History of Herbicide-Resistant Traits in Cotton in the U.S. and the Importance of Integrated Weed Management for Technology Stewardship
Abstract
:1. Economic Importance of Cotton to the U.S.
2. History of HR Traits in Cotton
2.1. BXN™ Cotton (Bromoxynil-Resistant Cotton)
2.2. Roundup Ready® Cotton (First-Generation GR Cotton)
2.3. Sulfonylurea-Resistant Cotton
2.4. LibertyLink® Cotton (Glufosinate-Resistant Cotton)
2.5. Roundup Ready® Flex Cotton (Second-Generation GR Cotton)
2.6. GlyTol® Cotton
2.7. GlyTol®-LibertyLink® Cotton
2.8. XtendFlex® Cotton (Dicamba-Resistant Cotton)
2.9. Enlist® Cotton (2,4-D-Resistant Cotton)
2.10. Isoxaflutole-Resistant Cotton
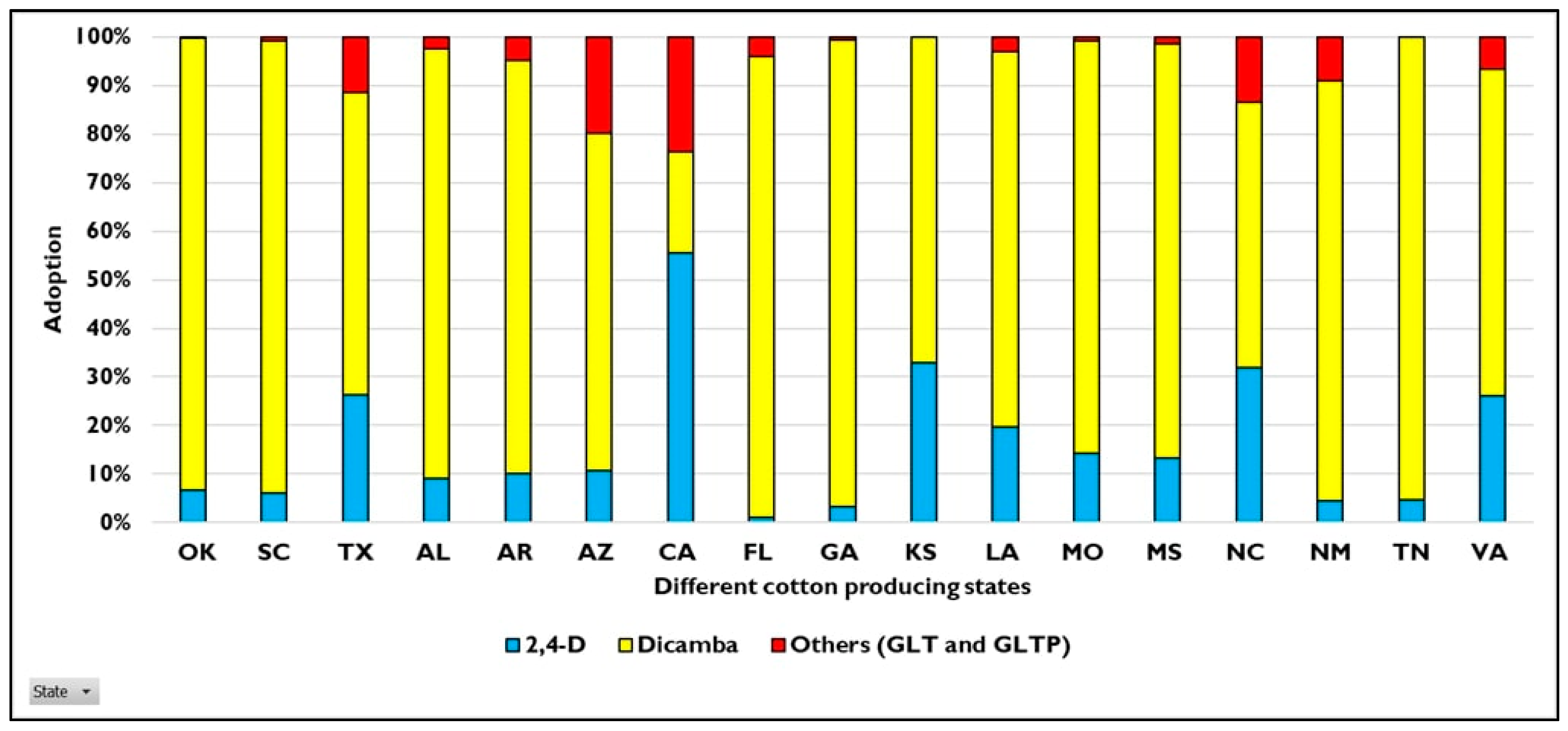
3. Benefits and Adoption of HR Cotton Traits 25 Years after Introduction
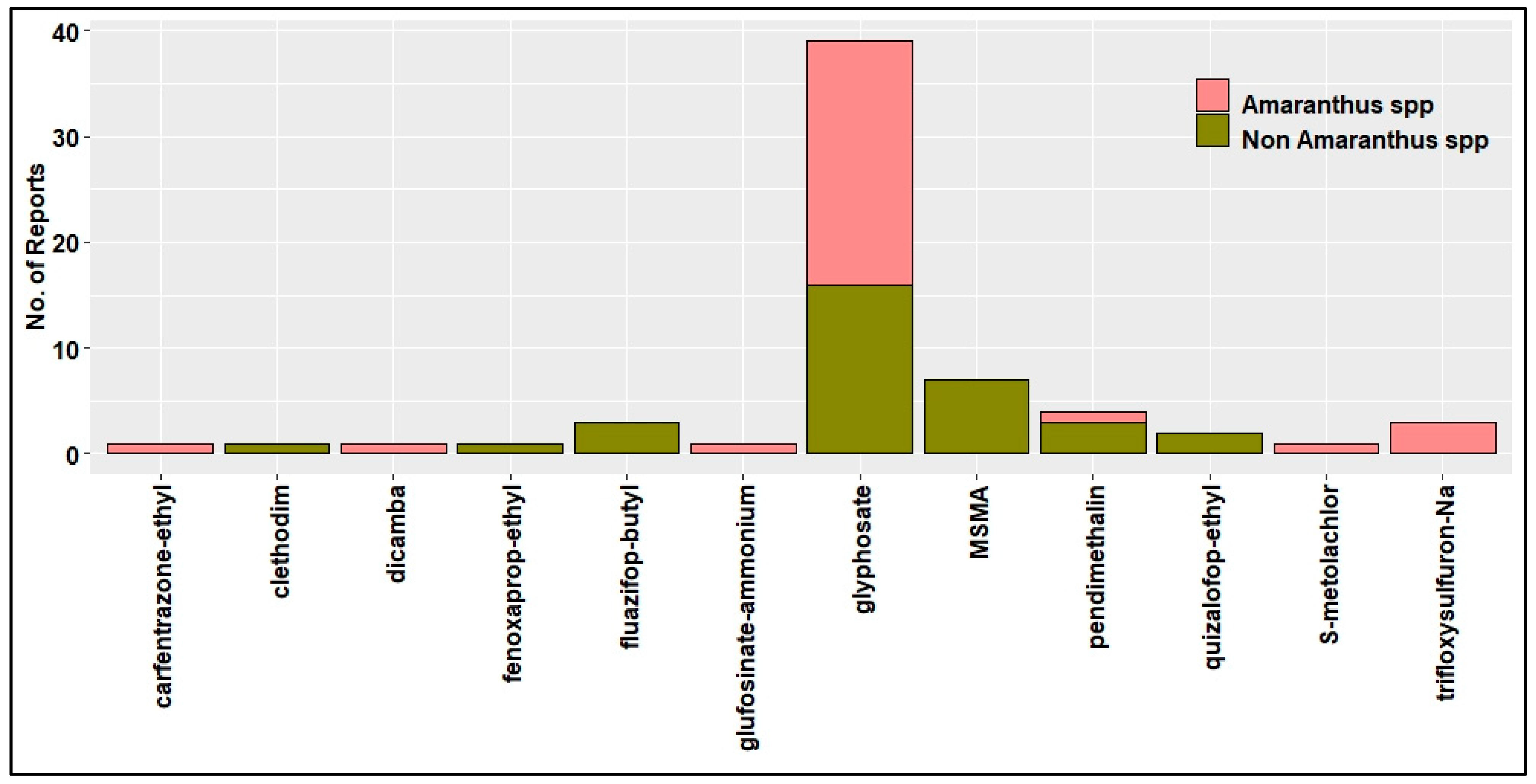
4. HR Weeds in U.S. Cotton Production
5. Non-Chemical Weed Control Options Available
5.1. Tillage Impacts on Weed Control
5.2. Cover Crops
5.3. Future Directions for Cover Cropping
5.4. Crop Rotation/Cropping Sequence Effects on Weed Population Dynamics
5.5. Crop Rotation Roadblocks
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Economic Research Service, U.S. Department of Agriculture. Overview of Cotton and Wool. Available online: https://www.ers.usda.gov/topics/crops/cotton-wool/ (accessed on 3 May 2021).
