Galectins and Cancer
A project collection of Biomolecules (ISSN 2218-273X). This project collection belongs to the section "Biomacromolecules: Proteins".
Papers displayed on this page all arise from the same project. Editorial decisions were made independently of project staff and handled by the Editor-in-Chief or qualified Editorial Board members.
Viewed by 15421
Share This Project Collection
Editor
 Prof. Dr. Lu-Gang Yu
Prof. Dr. Lu-Gang Yu
 Prof. Dr. Lu-Gang Yu
Prof. Dr. Lu-Gang Yu
E-Mail
Website
Guest Editor
Department of Biochemistry and Systems Biology, Institute of Systems, Molecular and Integrative Biology, University of Liverpool, Liverpool L69 3GE, UK
Interests: roles of cell glycosylation and carbohydrate-binding proteins in cancer
Special Issues, Collections and Topics in MDPI journals
Project Overview
Dear Colleagues,
Galectins are being increasingly recognized as an important family of regulators in cancer development, progression and metastasis. Changes in galectin expression are commonly seen in cancer and stroma cells and are often associated with poorer prognosis and metastasis. Galectins appear intracellularly, extracellularly, and in the circulation. Increasing evidence shows that galectins are involved in the regulation of almost every step in the process of cancer development, progression and metastasis, including cancer cell proliferation, adhesion, invasion, angiogenesis, immune response, and metastasis. Galectin interaction with divergent binding ligands such as growth factor receptors, adhesion molecules, mucin proteins and death receptors have been identified in various cancers and in the tumour-associated microenvironment. The development of galectin-targeted therapeutic agents for cancer treatment are currently being explored by a number of research labs and biotech/bio-pharma companies, and some have shown promising results in early clinical trials.
This Special Issue of Biomolecules, entitled “Galectins and cancer”, focuses on recent findings regarding the actions of galectin family members in the regulation of cancer development, progression or metastasis. We invite colleagues to submit their recent discoveries (reviews or research articles) to this Special Issue. Reports on the recent development of galectin inhibitors as cancer therapeutic agents are also encouraged.
Prof. Dr. Lu-Gang Yu
Guest Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Biomolecules is an international peer-reviewed open access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript.
The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs).
Submitted papers should be well formatted and use good English. Authors may use MDPI's
English editing service prior to publication or during author revisions.
Keywords
- galectins
- immune modulation
- immune cells
- endothelial cells
- cancer cell adhesion and invasion
- tumour-associated microenvironment
- angiogenesis
- metastasis
- inflammation
- apoptosis, galectin inhibitors
Published Papers (5 papers)
Open AccessReview
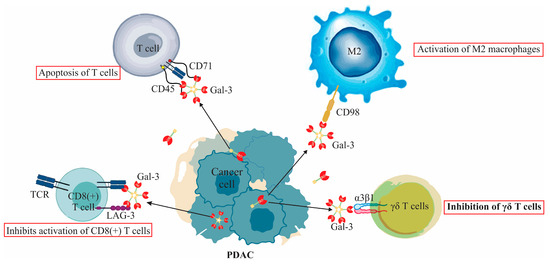
Galectin-3’s Complex Interactions in Pancreatic Ductal Adenocarcinoma: From Cellular Signaling to Therapeutic Potential
by
Milica Dimitrijevic Stojanovic, Bojan Stojanovic, Ivan Radosavljevic, Vojin Kovacevic, Ivan Jovanovic, Bojana S. Stojanovic, Nikola Prodanovic, Vesna Stankovic, Miodrag Jocic and Marina Jovanovic
Cited by 5 | Viewed by 2170
Abstract
Galectin-3 (Gal-3) plays a multifaceted role in the development, progression, and prognosis of pancreatic ductal adenocarcinoma (PDAC). This review offers a comprehensive examination of its expression in PDAC, its interaction with various immune cells, signaling pathways, effects on apoptosis, and therapeutic resistance. Additionally,
[...] Read more.
