Energy Consumption Analysis of a Diesel Hydrotreating Unit Using an Aspen Simulation
Abstract
:1. Introduction
2. Diesel Hydrotreating Unit
3. Aspen Simulation of a Diesel Hydrotreating Unit
3.1. Simulation Basis
3.2. Hydrogenation Reaction Kinetics



3.3. Aspen Simulation
3.4. Simulation Results and Comparison
4. Results and Discuss
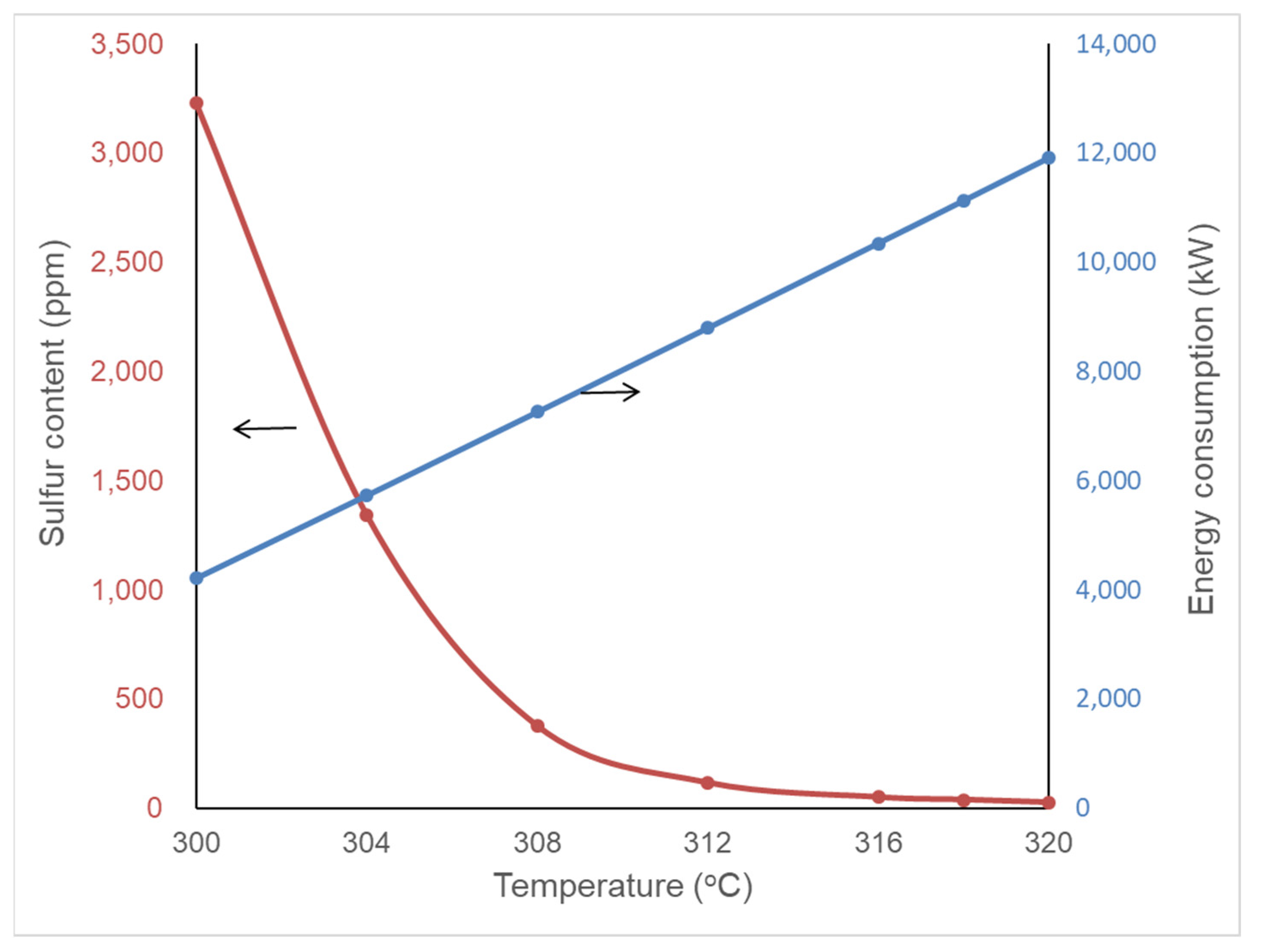
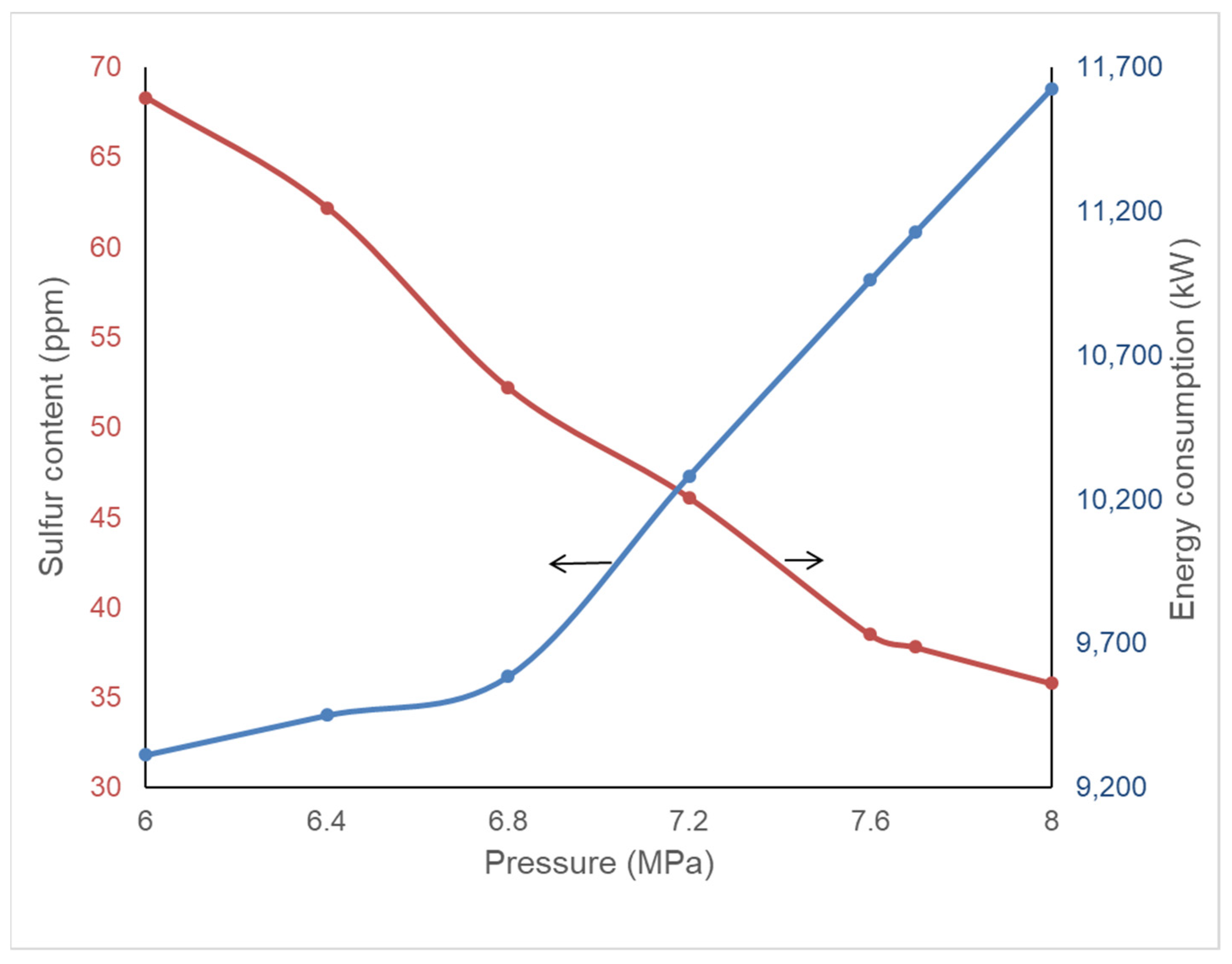
4.1. Effect of Reaction Temperature and Pressure on Product Quality and Energy Consumption
4.2. Energy Consumption of the Operating Condition Boundaries
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, S.; Feng, H.; Zheng, J.; Ye, J.; Song, Y.; Yang, H.; Zhou, M. Life Cycle Assessment and Economic Analysis of Biomass Energy Technology in China: A Brief Review. Processes 2020, 8, 1112. [Google Scholar] [CrossRef]
- Yang, K.; Liu, S.; He, C.; Zhang, B.; Chen, Q.; Pan, M. Improving energy saving of crude oil distillation units with optimal operations. J. Clean. Prod. 2020, 263, 121340. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Wang, Z.; Jin, Q. Optimization Study on Increasing Yield and Capacity of Fluid Catalytic Cracking (FCC) Units. Processes 2021, 9, 1497. [Google Scholar] [CrossRef]
- García-Sánchez, M.; Sales-Cruz, M.; Lopez-Arenas, T.; Viveros-García, T.; Pérez-Cisneros, E.S. An Intensified Reactive Separation Process for Bio-Jet Diesel Production. Processes 2019, 7, 655. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Deng, Z.; He, G.; Wang, H.; Zhang, X.; Lin, J.; Qi, Y.; Liang, X. Challenges and opportunities for carbon neutrality in China. Nat. Rev. Earth Environ. 2022, 3, 141–155. [Google Scholar] [CrossRef]
