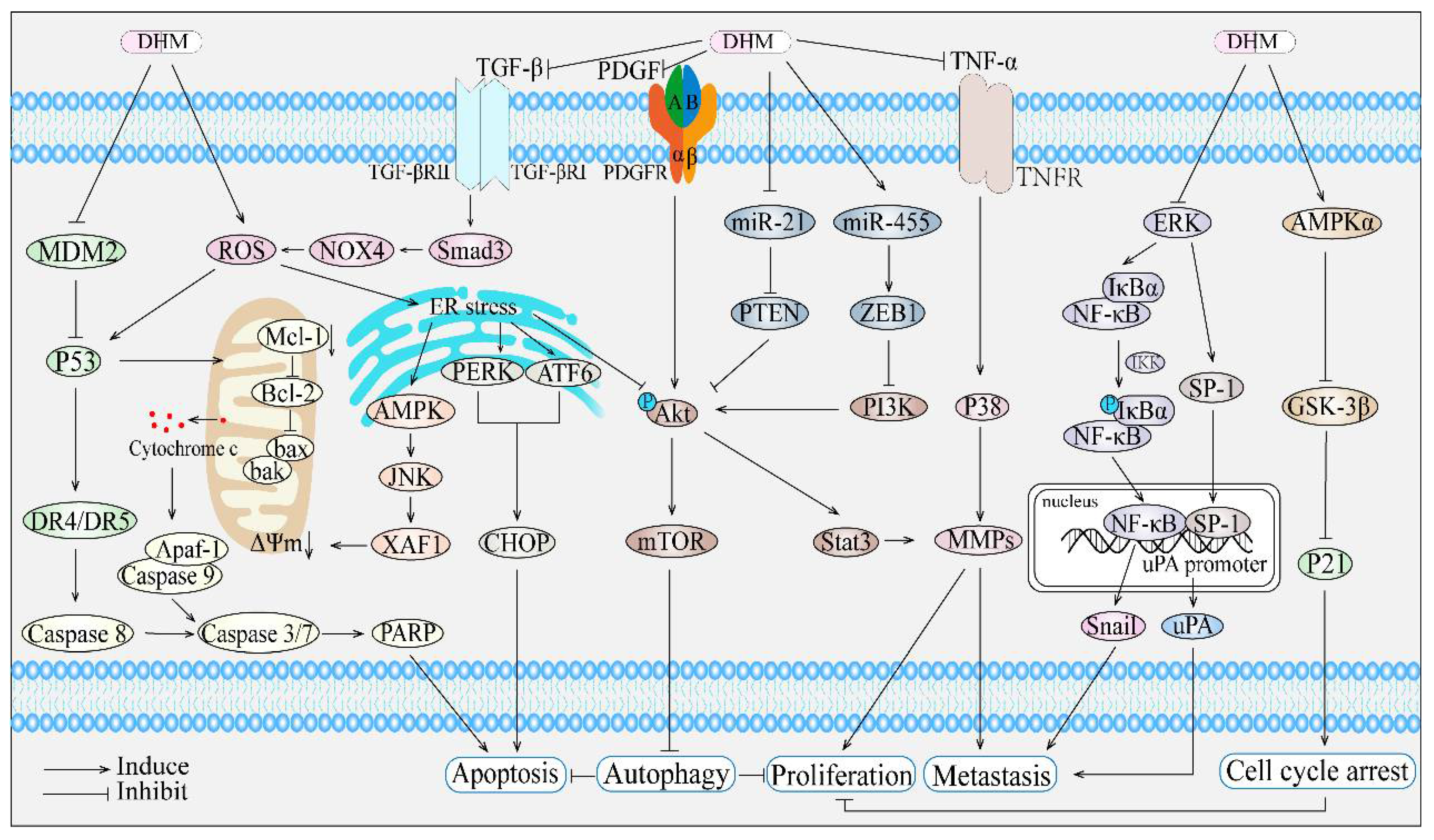
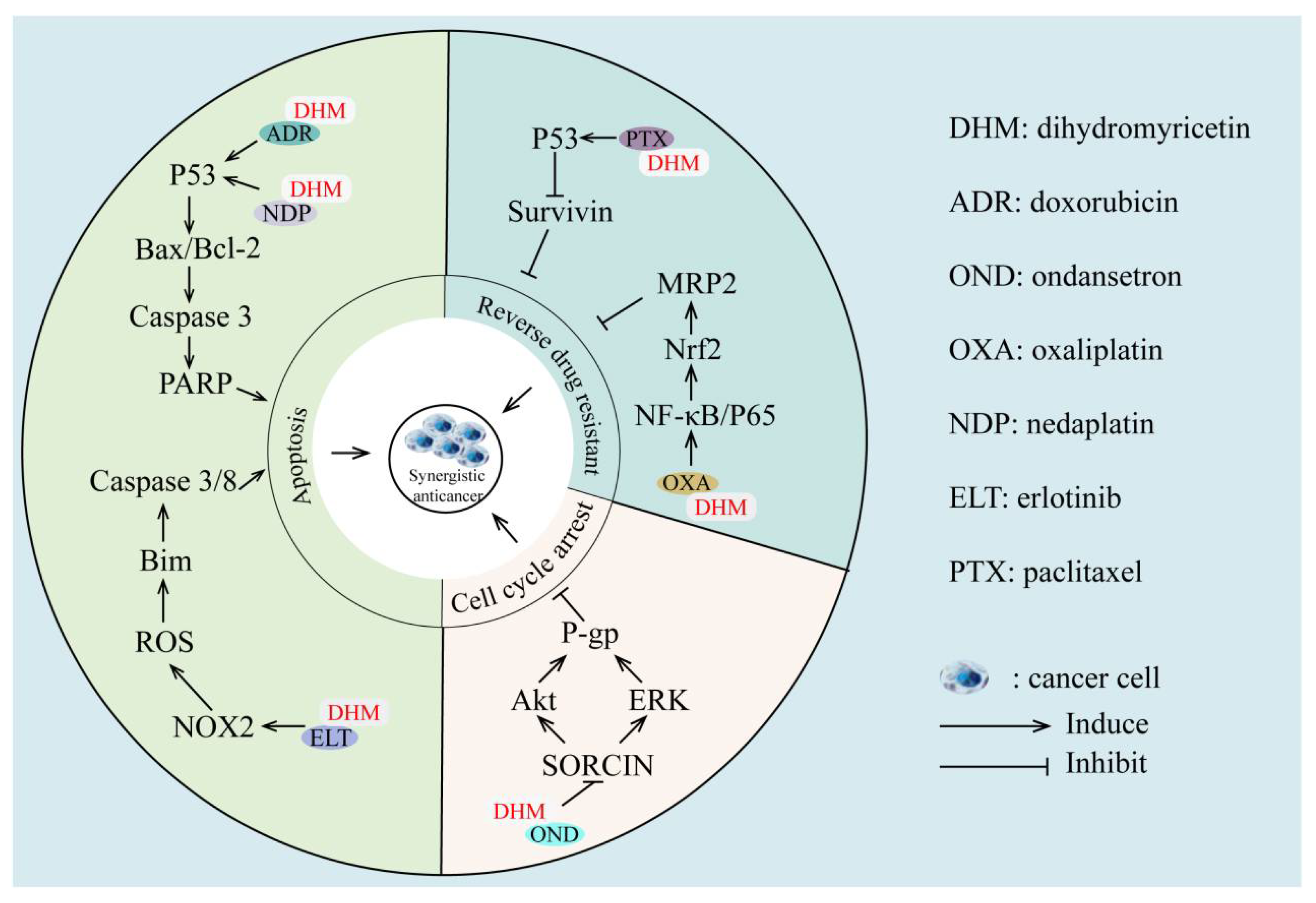
| Cancer Type | Cell Types (Animals) | Concentration of DHM | Upregulated Related Proteins | Downregulated Related Proteins | Effect of DHM | Ref |
|---|---|---|---|---|---|---|
| Lung cancer | A549, H1975 | 75 μM | Caspase-9/-7/-3; JNK1/2; ERK1/2 | PARP; Bcl-w | Apoptosis | [29] |
| 30 μM | XIAP; survivin; HDAC2; c-Myc; Skp2; FBW7α; FBW7γ; GSK-3β | Apoptosis proliferation inhibition | [35] | |||
| 10 μM | ERK1/2; Akt | Proliferation inhibition | [32] | |||
| Hepatocellular carcinoma | HepG2 | 50 μM | Beclin-1; LC3-II; PI3K; AMPK | p-ERK1/2; p-Akt; PDK1 | Invasion inhibition | [71] |
| 30 μM | Bax; caspase-3 | Bcl-2 | Apoptosis | [61] | ||
| 100 μg/mL | caspase-3/-9/-8; DR4; DR5; Bax; p53 | Bcl-2 | Apoptosis | [64] | ||
| HepG2, Hep3B | 200 μM | p-Chk1; p-Chk2; CDK1 | Cycle arrest | [7] | ||
| HepG2, QGY7701, Hepal-6 | 100 µM | p53; caspase-3 | Bcl-2 | Apoptosis | [62] | |
| QGY7701, HepG2 | 100 μM | Bax | Notch1; Hes1; Bcl 2 | Apoptosis | [67] | |
| Hepal-6 | 100 μM | TGF-β; TGF-βRII; Smad; p-Smad2/3; NOX4; ROS; ATP | Apoptosis | [70] | ||
| SK-Hep-1, MHCC97L | 50–100 μM | PKC-δ | MMP-9; P-ERK1/2; JNK | Invasion inhibition | [68] | |
| HepG2, HL7702 | 50 μM | caspase-9/-8/-3; HO-1; BAK | ROS; GSH; ATP; Bcl-2 | Apoptosis | [147] | |
| Cholangiocarcinoma | HCCC9810, TFK-1 | 156.8 µM | Caspase-3; Bad; PTEN | p-Akt; Bcl-2; MMP9; vimentin; miR-21 | Invasion inhibition | [83] |