- Drouillard, J.; Blasi, D. Composition and Feeding Value of Cottonseed Feed Products for Beef Cattle; Kansas State University: Manhattan, KS, USA, 2002. [Google Scholar]
- TNAU Agritech Portal Organic Farming. Organic Farming: Organic Inputs and Techniques. Available online: http://agritech.tnau.ac.in/org_farm/orgfarm_manure.html (accessed on 30 April 2021).
- Foreign Agricultural Service, U.S. Department of Agriculture. Cotton: World Markets and Trade. Available online: https://apps.fas.usda.gov/psdonline/circulars/cotton.pdf (accessed on 21 April 2021).
- United States Department of Agriculture—Economics, Statistics and Market Information System. Crop Production Monthly Report; USDA: Washington, DC, USA, 2021.
- Economic Research Service, U.S. Department of Agriculture. Adoption of Genetically Engineered Crops in the U.S. Data Set. Available online: https://www.ers.usda.gov/data-products/adoption-of-genetically-engineered-crops-in-the-us/ (accessed on 7 May 2021).
- Animal and Plant Health Inspection Service, U.S. Department of Agriculture. Petitions for Determination of Nonregulated Status in Cotton. Available online: https://www.aphis.usda.gov/aphis/ourfocus/biotechnology/permits-notifications-petitions/petitions/petition-status (accessed on 30 April 2021).
