TGF-Beta Signaling in Tissue Fibrosis and Cancer
Share This Topical Collection
Editors
 Dr. Paul J. Higgins
Dr. Paul J. Higgins
 Dr. Paul J. Higgins
Dr. Paul J. Higgins
E-Mail
Website
Collection Editor
Department of Regenerative and Cancer Cell Biology, Albany Medical College, 47 New Scotland Avenue, Albany, NY 12208-3479, USA
Interests: tumor microenvironment; tissue fibrosis; desmoplastic cancers; SERPINE1; transcriptomics/proteomics
Special Issues, Collections and Topics in MDPI journals
 Dr. Rohan Samarakoon
Dr. Rohan Samarakoon
 Dr. Rohan Samarakoon
Dr. Rohan Samarakoon
E-Mail
Website
Collection Editor
Department of Regenerative & Cancer Cell Biology, Albany Medical College, Albany, NY 12208, USA
Interests: hippo signaling; renal fibrosis; kidney cancer; EGFR transactivation; non-SMAD TGF-β pathway; tumor suppressor deregulation in fibrosis
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
TGF-β is a major contributor to fibrotic and neoplastic diseases in both humans and animals. Although this pleiotropic cytokine promotes epithelial cell plasticity/dedifferentiation/pEMT, cellular stemness, migration/invasion, and stimulates acquisition of a profibrotic phenotype within the tumor microenvironment (i.e., myofibroblasts and cancer-associated fibroblasts/CAFs), translation of emerging knowledge to successfully target TGF-β1 remains an unrealized clinical challenge. This Topical Collection welcomes original research articles as well as state-of-the-art reviews that address the following topics to further our understanding of the mechanistic and pathological basis of TGF-β involvement in organ fibrosis and cancer.
- TGF-β-mediated transcriptional (both SMAD and non-SMAD) networks, genetic reprogramming, and phenotypic responses (e.g., cell plasticity/stemness, cell cycle arrest, proliferation, migration) related to the onset or progression of fibrotic and neoplastic diseases.
- Non-transcriptional (e.g., microRNA, lncRNA, epigenetic) control of TGF-β1 signaling.
- TGF-β crosstalk with other receptors (e.g., tyrosine kinases and serine/threonine kinases) or tumor suppressors (e.g., p53, PTEN) in promoting or suppressing fibrotic and oncogenic behavior.
- Novel positive (e.g., inducers) and negative regulators (e.g., suppressors) of TGF-β1 pathways.
- Novel or potential therapeutic approaches (TGF-β ligand traps and neutralizing antibodies, signaling networks, or TGF-β collateral networks) to target aberrant TGF-β signaling in organ fibrosis and cancer.
- TGF-β1-induced metabolic alterations (e.g., glycolysis, Krebs cycle, oxidative phosphorylation, fatty acid oxidation) in tissue fibrosis and cancer.
- TGF-β1 control of inflammatory networks.
- Tissue or organ specificity of TGF-signaling.
- Evaluation of TGF-β1 signal transducers as biomarkers of cancer and fibrosis progression in animal models and humans.
Prof. Paul J. Higgins
Dr. Rohan Samarakoon
Collection Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Biomolecules is an international peer-reviewed open access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript.
The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs).
Submitted papers should be well formatted and use good English. Authors may use MDPI's
English editing service prior to publication or during author revisions.
Published Papers (7 papers)
Open AccessReview
The Synergistic Cooperation between TGF-β and Hypoxia in Cancer and Fibrosis
by
Pramod Mallikarjuna, Yang Zhou and Maréne Landström
Cited by 44 | Viewed by 8552
Abstract
Transforming growth factor β (TGF-β) is a multifunctional cytokine regulating homeostasis and immune responses in adult animals and humans. Aberrant and overactive TGF-β signaling promotes cancer initiation and fibrosis through epithelial–mesenchymal transition (EMT), as well as the invasion and metastatic growth of cancer
[...] Read more.
