Molecular Signaling Pathways and Networks in Cancer
Share This Topical Collection
Editor
 Dr. Shihori Tanabe
Dr. Shihori Tanabe
 Dr. Shihori Tanabe
Dr. Shihori Tanabe
E-Mail
Website
Collection Editor
Division of Risk Assessment, Center for Biological Safety and Research, National Institute of Health Sciences, Kawasaki 210-9501, Japan
Interests: molecular network in diseases; signaling pathway in cancer; molecular mechanism in cancer therapy; therapeutic response in cancer
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
Dynamic regulation in the activity of molecular signaling pathways and networks is critical in cancer. Cancer signaling in various types and conditions of cancers triggers, progresses, and promotes cancer development. Molecular signaling pathway networks involved in progression, metastasis, drug resistance, and development in cancer are the main focuses in this collection.
In the meantime, specific Adverse Outcome Pathways (AOPs) in cancer are also in the main scope of this collection. Any authors who have been developing AOPs in cancer are greatly welcome to submit their papers.
Dr. Shihori Tanabe
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cancers is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript.
The Article Processing Charge (APC) for publication in this open access journal is 2900 CHF (Swiss Francs).
Submitted papers should be well formatted and use good English. Authors may use MDPI's
English editing service prior to publication or during author revisions.
Keywords
- molecular signaling pathway
- adverse outcome pathway (AOP)
- signaling pathway network
Published Papers (7 papers)
Open AccessArticle
Exploring Tumor Heterogeneity: Radiogenomic Assessment of ADFP in Low WHO/ISUP Grade Clear Cell Renal Cell Carcinoma
by
Federico Greco, Andrea Panunzio, Valerio D’Andrea, Mariavittoria Vescovo, Alessandro Tafuri, Simone Carotti, Bruno Beomonte Zobel and Carlo Augusto Mallio
Viewed by 256
Abstract
This study aimed to investigate the association between metabolic lipid computed tomography (CT) features and adipose differentiation-related protein (ADFP) expression in clear cell renal cell carcinoma (ccRCC), providing insights into non-invasive methods for assessing ADFP expression and tumor characteristics. This study utilized data
[...] Read more.
This study aimed to investigate the association between metabolic lipid computed tomography (CT) features and adipose differentiation-related protein (ADFP) expression in clear cell renal cell carcinoma (ccRCC), providing insights into non-invasive methods for assessing ADFP expression and tumor characteristics. This study utilized data from The Cancer Genome Atlas and the Cancer Imaging Archive to analyze genetic alterations and imaging characteristics in ccRCC patients. Tumoral Hounsfield units (HU) analysis and quantification of abdominal adipose tissue compartments were performed using CT images. Statistical analyses were conducted to compare tumoral HU values according to ADFP gene expression and World Health Organization/International Society of Urological Pathology (WHO/ISUP) tumor grade, as well as to explore correlations between tumoral HU values and adipose tissue quantification. Among the 174 identified patients, those with ADFP gene expression showed significantly lower minimum tumoral HU values in low-grade cancers compared to high-grade cancers. Similarly, patients with low-grade cancers expressing ADFP exhibited lower minimum tumoral HU values compared to those without ADFP expression. Negative correlations were observed between minimum tumoral HU values and visceral adipose tissue, subcutaneous adipose tissue, and total adipose tissue in both ccRCC patients with and without ADFP expression. This study reveals a significant association between metabolic lipid CT features and ADFP expression in ccRCC patients. Lower minimum tumoral HU values, suggestive of higher intracellular lipid accumulation, were observed in tumors with low WHO/ISUP grade and ADFP expression.
Full article
►▼
Show Figures
Open AccessArticle
Analysis of Expression and Regulation of AKR1C2 in HPV-Positive and -Negative Oropharyngeal Squamous Cell Carcinoma
by
Maria Ziogas, Oliver Siefer, Nora Wuerdemann, Harini Balaji, Elena Gross, Uta Drebber, Jens Peter Klussmann and Christian U. Huebbers
Viewed by 378
Abstract
Head and Neck Squamous Cell Carcinoma (HNSCC), particularly Oropharyngeal Squamous Cell Carcinoma (OPSCC), is a major global health challenge due to its increasing incidence and high mortality rate. This study investigates the role of aldo-keto reductase 1C2 (AKR1C2) in OPSCC, focusing on its
[...] Read more.