- Martin, J.; Deceased, W.L.; Stamp, D.; Waldren, R. Principles of Field Crop Production, 4th ed.; Pearson: New York, NY, USA, 2006. [Google Scholar]
- Shaner, D.L. Herbicide Handbook; Weed Science Society of America: Westminster, CO, USA, 2014. [Google Scholar]
- Wilcut, J.W.; York, A.C.; Jordan, D.L.; Smith, A. Weed management systems for oil seed crops. In Handbook of Weed Management Systems; Marcel-Dekker: New York, NY, USA, 1995; pp. 343–400. [Google Scholar]
- Guthrie, D.S.; York, A.C. Cotton (Gossypium hirsutum) development and yield following fluometuron postemergence applied. Weed Technol. 1989, 3, 501–504. [Google Scholar] [CrossRef]
- Duke, S.O. Taking stock of herbicide-resistant crops ten years after introduction. Pest Manag. Sci. Former. Pestic. Sci. 2005, 61, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Duke, S.O.; Powles, S.B. Glyphosate: A once-in-a-century herbicide. Pest Manag. Sci. Former. Pestic. Sci. 2008, 64, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, D.I. Sustainable use of glyphosate in North American cropping systems. Pest Manag. Sci. Former. Pestic. Sci. 2008, 64, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Amrhein, N.; Deus, B.; Gehrke, P.; Steinrücken, H.C. The site of the inhibition of the shikimate pathway by glyphosate: II. Interference of glyphosate with chorismate formation in vivo and in vitro. Plant Physiol. 1980, 66, 830–834. [Google Scholar] [CrossRef] [Green Version]
- Green, J.M. The rise and future of glyphosate and glyphosate-resistant crops. Pest Manag. Sci. 2018, 74, 1035–1039. [Google Scholar] [CrossRef] [PubMed]
- Saroha, M.; Sridhar, P.; Malik, V. Glyphosate-tolerant crops: Genes and enzymes. J. Plant Biochem. Biotechnol. 1998, 7, 65–72. [Google Scholar] [CrossRef]
- Funke, T.; Han, H.; Healy-Fried, M.L.; Fischer, M.; Schönbrunn, E. Molecular basis for the herbicide resistance of Roundup Ready crops. Proc. Natl. Acad. Sci. USA 2006, 103, 13010–13015. [Google Scholar] [CrossRef] [Green Version]
- Widholm, J.M.; Chinnala, A.; Ryu, J.H.; Song, H.S.; Eggett, T.; Brotherton, J.E. Glyphosate selection of gene amplification in suspension cultures of 3 plant species. Physiol. Plant. 2001, 112, 540–545. [Google Scholar] [CrossRef]
- Lebrun, M.; Sailland, A.; Freyssinet, G.; DeGryse, E. Mutated 5-enolpyruvylshikimate-3-phosphate Synthase, Gene Coding for Said Protein and Transformed Plants Containing Said Gene. US Patent 6566587, 20 May 2003. [Google Scholar]
- Animal and Plant Health Inspection Service, U.S. Department of Agriculture. Petition for Determination of Nonregulated Status: Cotton with the Roundup Ready Gene, Lines 1445 and 1698; USDA: Washington, DC, USA, 1995.
- Chaleff, R.S.; Mauvais, C. Acetolactate synthase is the site of action of two sulfonylurea herbicides in higher plants. Science 1984, 224, 1443–1445. [Google Scholar] [CrossRef]
- Animal and Plant Health Inspection Service, U.S. Department of Agriculture. Petition for Determination of Non-Regulated Status: Sulfonylurea Resistant Cotton Line 19–51A; USDA: Washington, DC, USA, 1996.
- Heap, I. The International Survey of Herbicide Resistant Weeds. Available online: http://www.weedscience.org/ (accessed on 3 May 2021).
- Tachibana, K.; Kaneko, K. Development of a new herbicide, bialaphos. J. Pestic. Sci. 1986, 11, 297–304. [Google Scholar] [CrossRef] [Green Version]
- Dröge, W.; Broer, I.; Pühler, A. Transgenic plants containing the phosphinothricin-N-acetyltransferase gene metabolize the herbicide L-phosphinothricin (glufosinate) differently from untransformed plants. Planta 1992, 187, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Bellinder, R.R.; Hatzios, K.K.; Wilson, H.P. Mode of action investigations with the herbicides HOE-39866 and SC-0224. Weed Sci. 1985, 33, 779–785. [Google Scholar] [CrossRef]
- Takano, H.K.; Beffa, R.; Preston, C.; Westra, P.; Dayan, F.E. Reactive oxygen species trigger the fast action of glufosinate. Planta 2019, 249, 1837–1849. [Google Scholar] [CrossRef] [PubMed]
- Steckel, G.J.; Wax, L.M.; Simmons, F.W.; Phillips, W.H. Glufosinate efficacy on annual weeds is influenced by rate and growth stage. Weed Technol. 1997, 11, 484–488. [Google Scholar] [CrossRef]
- Culpepper, A.S.; Webster, T.M.; Sosnoskie, L.M.; York, A.C.; Nandula, V. Glyphosate-resistant Palmer amaranth in the United States. In Glyphosate Resistance in Crops and Weeds: History, Development, and Management; John Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 195–212. [Google Scholar]
- Coetzer, E.; Al-Khatib, K.; Peterson, D.E. Glufosinate efficacy on Amaranthus species in glufosinate-resistant soybean (Glycine max). Weed Technol. 2002, 16, 326–331. [Google Scholar] [CrossRef]
- Culpepper, A.S.; York, A.C.; Roberts, P.; Whitaker, J.R. Weed control and crop response to glufosinate applied to ‘PHY 485 WRF’cotton. Weed Technol. 2009, 23, 356–362. [Google Scholar] [CrossRef]
- Koger, C.H.; Burke, I.C.; Miller, D.K.; Kendig, J.A.; Reddy, K.N.; Wilcut, J.W. MSMA antagonizes glyphosate and glufosinate efficacy on broadleaf and grass weeds. Weed Technol. 2007, 21, 159–165. [Google Scholar] [CrossRef]
- Avila-Garcia, W.V.; Mallory-Smith, C. Glyphosate-resistant Italian ryegrass (Lolium perenne) populations also exhibit resistance to glufosinate. Weed Sci. 2011, 59, 305–309. [Google Scholar] [CrossRef] [Green Version]
- Brosnan, J.T.; Vargas, J.J.; Spesard, B.; Netzband, D.; Zobel, J.M.; Chen, J.; Patterson, E.L. Annual bluegrass (Poa annua) resistance to indaziflam applied early-postemergence. Pest Manag. Sci. 2020, 76, 2049–2057. [Google Scholar] [CrossRef] [PubMed]
- Barber, T.; Norsworthy, J.; Butts, T. Arkansas Palmer Amaranth Found Resistant to Field Rates of Glufosinate; University of Arkansas System: Fayetteville, NC, USA, 2021. [Google Scholar]
- Light, G.G.; Baughman, T.A.; Dotray, P.A.; Keeling, J.W.; Wester, D.B. Yield of glyphosate-tolerant cotton as affected by topical glyphosate applications on the Texas high plains and rolling plains. J. Cotton Sci. 2003, 7, 231–235. [Google Scholar]
- Chen, Y.C.S.; Hubmeier, C.; Tran, M.; Martens, A.; Cerny, R.E.; Sammons, R.D.; CaJacob, C. Expression of CP4 EPSPS in microspores and tapetum cells of cotton (Gossypium hirsutum) is critical for male reproductive development in response to late-stage glyphosate applications. Plant Biotechnol. J. 2006, 4, 477–487. [Google Scholar] [CrossRef]
- Main, C.L.; Jones, M.A.; Murdock, E.C. Weed response and tolerance of enhanced glyphosate-resistant cotton to glyphosate. J. Cotton Sci. 2007, 11, 104–109. [Google Scholar]
- Green, J.M. Evolution of glyphosate-resistant crop technology. Weed Sci. 2009, 57, 108–117. [Google Scholar] [CrossRef]
- Cabrera-Ponce, J.L.; Valencia-Lozano, E.; Trejo-Saavedra, D.L. Genetic modifications of Corn. In Corn; Elsevier: Amsterdam, The Netherlands, 2019; pp. 43–85. [Google Scholar]
- Burns, J. Petition for the Determination of Non-Regulated Status for Roundup Ready® Flex Cotton MON 88913; Report No. Petition; Monsanto: St. Louis, MO, USA, 2004. [Google Scholar]
- Reed, J.D.; Keeling, J.W.; Dotray, P.A. Palmer amaranth (Amaranthus palmeri) management in GlyTol® LibertyLink® cotton. Weed Technol. 2014, 28, 592–600. [Google Scholar] [CrossRef]
- Institute of Agriculture and Natural Resources, CropWatch. UNL Advances Dicamba-Resistance Research; Work Featured Internationally. Available online: https://cropwatch.unl.edu/unl-advances-dicamba-resistance-research-work-featured-internationally (accessed on 28 May 2021).