Transforming growth factor β (TGF-β) is a multifunctional cytokine regulating homeostasis and immune responses in adult animals and humans. Aberrant and overactive TGF-β signaling promotes cancer initiation and fibrosis through epithelial–mesenchymal transition (EMT), as well as the invasion and metastatic growth of cancer cells. TGF-β is a key factor that is active during hypoxic conditions in cancer and is thereby capable of contributing to angiogenesis in various types of cancer. Another potent role of TGF-β is suppressing immune responses in cancer patients. The strong tumor-promoting effects of TGF-β and its profibrotic effects make it a focus for the development of novel therapeutic strategies against cancer and fibrosis as well as an attractive drug target in combination with immune regulatory checkpoint inhibitors. TGF-β belongs to a family of cytokines that exert their function through signaling via serine/threonine kinase transmembrane receptors to intracellular Smad proteins via the canonical pathway and in combination with co-regulators such as the adaptor protein and E3 ubiquitin ligases TNF receptor-associated factor 4 (TRAF4) and TNF receptor-associated factor 6 (TRAF6) to promote non-canonical pathways. Finally, the outcome of gene transcription initiated by TGF-β is context-dependent and controlled by signals exerted by other growth factors such as EGF and Wnt. Here, we discuss the synergistic cooperation between TGF-β and hypoxia in development, fibrosis and cancer.
Full article
►▼
Show Figures
Open AccessReview
Actin Cytoskeleton and Regulation of TGFβ Signaling: Exploring Their Links
by
Roberta Melchionna, Paola Trono, Annalisa Tocci and Paola Nisticò
Cited by 29 | Viewed by 8636
Abstract
Human tissues, to maintain their architecture and function, respond to injuries by activating intricate biochemical and physical mechanisms that regulates intercellular communication crucial in maintaining tissue homeostasis. Coordination of the communication occurs through the activity of different actin cytoskeletal regulators, physically connected to
[...] Read more.
Human tissues, to maintain their architecture and function, respond to injuries by activating intricate biochemical and physical mechanisms that regulates intercellular communication crucial in maintaining tissue homeostasis. Coordination of the communication occurs through the activity of different actin cytoskeletal regulators, physically connected to extracellular matrix through integrins, generating a platform of biochemical and biomechanical signaling that is deregulated in cancer. Among the major pathways, a controller of cellular functions is the cytokine transforming growth factor β (TGFβ), which remains a complex and central signaling network still to be interpreted and explained in cancer progression. Here, we discuss the link between actin dynamics and TGFβ signaling with the aim of exploring their aberrant interaction in cancer.
Full article
►▼
Show Figures
Open AccessEditor’s ChoiceReview
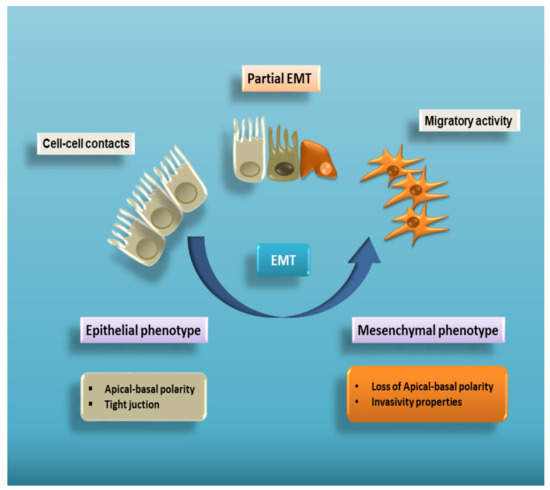
Organ Fibrosis and Autoimmunity: The Role of Inflammation in TGFβ-Dependent EMT
by
Margherita Sisto, Domenico Ribatti and Sabrina Lisi
Cited by 102 | Viewed by 6304
Abstract
Recent advances in our understanding of the molecular pathways that control the link of inflammation with organ fibrosis and autoimmune diseases point to the epithelial to mesenchymal transition (EMT) as the common association in the progression of these diseases characterized by an intense
[...] Read more.
Recent advances in our understanding of the molecular pathways that control the link of inflammation with organ fibrosis and autoimmune diseases point to the epithelial to mesenchymal transition (EMT) as the common association in the progression of these diseases characterized by an intense inflammatory response. EMT, a process in which epithelial cells are gradually transformed to mesenchymal cells, is a major contributor to the pathogenesis of fibrosis. Importantly, the chronic inflammatory microenvironment has emerged as a decisive factor in the induction of pathological EMT. Transforming growth factor-β (TGF-β), a multifunctional cytokine, plays a crucial role in the induction of fibrosis, often associated with chronic phases of inflammatory diseases, contributing to marked fibrotic changes that severely impair normal tissue architecture and function. The understanding of molecular mechanisms underlying EMT-dependent fibrosis has both a basic and a translational relevance, since it may be useful to design therapies aimed at counteracting organ deterioration and failure. To this end, we reviewed the recent literature to better elucidate the molecular response to inflammatory/fibrogenic signals in autoimmune diseases in order to further the specific regulation of EMT-dependent fibrosis in more targeted therapies.