- Potrč, S.; Čuček, L.; Martin, M.; Kravanja, Z. Synthesis of European Union Biorefinery Supply Networks Considering Sustainability Objectives. Processes 2020, 8, 1588. [Google Scholar] [CrossRef]
- Yadav, A.; Roy, S.; Aijaz, T. Modeling of three-phase radial flow reactor for diesel hydrotreating. Chem. Eng. Sci. 2022, 257, 117713. [Google Scholar] [CrossRef]
- Wei, W. Energy saving measures and effect of hydrofining units. Pet. Refin. Eng. 2022, 52, 60. [Google Scholar]
- Al-Hadhrami, L.M.; Ahmad, A.; Al-Qahtani, A. Performance analysis of heat exchangers of an existing naphtha hydrotreating plant: A case study. Appl. Therm. Eng. 2010, 30, 1029–1033. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, Q.; Hu, S.; Gu, W.; Hui, C.-W. Simultaneous optimization of energy and materials based on heat exchanger network simulation for diesel hydrotreating units. Chem. Eng. Res. Des. 2010, 88, 513–519. [Google Scholar] [CrossRef]
- Agbo, A.F.; Aboje, A.A.; Obayomi, K.S. Exergy analysis of Naphtha Hydrotreating Unit (NHU). J. Phys. Conf. Ser. 2019, 1299, 012025. [Google Scholar] [CrossRef]
- Bandyopadhyay, R.; Alkilde, O.F.; Upadhyayula, S. Applying pinch and exergy analysis for energy efficient design of diesel hydrotreating unit. J. Clean. Prod. 2019, 232, 337–349. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, F.; Liu, X.; Sun, L. Heat Exchanger Network Retrofit of Diesel Hydrotreating Unit Using Pinch Analysis. China Pet. Process. Petrochem. Technol. 2021, 23, 34. [Google Scholar]