- Behrens, M.R.; Mutlu, N.; Chakraborty, S.; Dumitru, R.; Jiang, W.Z.; LaVallee, B.J.; Herman, P.L.; Clemente, T.E.; Weeks, D.P. Dicamba resistance: Enlarging and preserving biotechnology-based weed management strategies. Science 2007, 316, 1185–1188. [Google Scholar] [CrossRef] [Green Version]
- Bunch, T.; Gervais, J.; Buhl, K.; Stone, D. Dicamba Technical Fact Sheet. National Pesticide Information Center, Oregon State University Extension Services. 2012. Available online: http://npic.orst.edu/factsheets/dicambatech.pdf (accessed on 3 May 2021).
- Sciumbato, A.S.; Chandler, J.M.; Senseman, S.A.; Bovey, R.W.; Smith, K.L. Determining Exposure to Auxin-Like Herbicides. I. Quantifying Injury to Cotton and Soybean1. Weed Technol. 2004, 18, 1125–1134. [Google Scholar] [CrossRef]
- Strachan, S.D.; Casini, M.S.; Heldreth, K.M.; Scocas, J.A.; Nissen, S.J.; Bukun, B.; Lindenmayer, R.B.; Shaner, D.L.; Westra, P.; Brunk, G. Vapor movement of synthetic auxin herbicides: Aminocyclopyrachlor, aminocyclopyrachlor-methyl ester, dicamba, and aminopyralid. Weed Sci. 2010, 58, 103–108. [Google Scholar] [CrossRef]
- Malven, M.; Arackal, S.; Comstock, B.; Chandu, D.; Deffenbaugh, A.; Eskelsen, S.; Howard, D.; Malven, M.; Soteres, J. Petition for the Determination of Nonregulated Status for Dicamba and Glufosinate-Tolerant Cotton MON 88701; Monsanto: St. Louis, MO, USA, 2015. [Google Scholar]
- Randell, T.M.; Hand, L.C.; Vance, J.C.; Culpepper, A.S. Interval between sequential glufosinate applications influences weed control in cotton. Weed Technol. 2020, 34, 528–533. [Google Scholar] [CrossRef]
- Raper, T.B.; Butler, S.A.; Denton, S.; Steckel, L.E.; Hayes, R.M. LibertyLink®, WideStrike® and XtendFlex® Tolerance to Late Postemergence Applications of Glufosinate and S-Metolachlor. J. Cotton Sci. 2019, 23, 262–269. [Google Scholar]
- Underwood, M.G.; Soltani, N.; Hooker, D.C.; Robinson, D.E.; Vink, J.P.; Swanton, C.J.; Sikkema, P.H. The addition of dicamba to POST applications of quizalofop-p-ethyl or clethodim antagonizes volunteer glyphosate-resistant corn control in dicamba-resistant soybean. Weed Technol. 2015, 30, 639–647. [Google Scholar] [CrossRef]
- Mueller, T.C.; Steckel, L.E. Dicamba volatility in humidomes as affected by temperature and herbicide treatment. Weed Technol. 2019, 33, 541–546. [Google Scholar] [CrossRef] [Green Version]
- Timmons, F. A history of weed control in the United States and Canada. Weed Sci. 1970, 18, 294–307. [Google Scholar] [CrossRef]
- Bayley, C.; Trolinder, N.; Ray, C.; Morgan, M.; Quisenberry, J.; Ow, D. Engineering 2,4-D resistance into cotton. Theor. Appl. Genet. 1992, 83, 645–649. [Google Scholar] [CrossRef]
- Wright, T.R.; Shan, G.; Walsh, T.A.; Lira, J.M.; Cui, C.; Song, P.; Zhuang, M.; Arnold, N.L.; Lin, G.; Yau, K. Robust crop resistance to broadleaf and grass herbicides provided by aryloxyalkanoate dioxygenase transgenes. Proc. Natl. Acad. Sci. USA 2010, 107, 20240–20245. [Google Scholar] [CrossRef] [Green Version]
- Animal and Plant Health Inspection Service, U.S. Department of Agriculture. Petition for Determination of Nonregulated Status for Herbicide Tolerant DAS-8191Ø-7 Cotton; USDA: Washington, DC, USA, 2015.