| Colon cancer | Colo-205 (male Balb/c nude mice) | 64 Μm (100 mg/kg) | GSH; CAT; SOD; GPX; HO-1 | Sema4D; ROS; MDA; COX-2; iNOS | Proliferation inhibition | [94] |
| HCT-116, HCT-8, HT-29 | 100 μM | GRP78; CHOP; p-AMPK; XAF1 | p-p38; p-JNK; Bcl-2; Mcl-1 | Apoptosis | [87] | |
| Gastric cancer | AGS | 25–100 μM | p53 mRNA | Bcl-2 mRNA | Apoptosis | [74] |
| BGC-823 | 80 μg/mL | HMGB1 | Proliferation inhibition | [77] | ||
| Breast cancer | MCF-7, MDA-MB-231 | 80 μM | ROS; GRP78; p-PERK; CHOP | Apoptosis | [37] | |
| 60 μM | p-elF2α; cleaved ATF6α | p-Akt; p-mTOR; p-p70S6K | Autophagy | [42] | ||
| Human melanoma | SK-MEL-28 | 100 μM | Caspase-3; ROS; LC3; p62; Beclin-1 | Apoptosis | [97] | |
| 100 μM | p53; p21 | Cdc25A; Cdc2; P-Cdc2 | Cycle arrest | [96] | ||
| Human ovarian cancer | A2780 | 50 µM | E-cadherin; p65 | N-cadherin; vimentin; Snail | Invasion inhibition | [54] |
| SKOV3, A2780 | 120, 80 μM | caspase-3; Bax | Bcl-2; GRASP65 | Apoptosis | [55] | |
| Prostate cancer | LNCaP, PC-3 (male severe combined immune-deficient mice) | 25 µM, 60 µM (300 mg/kg) | CDK2; Cdc2; Bcl-2; CXCR4 | Invasion inhibition | [60] | |
| Choriocarcinoma | JAR | 100 mg/L | Smad3; p-Smad3; Smad4; cyclin A1; cyclinD1 | Proliferation inhibition | [58] | |
| 100 mg/L | caspase-3; Bax; Bcl-2 | Apoptosis | [59] | |||
| Osteosarcoma cells | MG63 | 30 µM | Bcl-2 | caspase-3/-9 | Apoptosis | [44] |
| U2OS, MG63, Saos2, HOS, 143B cells (athymic nude (nu/nu) mice) | 60 μM (300 mg/kg) | p21; AMPKα; p38MAPK; GSK-3β; JNK | Sox2 | Proliferation inhibition | [49] | |