- Gabbar, H.A.; Aboughaly, M. Conceptual Process Design, Energy and Economic Analysis of Solid Waste to Hydrocarbon Fuels via Thermochemical Processes. Processes 2021, 9, 2149. [Google Scholar] [CrossRef]
- Samoilov, N.A. Mathematical Modeling and Optimization of Diesel-Fuel Hydrotreatment. Theor. Found. Chem. Eng. 2021, 55, 91–100. [Google Scholar] [CrossRef]
- Ahmad, M.I.; Zhang, N.; Jobson, M. Integrated design of diesel hydrotreating processes. Chem. Eng. Res. Des. 2011, 89, 1025–1036. [Google Scholar] [CrossRef]
- da Rocha Novaes, L.; De Resende, N.S.; Salim, V.M.; Secchi, A. Modeling, simulation and kinetic parameter estimation for diesel hydrotreating. Fuel 2017, 209, 184–193. [Google Scholar] [CrossRef]
- Kang, L.; Liu, Y. Design of flexible multiperiod heat exchanger networks with debottlenecking in subperiods. Chem. Eng. Sci. 2018, 185, 116–126. [Google Scholar] [CrossRef]
- De la Paz-Zavala, C.; Burgos-Vázquez, E.; Rodríguez-Rodríguez, J.E.; Ramírez-Verduzco, L.F. Ultra low sulfur diesel simulation. Application to commercial units. Fuel 2013, 110, 227–234. [Google Scholar] [CrossRef]
- Froment, G.F.; Depauw, G.A.; Vanrysselberghe, V. Kinetic modeling and reactor simulation in hydrodesulfurization of oil fractions. Ind. Eng. Chem. Res. 1994, 33, 2975–2988. [Google Scholar] [CrossRef]