Full article
►▼
Show Figures
Open AccessReview
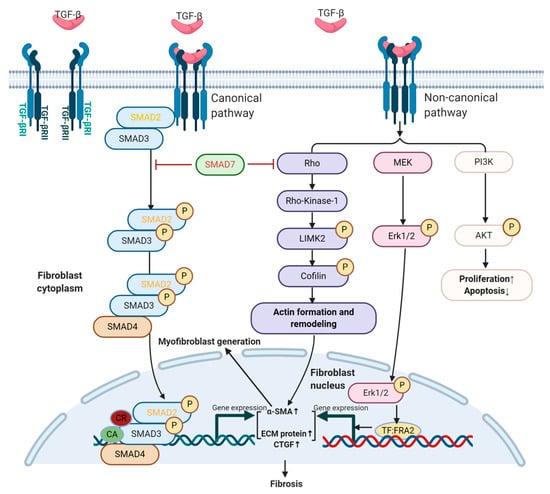
Role of TGF-Beta and Smad7 in Gut Inflammation, Fibrosis and Cancer
by
Carmine Stolfi, Edoardo Troncone, Irene Marafini and Giovanni Monteleone
Cited by 62 | Viewed by 12088
Abstract
The human gastrointestinal tract contains the largest population of immune cells in the body and this is a reflection of the fact that it is continuously exposed to a myriad of dietary and bacterial antigens. Although these cells produce a variety of inflammatory
[...] Read more.
The human gastrointestinal tract contains the largest population of immune cells in the body and this is a reflection of the fact that it is continuously exposed to a myriad of dietary and bacterial antigens. Although these cells produce a variety of inflammatory cytokines that could potentially promote tissue damage, in normal conditions the mucosal immune response is tightly controlled by counter-regulatory factors, which help induce and maintain gut homeostasis and tolerance. One such factor is transforming growth factor (TGF)-β1, a cytokine produced by multiple lineages of leukocytes, stromal cells and epithelial cells, and virtually targets all the gut mucosal cell types. Indeed, studies in animals and humans have shown that defects in TGF-β1 production and/or signaling can lead to the development of immune-inflammatory pathologies, fibrosis and cancer in the gut. Here, we review and discuss the available evidence about the role of TGF-β1 and Smad7, an inhibitor of TGF-β1 activity, in gut inflammation, fibrosis and cancer with particular regard to the contribution of these two molecules in the pathogenesis of inflammatory bowel diseases and colon cancer.
Full article
►▼
Show Figures
Open AccessReview
Transforming Growth Factor-β Signaling in Fibrotic Diseases and Cancer-Associated Fibroblasts
by
Xueke Shi, Christian D. Young, Hongmei Zhou and Xiao-Jing Wang
Cited by 142 | Viewed by 20881
Abstract
Transforming growth factor-β (TGF-β) signaling is essential in embryo development and maintaining normal homeostasis. Extensive evidence shows that TGF-β activation acts on several cell types, including epithelial cells, fibroblasts, and immune cells, to form a pro-fibrotic environment, ultimately leading to fibrotic diseases. TGF-β
[...] Read more.
Transforming growth factor-β (TGF-β) signaling is essential in embryo development and maintaining normal homeostasis. Extensive evidence shows that TGF-β activation acts on several cell types, including epithelial cells, fibroblasts, and immune cells, to form a pro-fibrotic environment, ultimately leading to fibrotic diseases. TGF-β is stored in the matrix in a latent form; once activated, it promotes a fibroblast to myofibroblast transition and regulates extracellular matrix (ECM) formation and remodeling in fibrosis. TGF-β signaling can also promote cancer progression through its effects on the tumor microenvironment. In cancer, TGF-β contributes to the generation of cancer-associated fibroblasts (CAFs) that have different molecular and cellular properties from activated or fibrotic fibroblasts. CAFs promote tumor progression and chronic tumor fibrosis via TGF-β signaling. Fibrosis and CAF-mediated cancer progression share several common traits and are closely related. In this review, we consider how TGF-β promotes fibrosis and CAF-mediated cancer progression. We also discuss recent evidence suggesting TGF-β inhibition as a defense against fibrotic disorders or CAF-mediated cancer progression to highlight the potential implications of TGF-β-targeted therapies for fibrosis and cancer.