- Meyer, C.J.; Norsworthy, J.K. Influence of weed size on herbicide interactions for Enlist™ and Roundup Ready® Xtend® technologies. Weed Technol. 2019, 33, 569–577. [Google Scholar] [CrossRef] [Green Version]
- Perotti, V.E.; Larran, A.S.; Palmieri, V.E.; Martinatto, A.K.; Permingeat, H.R. Herbicide resistant weeds: A call to integrate conventional agricultural practices, molecular biology knowledge and new technologies. Plant Sci. 2020, 290, 110255. [Google Scholar] [CrossRef]
- Animal and Plant Health Inspection Service, U.S. Department of Agriculture. Petition for a Determination of Nonregulated Status for Herbicide Tolerant Cotton Transformation Event GHB811; USDA: Washington, DC, USA, 2018.
- Viviani, F.; Little, J.; Pallett, K. The mode of action of isoxaflutole II. Characterization of the inhibition of carrot 4-hydroxyphenylpyruvate dioxygenase by the diketonitrile derivative of isoxaflutole. Pestic. Biochem. Physiol. 1998, 62, 125–134. [Google Scholar] [CrossRef]
- Pallett, K.; Little, J.; Sheekey, M.; Veerasekaran, P. The mode of action of isoxaflutole: I. Physiological effects, metabolism, and selectivity. Pestic. Biochem. Physiol. 1998, 62, 113–124. [Google Scholar] [CrossRef]
- Rice, P.J.; Koskinen, W.C.; Carrizosa, M.J. Effect of soil properties on the degradation of isoxaflutole and the sorption− desorption of isoxaflutole and its diketonitrile degradate. J. Agric. Food Chem. 2004, 52, 7621–7627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beltrán, E.; Fenet, H.; Cooper, J.-F.; Coste, C.-M. Fate of isoxaflutole in soil under controlled conditions. J. Agric. Food Chem. 2003, 51, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, D.J.; Jordan, D.L.; Roma-Burgos, N.; Jennings, K.M.; Leon, R.G.; Vann, M.C.; Everman, W.J.; Cahoon, C.W. Susceptibility of Palmer amaranth (Amaranthus palmeri) to herbicides in accessions collected from the North Carolina Coastal Plain. Weed Sci. 2020, 68, 582–593. [Google Scholar] [CrossRef]
- Garetson, R.; Singh, V.; Singh, S.; Dotray, P.; Bagavathiannan, M. Distribution of herbicide-resistant Palmer amaranth (Amaranthus palmeri) in row crop production systems in Texas. Weed Technol. 2019, 33, 355–365. [Google Scholar] [CrossRef]
- Singh, V.; Garetson, R.; McGinty, J.; Dotray, P.; Morgan, G.; Nolte, S.; Bagavathiannan, M. Distribution of herbicide-resistant waterhemp (Amaranthus tuberculatus) across row crop production systems in Texas. Weed Technol. 2020, 34, 129–139. [Google Scholar] [CrossRef]
- International, C. Database of the Safety and Benefits of Biotechnology. Available online: http://biotechbenefits.croplife.org/ (accessed on 31 May 2021).
- Green, J.M. The benefits of herbicide-resistant crops. Pest Manag. Sci. 2012, 68, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Busi, R.; Powles, S.B.; Beckie, H.J.; Renton, M. Rotations and mixtures of soil-applied herbicides delay resistance. Pest Manag. Sci. 2020, 76, 487–496. [Google Scholar] [CrossRef] [PubMed]
- International Service for the Acquisition of Agri-biotech Applications. Global Status of Commercialized Biotech/GM Crops in 2017: Biotech Crop Adoption Surges as Economic Benefits Accumulate in 22 Years; International Service for the Acquisition of Agri-Biotech Applications: Ithaca, NY, USA, 2017. [Google Scholar]
- Brookes, G.; Barfoot, P. Farm income and production impacts of using GM crop technology 1996–2016. GM Crop. Food 2018, 9, 59–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brookes, G.; Barfoot, P. GM crop technology use 1996–2018: Farm income and production impacts. GM Crop. Food 2020, 11, 242–261. [Google Scholar] [CrossRef] [PubMed]
- Kniss, A. Have Genetically Engineered Herbicide-Resistant Crops Increased or Decreased Herbicide Use? Available online: https://plantoutofplace.com/2018/12/have-genetically-engineered-herbicide-resistant-crops-increased-or-decreased-herbicide-use/ (accessed on 1 June 2021).
- Brookes, G.; Barfoot, P. Environmental impacts of genetically modified (GM) crop use 1996–2015: Impacts on pesticide use and carbon emissions. GM Crop. Food 2017, 8, 117–147. [Google Scholar] [CrossRef] [Green Version]
- United States Department of Agriculture—Agricultural Marketing Service—Cotton and Tobacco Program. Cotton Varieties Planted 2020 Crop; United States Department of Agriculture: Memphis, TA, USA, 2020.
- United States Department of Agriculture—Agricultural Marketing Service. Cotton and Tobacco Program. In Cotton Varieties Planted 2019 Crop; United States Department of Agriculture: Memphis, TA, USA, 2019. [Google Scholar]
- National Agricultural Statistics Service (NASS), Agricultural Statistics Board, United States Department of Agriculture (USDA). Acreage-Cotton Area Planted and Harvested by Type—States and United States: 2019 and 2020; USDA: Washington, DC, USA, 2020.
- Peterson, M.A.; Collavo, A.; Ovejero, R.; Shivrain, V.; Walsh, M.J. The challenge of herbicide resistance around the world: A current summary. Pest Manag. Sci. 2018, 74, 2246–2259. [Google Scholar] [CrossRef]
- Norsworthy, J.K.; McClelland, M.; Griffith, G.M. Conyza canadensis (L.) Cronquist response to pre-plant application of residual herbicides in cotton (Gossypium hirsutum L.). Crop Prot. 2009, 28, 62–67. [Google Scholar] [CrossRef]
- Salas, R.A.; Burgos, N.R.; Tranel, P.J.; Singh, S.; Glasgow, L.; Scott, R.C.; Nichols, R.L. Resistance to PPO-inhibiting herbicide in Palmer amaranth from Arkansas. Pest Manag. Sci. 2016, 72, 864–869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tehranchian, P.; Norsworthy, J.K.; Powles, S.; Bararpour, M.T.; Bagavathiannan, M.V.; Barber, T.; Scott, R.C. Recurrent sublethal-dose selection for reduced susceptibility of Palmer amaranth (Amaranthus palmeri) to dicamba. Weed Sci. 2017, 65, 206–212. [Google Scholar] [CrossRef]
- Peterson, D.; Jugulam, M.; Shyam, C.; Borgato, E. Palmer Amaranth Resistance to 2,4-D and Dicamba Confirmed in Kansas. Available online: https://webapp.agron.ksu.edu/agr_social/m_eu_article.throck?article_id=2110&eu_id=322 (accessed on 15 December 2021).