- Houalla, M.; Broderick, D.H.; Sapre, A.V.; Nag, N.K.; De Beer, V.H.J.; Gates, B.C.; Kwart, H. Hydrodesulfurization of methyl-substituted dibenzothiophenes catalyzed by sulfided Co-Moγ-Al2O3. J. Catal. 1980, 61, 523–527. [Google Scholar] [CrossRef]
- Liang, X.; Liu, Y. Simulation of a Was oil Hydrotreating Unit and calculations of Hydrogen Consumption at Off-design Conditions. J. East China Univ. Sci. Technol. 2012, 38, 718–723. (In Chinese) [Google Scholar]
- Bej, S.K.; Dalai, A.K.; Adjaye, J. Comparison of hydrodenitrogenation of basic and nonbasic nitrogen compounds present in oil sands derived heavy gas oil. Energy Fuels 2001, 15, 377–383. [Google Scholar] [CrossRef]
- Cheng, Z.; Fang, X.; Zeng, R.; Han, B.; Huang, L.; Yuan, W. Deep removal of sulfur and aromatics from diesel through two-stage concurrently and countercurrently operated fixed-bed reactors. Chem. Eng. Sci. 2004, 59, 5465–5472. [Google Scholar] [CrossRef]

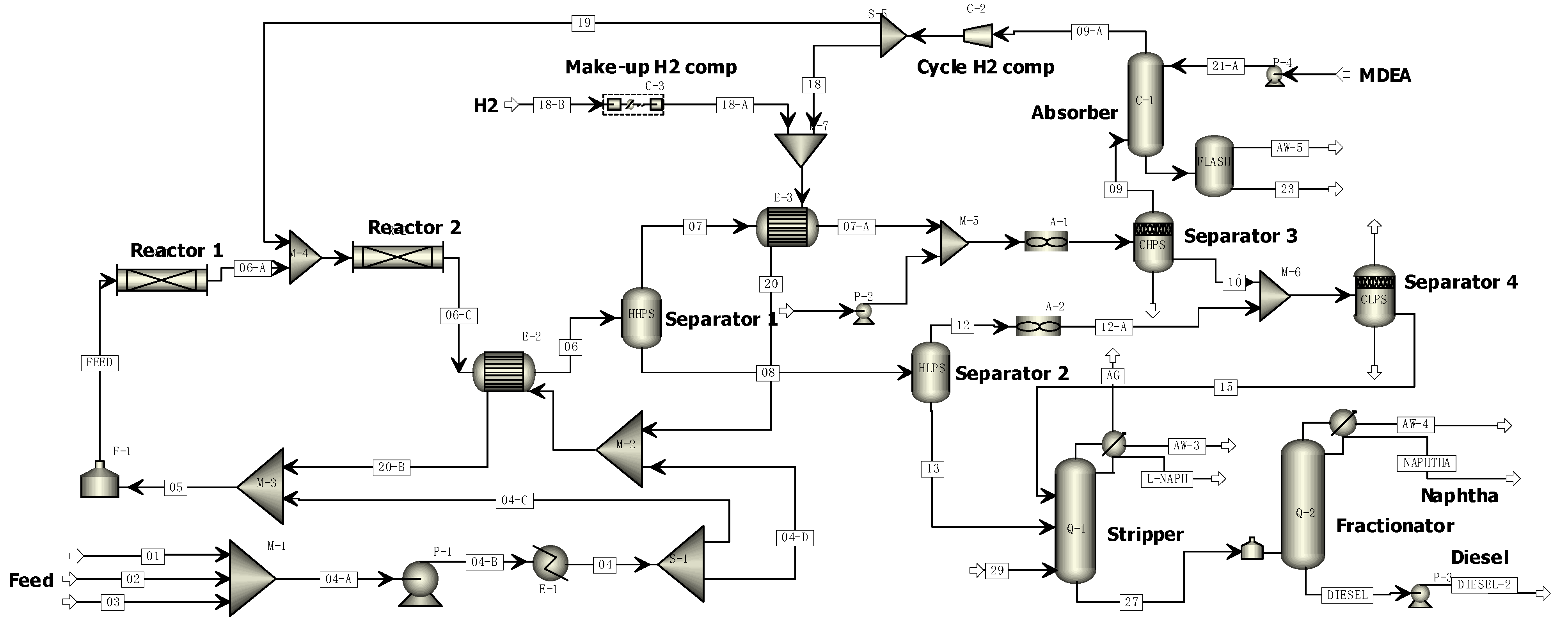
| Actual Equipment | Simulation Module | Operating Conditions 1 |
|---|---|---|
| HDT reactor | Rplug | 320 °C ≤ T ≤ 360 °C 6 MPa ≤ P ≤ 8 MPa |
| Make-up H2 compressor | Mcompr | T = 40 °C P = 2.1 MPa |
| Recycle H2 compressor | Compr | T = 50 °C P = 7 MPa |
| Separator 1, 2, 3, 4 | Flash2/Flash3 | T1 = T2 = 220 °C; T3 = T4 = 45 °C P1 = 7.2 MPa; P2 = 2.5 MPa; P3 = 7.1 MPa; P4 = 2.4 MPa |
| Absorber | RadFrac | P = 7.05 MPa |
| Stripper | PetroFrac | P = 0.8 MPa |
| Fractionator | PetroFrac | P = 0.7 MPa |