| U-2OS, HOS | 100 μM | IκBα | SP-1; NF-κB; uPA, ERK2 | Invasion inhi-bition | [46] | |
| U2OS, MG63, HOS | 60 μM | p21; AMPKα | GSK-3β; Sox2 | Invasion inhi-bition | [48] | |
| Nasopharyngeal carcinoma | CNE-2 | 160 μg/mL | p-IKKβ; p-IKKα | Bcl-2; pro-caspase-3 | Apoptosis | [105] |
| HONE-1, NPC-BM, NPC-39 | 100 μM | ERK1/2; MMP-2 | Invasion inhibition | [106] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Xiao, Z.; Li, H.; Zhu, N.; Gu, J.; Wang, W.; Liu, C.; Wang, W.; Qin, L. Present Status, Challenges, and Prospects of Dihydromyricetin in the Battle against Cancer. Cancers 2022, 14, 3487. https://doi.org/10.3390/cancers14143487
Wu J, Xiao Z, Li H, Zhu N, Gu J, Wang W, Liu C, Wang W, Qin L. Present Status, Challenges, and Prospects of Dihydromyricetin in the Battle against Cancer. Cancers. 2022; 14(14):3487. https://doi.org/10.3390/cancers14143487
Chicago/Turabian StyleWu, Jiajun, Zuowei Xiao, Hongfang Li, Neng Zhu, Jia Gu, Wenmao Wang, Chao Liu, Wei Wang, and Li Qin. 2022. "Present Status, Challenges, and Prospects of Dihydromyricetin in the Battle against Cancer" Cancers 14, no. 14: 3487. https://doi.org/10.3390/cancers14143487
APA StyleWu, J., Xiao, Z., Li, H., Zhu, N., Gu, J., Wang, W., Liu, C., Wang, W., & Qin, L. (2022). Present Status, Challenges, and Prospects of Dihydromyricetin in the Battle against Cancer. Cancers, 14(14), 3487. https://doi.org/10.3390/cancers14143487