- Steckel, L. Dicamba-Resistant Palmer Amaranth in Tennessee: Stewardship Even More Important. Available online: https://news.utcrops.com/2020/07/dicamba-resistant-palmer-amaranth-in-tennessee-stewardship-even-more-important/ (accessed on 21 December 2021).
- Norsworthy, J.K.; Griffith, G.; Griffin, T.; Bagavathiannan, M.; Gbur, E.E. In-field movement of glyphosate-resistant Palmer amaranth (Amaranthus palmeri) and its impact on cotton lint yield: Evidence supporting a zero-threshold strategy. Weed Sci. 2014, 62, 237–249. [Google Scholar] [CrossRef]
- Larran, A.S.; Palmieri, V.E.; Perotti, V.E.; Lieber, L.; Tuesca, D.; Permingeat, H.R. Target-site resistance to acetolactate synthase (ALS)-inhibiting herbicides in Amaranthus palmeri from Argentina. Pest Manag. Sci. 2017, 73, 2578–2584. [Google Scholar] [CrossRef] [Green Version]
- Sosnoskie, L.M.; Webster, T.M.; Kichler, J.M.; MacRae, A.W.; Grey, T.L.; Culpepper, A.S. Pollen-mediated dispersal of glyphosate-resistance in Palmer amaranth under field conditions. Weed Sci. 2012, 60, 366–373. [Google Scholar] [CrossRef]
- Ganie, Z.A.; Jhala, A.J. Interaction of 2, 4-D or dicamba with glufosinate for control of glyphosate-resistant giant ragweed (Ambrosia trifida L.) in glufosinate-resistant maize (Zea mays L.). Front. Plant Sci. 2017, 8, 1207. [Google Scholar] [CrossRef] [PubMed]
- Barnett, K.A.; Mueller, T.C.; Steckel, L.E. Glyphosate-resistant giant ragweed (Ambrosia trifida) control with glufosinate or fomsafen combined with growth regulator herbicides. Weed Technol. 2013, 27, 454–458. [Google Scholar] [CrossRef]
- Chepil, W. Germination of weed seeds: II. The influence of tillage treatments on germination. Sci. Agric. 1946, 26, 347–357. [Google Scholar]
- Roberts, H. Emergence and longevity in cultivated soil of seeds of some annual weeds. Weed Res. 1964, 4, 296–307. [Google Scholar] [CrossRef]
- Boyer, C.N.; Lambert, D.M.; Larson, J.A.; Tyler, D.D. Investment analysis of cover crop and no-tillage systems on Tennessee cotton. Agron. J. 2018, 110, 331–338. [Google Scholar] [CrossRef]
- Young, M.; Foster, J.; McGinty, J.; Klose, S.; Maeda, A. No-Till Farming Practices Offer Cost Savings and More Profit Potential to Cotton and Grain Sorghum Producers; Department of Agricultural Economics Texas A&M AgriLife Extension Service: Amarillo, TX, USA, 2018. [Google Scholar]
- Refsell, D.; Hartzler, R. Effect of tillage on common waterhemp (Amaranthus rudis) emergence and vertical distribution of seed in the soil. Weed Technol. 2009, 23, 129–133. [Google Scholar] [CrossRef]
- Swanton, C.J.; Shrestha, A.; Roy, R.C.; Ball-Coelho, B.R.; Knezevic, S.Z. Effect of tillage systems, N, and cover crop on the composition of weed flora. Weed Sci. 1999, 47, 454–461. [Google Scholar] [CrossRef]
- Moyer, J.; Roman, E.; Lindwall, C.; Blackshaw, R. Weed management in conservation tillage systems for wheat production in North and South America. Crop Prot. 1994, 13, 243–259. [Google Scholar] [CrossRef]
- Tuesca, D.; Puricelli, E.; Papa, J. A long-term study of weed flora shifts in different tillage systems. Weed Res. 2001, 41, 369–382. [Google Scholar] [CrossRef]
- Barberi, P.; Lo Cascio, B. Long-term tillage and crop rotation effects on weed seedbank size and composition. Weed Res. 2001, 41, 325–340. [Google Scholar] [CrossRef]
- Highlights, United States Department of Agriculture—Census of Agriculture. Conservation; 2012. Available online: https://www.nass.usda.gov/Publications/Highlights/2014/Highlights_Conservation.pdf (accessed on 10 December 2021).