| Reactor 1 (°C) | Reactor 2 (°C) | Pressure (MPa) | H2:Oil Ratio | Make-Up H2 (Nm3/h) | Fractionator (°C) | |
|---|---|---|---|---|---|---|
| Actuals | 318.0–343.0 | 335.0–355.0 | 7.7 | 300:1 | 33,443 | 161, 251, 302 1 |
| Simulation | 318.0–343.5 | 336.0–355.7 | 7.7 | 283:1 | 33,779 | 152, 251, 296 |
| Flowrate (tonne/h) | Density (kg/m−3) | Distillation Curves (°C) | S in Diesel (ppm) | N in Diesel (ppm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IBP 1 | 10 | 30 | 50 | 70 | 90 | FBP 2 | ||||||
| Feed 1 | Actuals | 283.2 | 789.1 | 253 | 256 | 269 | 285 | 307 | 327 | 341 | 14,900 | 42 |
| Simulation | 283.2 | 800.3 | 255 | 261 | 279 | 292 | 312 | 326 | 344 | 14,901 | 42 | |
| Feed 2 | Actuals | 40.9 | 683.5 | 48 | 74 | 96 | 117 | 138 | 159 | 181 | 7000 | 110 |
| Simulation | 40.9 | 686.7 | 46 | 73 | 98 | 118 | 139 | 165 | 183 | 7009 | 110 | |
| Feed 3 | Actuals | 56.8 | 790.2 | 188 | 214 | 242 | 272 | 302 | 331 | 350 | 26,000 | 750 |
| Simulation | 56.8 | 826.9 | 184 | 213 | 247 | 279 | 314 | 330 | 358 | 26,018 | 750 | |
| Diesel | Actuals | 333.7 | 618.5 | 188 | 231 | 252 | 274 | 297 | 330 | 355 | 40 | 15 |
| Simulation | 334.0 | 617.3 | 187 | 254 | 264 | 279 | 300 | 324 | 347 | 38.2 | 14.2 | |
| Gasoline | Actuals | 41.2 | 717.2 | 45 | 74 | 95 | 116 | 137 | 159 | 180 | <10 | <5 |
| Simulation | 38.1 | 706.4 | 45 | 87 | 99 | 119 | 137 | 160 | 183 | 0 | 0 | |
| S in Diesel (ppm) | Recycle H2 Compressor (kW) | Feed Oil Pump (kW) | Make-Up H2 Compressor (kW) | Furnace (kW) | |