Galectin-3 (Gal-3) plays a multifaceted role in the development, progression, and prognosis of pancreatic ductal adenocarcinoma (PDAC). This review offers a comprehensive examination of its expression in PDAC, its interaction with various immune cells, signaling pathways, effects on apoptosis, and therapeutic resistance. Additionally, the prognostic significance of serum levels of Gal-3 is discussed, providing insights into its potential utilization as a biomarker. Critical analysis is also extended to the inhibitors of Gal-3 and their potential therapeutic applications in PDAC, offering new avenues for targeted treatments. The intricate nature of Gal-3’s role in PDAC reveals a complex landscape that demands a nuanced understanding for potential therapeutic interventions and monitoring.
Full article
►▼
Show Figures
Open AccessReview
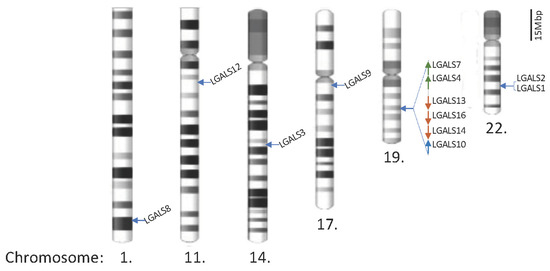
Chimera and Tandem-Repeat Type Galectins: The New Targets for Cancer Immunotherapy
by
Frankie Chi Fat Ko, Sheng Yan, Ka Wai Lee, Sze Kwan Lam and James Chung Man Ho
Cited by 5 | Viewed by 2868
Abstract
In humans, a total of 12 galectins have been identified. Their intracellular and extracellular biological functions are explored and discussed in this review. These galectins play important roles in controlling immune responses within the tumour microenvironment (TME) and the infiltration of immune cells,
[...] Read more.
In humans, a total of 12 galectins have been identified. Their intracellular and extracellular biological functions are explored and discussed in this review. These galectins play important roles in controlling immune responses within the tumour microenvironment (TME) and the infiltration of immune cells, including different subsets of T cells, macrophages, and neutrophils, to fight against cancer cells. However, these infiltrating cells also have repair roles and are hijacked by cancer cells for pro-tumorigenic activities. Upon a better understanding of the immunomodulating functions of galectin-3 and -9, their inhibitors, namely, GB1211 and LYT-200, have been selected as candidates for clinical trials. The use of these galectin inhibitors as combined treatments with current immune checkpoint inhibitors (ICIs) is also undergoing clinical trial investigations. Through their network of binding partners, inhibition of galectin have broad downstream effects acting on CD8
+ cytotoxic T cells, regulatory T cells (Tregs), Natural Killer (NK) cells, and macrophages as well as playing pro-inflammatory roles, inhibiting T-cell exhaustion to support the fight against cancer cells. Other galectin members are also included in this review to provide insight into potential candidates for future treatment(s). The pitfalls and limitations of using galectins and their inhibitors are also discussed to cognise their clinical application.
Full article
►▼
Show Figures
Open AccessFeature PaperEditor’s ChoiceArticle
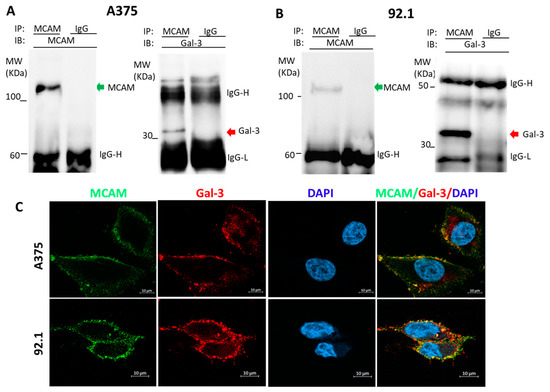
Galectin-3 Is a Natural Binding Ligand of MCAM (CD146, MUC18) in Melanoma Cells and Their Interaction Promotes Melanoma Progression
by
Yaoyu Pang, Ellen Maxwell, Paulina Sindrewicz-Goral, Andrew Shapanis, Shun Li, Mark Morgan and Lu-Gang Yu
Cited by 6 | Viewed by 4244
Abstract
Melanoma cell adhesion molecule (MCAM, CD146, MUC18) is a heavily glycosylated transmembrane protein and a marker of melanoma metastasis. It is expressed in advanced primary melanoma and metastasis but rarely in benign naevi or normal melanocytes. More and more evidence has shown that
[...] Read more.