- Farmer, J.A.; Bradley, K.W.; Young, B.G.; Steckel, L.E.; Johnson, W.G.; Norsworthy, J.K.; Davis, V.M.; Loux, M.M. Influence of tillage method on management of Amaranthus species in soybean. Weed Technol. 2017, 31, 10–20. [Google Scholar] [CrossRef] [Green Version]
- Young, F.; Thorne, M. Weed-species dynamics and management in no-till and reduced-till fallow cropping systems for the semi-arid agricultural region of the Pacific Northwest, USA. Crop Prot. 2004, 23, 1097–1110. [Google Scholar] [CrossRef]
- Govindasamy, P.; Sarangi, D.; Provin, T.; Hons, F.; Bagavathiannan, M. Thirty-six years of no-tillage regime altered weed population dynamics in soybean. Agron. J. 2021, 113, 2926–2937. [Google Scholar] [CrossRef]
- Govindasamy, P.; Sarangi, D.; Provin, T.; Hons, F.; Bagavathiannan, M. No-tillage altered weed species dynamics in a long-term (36-year) grain sorghum experiment in southeast Texas. Weed Sci. 2020, 68, 476–484. [Google Scholar] [CrossRef]
- Steckel, L.E.; Sprague, C.L.; Stoller, E.W.; Wax, L.M.; Simmons, F.W. Tillage, cropping system, and soil depth effects on common waterhemp (Amaranthus rudis) seed-bank persistence. Weed Sci. 2007, 55, 235–239. [Google Scholar] [CrossRef]
- Bajwa, A.A.; Mahajan, G.; Chauhan, B.S. Nonconventional weed management strategies for modern agriculture. Weed Sci. 2015, 63, 723–747. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Antle, J.; Garrett, K.A.; Izaurralde, R.C.; Mader, T.; Marshall, E.; Nearing, M.; Robertson, G.P.; Ziska, L. Indicators of climate change in agricultural systems. Clim. Change 2020, 163, 1719–1732. [Google Scholar] [CrossRef] [Green Version]
- Sustainable Agriculture Research and Education. National Cover Crop Survey—Annual Report of 2019–2020; Sustainable Agriculture Research and Education: College Park, MD, USA, 2020. [Google Scholar]
- Bagavathiannan, M.V.; Norsworthy, J.K. Late-season seed production in arable weed communities: Management implications. Weed Sci. 2012, 60, 325–334. [Google Scholar] [CrossRef]
- Wayman, S.; Cogger, C.; Benedict, C.; Collins, D.; Burke, I.; Bary, A. Cover crop effects on light, nitrogen, and weeds in organic reduced tillage. Agroecol. Sustain. Food Syst. 2015, 39, 647–665. [Google Scholar] [CrossRef]
- Osipitan, O.A.; Dille, J.A.; Assefa, Y.; Knezevic, S.Z. Cover crop for early season weed suppression in crops: Systematic review and meta-analysis. Agron. J. 2018, 110, 2211–2221. [Google Scholar] [CrossRef] [Green Version]
- Pullaro, T.C.; Marino, P.C.; Jackson, D.M.; Harrison, H.F.; Keinath, A.P. Effects of killed cover crop mulch on weeds, weed seeds, and herbivores. Agric. Ecosyst. Environ. 2006, 115, 97–104. [Google Scholar] [CrossRef]
- Lemessa, F.; Wakjira, M. Cover crops as a means of ecological weed management in agroecosystems. J. Crop Sci. Biotechnol. 2015, 18, 123–135. [Google Scholar] [CrossRef]
- Burgos, N.R.; Talbert, R.E.; Mattice, J.D. Cultivar and age differences in the production of allelochemicals by Secale cereale. Weed Sci. 1999, 47, 481–485. [Google Scholar] [CrossRef]
- Creamer, N.G.; Bennett, M.A.; Stinner, B.R. Evaluation of cover crop mixtures for use in vegetable production systems. HortScience 1997, 32, 866–870. [Google Scholar] [CrossRef]
- Al-Khatib, K.; Libbey, C.; Boydston, R. Weed suppression with Brassica green manure crops in green pea. Weed Sci. 1997, 45, 439–445. [Google Scholar] [CrossRef]
- Korres, N.E.; Norsworthy, J.K. Influence of a rye cover crop on the critical period for weed control in cotton. Weed Sci. 2015, 63, 346–352. [Google Scholar] [CrossRef]
- Sainju, U.M.; Whitehead, W.F.; Singh, B.P. Biculture legume–cereal cover crops for enhanced biomass yield and carbon and nitrogen. Agron. J. 2005, 97, 1403–1412. [Google Scholar] [CrossRef] [Green Version]
- Rochester, I.; Peoples, M.; Hulugalle, N.; Gault, R.; Constable, G. Using legumes to enhance nitrogen fertility and improve soil condition in cotton cropping systems. Field Crop. Res. 2001, 70, 27–41. [Google Scholar] [CrossRef]
- Touchton, J.; Rickerl, D.; Walker, R.; Snipes, C. Winter legumes as a nitrogen source for no-tillage cotton. Soil Tillage Res. 1984, 4, 391–401. [Google Scholar] [CrossRef]
- Meisinger, J.; Hargrove, W.; Mikkelsen, R.; Williams, J.; Benson, V. Effects of cover crops on groundwater quality. Cover Crop. Clean Water 1991, 57–68. [Google Scholar]
- Norsworthy, J.K.; McClelland, M.; Griffith, G.; Bangarwa, S.K.; Still, J. Evaluation of cereal and Brassicaceae cover crops in conservation-tillage, enhanced, glyphosate-resistant cotton. Weed Technol. 2011, 25, 6–13. [Google Scholar] [CrossRef]
- Norsworthy, J.K.; Meehan, J.T., IV. Use of isothiocyanates for suppression of Palmer amaranth (Amaranthus palmeri), pitted morningglory (Ipomoea lacunosa), and yellow nutsedge (Cyperus esculentus). Weed Sci. 2005, 53, 884–890. [Google Scholar] [CrossRef]
- Price, A.J.; Kelton, J.; Mosjidis, J.A. Utilization of Sunn Hemp for Cover Crops and Weed Control in Temperate Climates; United States Department of Agriculture and Auburn University: Auburn, AL, USA, 2012.