|---|---|---|---|---|---|
| Actuals | 40 | 1960 | 2308 | 2836 | 6485 |
| Simulation | 38.2 | 1829 | 2277.07 | 2794.73 | 6315 |
| Temperature (°C) | Pressure (MPa) | S in Diesel (ppm) | Furnace (kW) | Feed Oil Pump (kW) | Make-Up H2 Compressor (kW) | Recycle H2 Compressor (kW) | Total Energy Consumption (kW) | |
|---|---|---|---|---|---|---|---|---|
| Original condition | 318 | 7.7 | 37.5 | 6316.3 | 2277.07 | 2794.73 | 1829.41 | 13,217.51 |
| Pressure boundaries | 317.5 | 8.0 | 37.1 | 6111.56 | 2354.7 | 2878.07 | 1760.31 | 13,104.64 |
| Temperature boundaries | 320 | 6.0 | 36.8 | 7334.68 | 1837.18 | 2271.62 | 2361.29 | 13,804.77 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, R.; Xu, W.; Li, Y.; Tian, J.; Wu, L. Energy Consumption Analysis of a Diesel Hydrotreating Unit Using an Aspen Simulation. Processes 2022, 10, 2055. https://doi.org/10.3390/pr10102055
Tian R, Xu W, Li Y, Tian J, Wu L. Energy Consumption Analysis of a Diesel Hydrotreating Unit Using an Aspen Simulation. Processes. 2022; 10(10):2055. https://doi.org/10.3390/pr10102055
Chicago/Turabian StyleTian, Ruijie, Weibin Xu, Yongchao Li, Jun Tian, and Le Wu. 2022. "Energy Consumption Analysis of a Diesel Hydrotreating Unit Using an Aspen Simulation" Processes 10, no. 10: 2055. https://doi.org/10.3390/pr10102055
APA StyleTian, R., Xu, W., Li, Y., Tian, J., & Wu, L. (2022). Energy Consumption Analysis of a Diesel Hydrotreating Unit Using an Aspen Simulation. Processes, 10(10), 2055. https://doi.org/10.3390/pr10102055