Melanoma cell adhesion molecule (MCAM, CD146, MUC18) is a heavily glycosylated transmembrane protein and a marker of melanoma metastasis. It is expressed in advanced primary melanoma and metastasis but rarely in benign naevi or normal melanocytes. More and more evidence has shown that activation of the MCAM on cell surface plays a vital role in melanoma progression and metastasis. However, the natural MCAM binding ligand that initiates MCAM activation in melanoma so far remains elusive. This study revealed that galectin-3, a galactoside-binding protein that is commonly overexpressed in many cancers including melanoma, is naturally associated with MCAM on the surface of both skin and uveal melanoma cells. Binding of galectin-3 to MCAM, via
O-linked glycans on the MCAM, induces MCAM dimerization and clustering on cell surface and subsequent activation of downstream AKT signalling. This leads to the increases of a number of important steps in melanoma progression of cell proliferation, adhesion, migration, and invasion. Thus, galectin-3 is a natural binding ligand of MCAM in melanoma, and their interaction activates MCAM and promotes MCAM-mediated melanoma progression. Targeting the galectin-3–MCAM interaction may potentially be a useful therapeutic strategy for melanoma treatment.
Full article
►▼
Show Figures
Open AccessFeature PaperEditor’s ChoiceReview
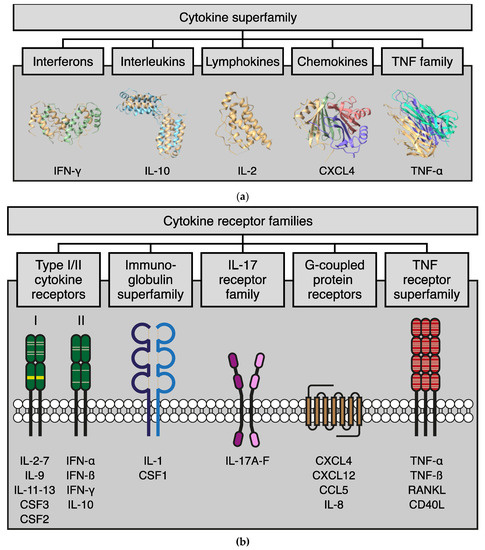
Galectokines: The Promiscuous Relationship between Galectins and Cytokines
by
Lucía Sanjurjo, Esmee C. Broekhuizen, Rory R. Koenen and Victor L. J. L. Thijssen
Cited by 14 | Viewed by 2870
Abstract
Galectins, a family of glycan-binding proteins, are well-known for their role in shaping the immune microenvironment. They can directly affect the activity and survival of different immune cell subtypes. Recent evidence suggests that galectins also indirectly affect the immune response by binding to
[...] Read more.
Galectins, a family of glycan-binding proteins, are well-known for their role in shaping the immune microenvironment. They can directly affect the activity and survival of different immune cell subtypes. Recent evidence suggests that galectins also indirectly affect the immune response by binding to members of another immunoregulatory protein family, i.e., cytokines. Such galectin-cytokine heterodimers, here referred to as galectokines, add a new layer of complexity to the regulation of immune homeostasis. Here, we summarize the current knowledge with regard to galectokine formation and function. We describe the known and potential mechanisms by which galectokines can help to shape the immune microenvironment. Finally, the outstanding questions and challenges for future research regarding the role of galectokines in immunomodulation are discussed.