- Atwell, R.A.; Reberg-Horton, S.C.; Edmisten, K.L.; York, A.C. Utilizing cover crop mulches for weed control in conventional and organic cotton production. Presented at the 2016 Annual Meeting Abstracts, ASA/CSSA/SSSA, Phoenix, AZ, USA, 6–9 November 2016. [Google Scholar]
- Palhano, M.G.; Norsworthy, J.K.; Barber, T. Cover crops suppression of Palmer amaranth (Amaranthus palmeri) in cotton. Weed Technol. 2018, 32, 60–65. [Google Scholar] [CrossRef]
- Palhano, M.G.; Norsworthy, J.K.; Barber, T. Evaluation of chemical termination options for cover crops. Weed Technol. 2018, 32, 227–235. [Google Scholar] [CrossRef]
- Davis, S.; Raper, T.B.; Shekoofa, A.; Stewart, S. Impact of Cover Crop Termination Timing and Method on Cotton Production Systems. In Proceedings of the 2019 ASA, CSSA and SSSA International Annual Meetings, San Antonio, TX, USA, 10–13 November 2019. [Google Scholar]
- Wiggins, M.S.; Hayes, R.M.; Steckel, L.E. Evaluating cover crops and herbicides for glyphosate-resistant Palmer amaranth (Amaranthus palmeri) control in cotton. Weed Technol. 2016, 30, 415–422. [Google Scholar] [CrossRef]
- Balkcom, K.S.; Duzy, L.M.; Kornecki, T.S.; Price, A.J. Timing of cover crop termination: Management considerations for the Southeast. Crop Forage Turfgrass Manag. 2015, 1, 1–7. [Google Scholar] [CrossRef]
- Ball, D.A. Weed seedbank response to tillage, herbicides, and crop rotation sequence. Weed Sci. 1992, 40, 654–659. [Google Scholar] [CrossRef]
- Martin, R.; Felton, W. Effect of crop rotation, tillage practice, and herbicides on the population dynamics of wild oats in wheat. Aust. J. Exp. Agric. 1993, 33, 159–165. [Google Scholar] [CrossRef]
- Liebman, M.; Dyck, E. Crop rotation and intercropping strategies for weed management. Ecol. Appl. 1993, 3, 92–122. [Google Scholar] [CrossRef]
- Schreiber, M.M. Influence of tillage, crop rotation, and weed management on giant foxtail (Setaria faberi) population dynamics and corn yield. Weed Sci. 1992, 40, 645–653. [Google Scholar] [CrossRef]
- Beckie, H.J. Herbicide-resistant weeds: Management tactics and practices. Weed Technol. 2006, 20, 793–814. [Google Scholar] [CrossRef]
- Beckie, H.; Hall, L.; Tardif, F.; Seguin-Swartz, G. Acetolactate synthase inhibitor-resistant stinkweed (Thlaspi arvense L.) in Alberta. Can. J. Plant Sci. 2007, 87, 965–972. [Google Scholar] [CrossRef] [Green Version]
- Aulakh, J.S.; Price, A.J.; Enloe, S.F.; Wehtje, G.; Patterson, M.G. Integrated Palmer amaranth management in glufosinate-resistant cotton: II. Primary, secondary and conservation tillage. Agronomy 2013, 3, 28–42. [Google Scholar] [CrossRef] [Green Version]
- Aulakh, J.S.; Price, A.J.; Enloe, S.F.; Santen, E.V.; Wehtje, G.; Patterson, M.G. Integrated Palmer amaranth management in glufosinate-resistant cotton: I. Soil-inversion, high-residue cover crops and herbicide regimes. Agronomy 2012, 2, 295–311. [Google Scholar] [CrossRef] [Green Version]
- Tingle, C.; Chandler, J. The effect of herbicides and crop rotation on weed control in glyphosate-resistant crops. Weed Technol. 2004, 18, 940–946. [Google Scholar] [CrossRef]




| Trait Name | Transgene (s) | Herbicide(s) Resistant to | MOA | Company | Year Deregulated |
|---|---|---|---|---|---|
| BXN | nitrilase | Bromoxynil | PS-II inhibitor | Calgene | 1994 |
| Roundup Ready® | Cp4-EPSPS | Glyphosate during vegetative phase only | EPSPS inhibitor | Monsanto | 1995 |
| Sulfonylurea-resistant cotton | Mutant form of Acetolactate synthase (ALS) | Pyrithiobac | ALS inhibitor | DuPont | 1995 |
| LibertyLink® | Bar | Glufosinate | Glutamine synthetase inhibitor | Aventis | 2003 |
| Roundup Ready® Flex | 2 cp4-EPSPS genes | Glyphosate during both vegetative and reproductive stage | EPSPS inhibitor | Monsanto | 2004 |
| GlyTol® | 2m-EPSPS | Bayer CropScience | 2009 | ||
| XtendFlex® | dmo, EPSPS, bar | Dicamba, glyphosate, and glufosinate | Synthetic auxin, EPSPS, and glutamine synthetase inhibitors | Monsanto | 2015 |
| Enlist® | tfdA, EPSPS, bar | 2,4-D, glyphosate, and glufosinate | Synthetic auxin, EPSPS, and glutamine synthetase inhibitors | Dow Agro-Sciences | 2015 |
| IFT | HPPDPfW336-1Pa, 2mEPSPS | Isoxaflutole | HPPD inhibitor | Bayer CropScience | 2018 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vulchi, R.; Bagavathiannan, M.; Nolte, S.A. History of Herbicide-Resistant Traits in Cotton in the U.S. and the Importance of Integrated Weed Management for Technology Stewardship. Plants 2022, 11, 1189. https://doi.org/10.3390/plants11091189
Vulchi R, Bagavathiannan M, Nolte SA. History of Herbicide-Resistant Traits in Cotton in the U.S. and the Importance of Integrated Weed Management for Technology Stewardship. Plants. 2022; 11(9):1189. https://doi.org/10.3390/plants11091189
Chicago/Turabian StyleVulchi, Rohith, Muthukumar Bagavathiannan, and Scott A. Nolte. 2022. "History of Herbicide-Resistant Traits in Cotton in the U.S. and the Importance of Integrated Weed Management for Technology Stewardship" Plants 11, no. 9: 1189. https://doi.org/10.3390/plants11091189
APA StyleVulchi, R., Bagavathiannan, M., & Nolte, S. A. (2022). History of Herbicide-Resistant Traits in Cotton in the U.S. and the Importance of Integrated Weed Management for Technology Stewardship. Plants, 11(9), 1189. https://doi.org/10.3390/plants11091189