Full article
►▼
Show Figures
Open AccessArticle
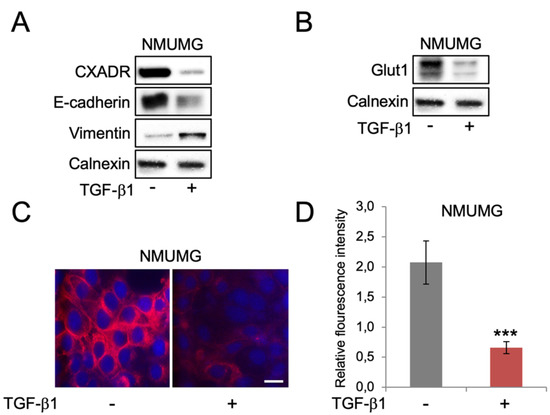
Different Regulation of Glut1 Expression and Glucose Uptake during the Induction and Chronic Stages of TGFβ1-Induced EMT in Breast Cancer Cells
by
Azadeh Nilchian, Nikolina Giotopoulou, Wenwen Sun and Jonas Fuxe
Cited by 17 | Viewed by 3777
Abstract
Transforming growth factor beta 1 (TGF-β1) is associated with epithelial-mesenchymal transition (EMT), lymph metastasis, and poor prognosis in breast cancer. Paradoxically, TGF-β1 is also a potent inhibitor of cell proliferation. TGF-β1-induced EMT involves activation of several pathways including AKT, which also regulates glucose
[...] Read more.
Transforming growth factor beta 1 (TGF-β1) is associated with epithelial-mesenchymal transition (EMT), lymph metastasis, and poor prognosis in breast cancer. Paradoxically, TGF-β1 is also a potent inhibitor of cell proliferation. TGF-β1-induced EMT involves activation of several pathways including AKT, which also regulates glucose uptake. Recent data show that prolonged TGF-β1 exposure leads to a more stable EMT phenotype in breast cancer cells. However, whether this is linked to changes in glucose metabolism is not clear. Here, we used a model of TGF-β1-induced EMT in mammary epithelial cells to study the regulation of Glut1 and EMT markers during the induction compared to a prolonged phase of EMT by western blot, immunofluorescence and qPCR analysis. We also measured cell proliferation and uptake of the glucose analogue 2-NDBG. We found that EMT induction was associated with decreased Glut1 expression and glucose uptake. These effects were linked to reduced cell proliferation rather than EMT. Knockdown of Glut1 resulted in growth inhibition and less induction of vimentin during TGF-β1-induced EMT. Intriguingly, Glut1 levels, glucose uptake and cell proliferation were restored during prolonged EMT. The results link Glut1 repression to the anti-proliferative response of TGF-β1 and indicate that re-expression of Glut1 during chronic TGF-β1 exposure allows breast cancer cells to develop stable EMT and proliferate, in parallel.
Full article
►▼
Show Figures
Open AccessArticle
Activin A-Mediated Regulation of XT-I in Human Skin Fibroblasts
by
Thanh-Diep Ly, Ricarda Plümers, Bastian Fischer, Vanessa Schmidt, Doris Hendig, Joachim Kuhn, Cornelius Knabbe and Isabel Faust
Cited by 9 | Viewed by 3368
Abstract
Fibrosis is a fundamental feature of systemic sclerosis (SSc) and is characterized by excessive accumulation of extracellular matrix components like proteoglycans (PG) or collagens in skin and internal organs. Serum analysis from SSc patients showed an increase in the enzyme activity of xylosyltransferase
[...] Read more.
Fibrosis is a fundamental feature of systemic sclerosis (SSc) and is characterized by excessive accumulation of extracellular matrix components like proteoglycans (PG) or collagens in skin and internal organs. Serum analysis from SSc patients showed an increase in the enzyme activity of xylosyltransferase (XT), the initial enzyme in PG biosynthesis. There are two distinct XT isoforms—XT-I and XT-II—in humans, but until now only XT-I is associated with fibrotic remodelling for an unknown reason. The aim of this study was to identify new XT mediators and clarify the underlying mechanisms, in view of developing putative therapeutic anti-fibrotic interventions in the future. Therefore, we used different cytokines and growth factors, small molecule inhibitors as well as small interfering RNAs, and assessed the cellular XT activity and
XYLT1 expression in primary human dermal fibroblasts by radiochemical activity assays and qRT-PCR. We identified a new function of activin A as a regulator of
XYLT1 mRNA expression and XT activity. While the activin A-induced XT-I increase was found to be mediated by activin A receptor type 1B, MAPK and Smad pathways, the activin A treatment did not alter the
XYLT2 expression. Furthermore, we observed a reciprocal regulation of
XYLT1 and
XYLT2 transcription after inhibition of the activin A pathway components. These results improve the understanding of the differential expression regulation of
XYLT isoforms under pathological fibroproliferative conditions.
Full article
►▼
Show Figures