Full article
►▼
Show Figures
Open AccessCommunication
Galectins Differentially Regulate the Surface Glycosylation of Human Monocytes
by
Dina B. AbuSamra, Rafael Martínez-Carrasco and Pablo Argüeso
Cited by 2 | Viewed by 1875
Abstract
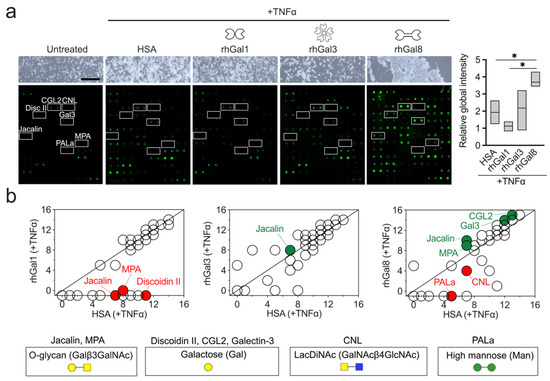
Monocytes are circulating blood cells that rapidly mobilize to inflamed sites where they serve diverse effector functions shaped in part by microenvironmental cues. The establishment of specific glycosylation patterns on the immune cell glycocalyx is fundamental to direct the inflammatory response, but relatively
[...] Read more.
Monocytes are circulating blood cells that rapidly mobilize to inflamed sites where they serve diverse effector functions shaped in part by microenvironmental cues. The establishment of specific glycosylation patterns on the immune cell glycocalyx is fundamental to direct the inflammatory response, but relatively little is known about the mechanisms whereby the microenvironment controls this process. Here, we report that galectins differentially participate in remodeling the surface glycosylation of human primary CD14
+CD16
− monocytes under proinflammatory conditions. Using a lectin array on biotinylated protein, we found that the prototypic galectin-1 negatively influenced the expression of galactose epitopes on the surface of monocytic cells. On the other hand, the tandem-repeat galectin-8 and, to a certain extent, the chimeric galectin-3 promoted the expression of these residues. Jacalin flow cytometry and pull-down experiments further demonstrated that galectin-8 causes a profound upregulation of mucin-type O-glycosylation in cell surface proteins from primary monocytes and THP-1 cells. Overall, these results highlight the emerging role of the galectin signature on inflamed tissues and provide new insights into the contribution of extracellular galectins to the composition of the glycocalyx in human monocytes.
Full article
►▼
Show Figures
Planned Papers
The below list represents only planned manuscripts. Some of these
manuscripts have not been received by the Editorial Office yet. Papers
submitted to MDPI journals are subject to peer-review.
Title: Inhibition of Galectins in Cancer: Biological Challenges for their Clinical Application
Authors: Diego Laderach; et al.
Affiliation: Universidad de Buenos Aires
Abstract: Galectins play relevant roles in tumor development, progression and metastasis. Accordingly, Galectins’ inhibition constitutes attractive strategies for cancer treatment. To date, however, clinical trials based on the use of Galectin’s inhibitors reported inconclusive results. This review summarizes the strategies that are currently being evaluated for Galectin inhibition and also discusses some biological challenges that need to be addressed to improve these strategies for the benefit of cancer patients.
Title: Galectin-1 and galectin-3 differentially modulate Notch signaling in gastric cancer
Authors: Sofia N. dos Santos; et al.
Affiliation: Departmento de Analises Clinicas, Toxicologicas e Bromatologicas, Faculdade de Ciencias Farmaceuticas de Ribeirao Preto, Universidade de Sao Paulo, Ribeirao Preto, Brazil
Abstract: Notch signaling pathway has emerged as an important pathway in maintaining the balance between cell proliferation, differentiation, and apoptosis in cancer cells. Galectin-3, a glycan-binding protein, has been reported as a regulator of Notch signaling activation. Still, the precise mechanism by which this regulation is accomplished is not fully understood. Here we demonstrate that galectin-1-DLL4 and galectin-3-Jagged1 expressions are positively correlated in human gastric cancer. Moreover, galectin-1 and galectin-3 interact with the Notch-1 receptor and preferentially activate Notch signaling by DLL4 and Jagged1 ligands, respectively. Galectin-3, which is mainly found in tumor cells, regulates the cell surface expression of Notch ligands and Notch-1 receptor and, when present extracellularly, can rescue Notch-1 activation in the absence of endogenous galectin-3. This knowledge open novel perspectives for future combined Notch-targeted therapy