Head and Neck Squamous Cell Carcinoma (HNSCC), particularly Oropharyngeal Squamous Cell Carcinoma (OPSCC), is a major global health challenge due to its increasing incidence and high mortality rate. This study investigates the role of aldo-keto reductase 1C2 (AKR1C2) in OPSCC, focusing on its expression, correlation with Human Papillomavirus (HPV) status, oxidative stress status, and clinical outcomes, with an emphasis on sex-specific differences. We analyzed AKR1C2 expression using immunohistochemistry in formalin-fixed, paraffin-embedded tissue samples from 51 OPSCC patients. Additionally, we performed RT-qPCR in cultured HPV16-E6*I and HPV16-E6 overexpressing HEK293 cell lines (p53
WT). Statistical analyses were performed to assess the correlation between AKR1C2 expression and patient data. Our results indicate a significant association between increased AKR1C2 expression and higher AJCC classification (
p = 0.009) as well as positive HPV status (
p = 0.008). Prognostic implications of AKR1C2 varied by sex, whereby female patients with high AKR1C2 expression had better overall survival, whereas male patients exhibited poorer outcomes. Additionally, AKR1C2 expression was linked to HPV status, suggesting a potential HPV-specific regulatory mechanism. These findings underscore the complex interplay among AKR1C2, HPV, and patient sex, highlighting the need for personalized treatment strategies for OPSCC. Targeted inhibition of AKR1C2, considering sex-specific differences, may enhance therapeutic outcomes. Future research should investigate these mechanisms to enhance treatment efficacy.
Full article
►▼
Show Figures
Open AccessArticle
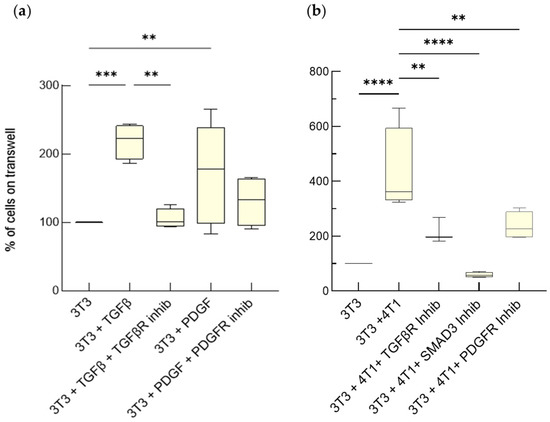
Advanced Cell Culture Models Illuminate the Interplay between Mammary Tumor Cells and Activated Fibroblasts
by
Martina Del Nero, Alessandro Colombo, Stefania Garbujo, Chiara Baioni, Linda Barbieri, Metello Innocenti, Davide Prosperi, Miriam Colombo and Luisa Fiandra
Cited by 4 | Viewed by 2406
Abstract
The interaction between tumor cells and activated fibroblasts determines malignant features of desmoplastic carcinomas such as rapid growth, progression towards a metastatic phenotype, and resistance to chemotherapy. On one hand, tumor cells can activate normal fibroblasts and even reprogram them into CAFs through
[...] Read more.
The interaction between tumor cells and activated fibroblasts determines malignant features of desmoplastic carcinomas such as rapid growth, progression towards a metastatic phenotype, and resistance to chemotherapy. On one hand, tumor cells can activate normal fibroblasts and even reprogram them into CAFs through complex mechanisms that also involve soluble factors. Among them, transforming growth factor beta (TGF-β) and Platelet-Derived Growth Factor (PDGF) have an established role in the acquisition of pro-tumorigenic phenotypes by fibroblasts. On the other hand, activated fibroblasts release Interleukin-6 (IL-6), which increases tumor-cell invasiveness and chemoresistance. However, the interplay between breast cancer cells and fibroblasts, as well as the modes of action of TGF-β, PDGF, and IL-6, are difficult to investigate in vivo. Here, we validated the usage of advanced cell culture models as tools to study the interplay between mammary tumor cells and fibroblasts, taking mouse and human triple-negative tumor cells and fibroblasts as a case study. We employed two different settings, one permitting only paracrine signaling, the other both paracrine and cell-contact-based signaling. These co-culture systems allowed us to unmask how TGF-β, PDGF and IL-6 mediate the interplay between mammary tumor cells and fibroblasts. We found that the fibroblasts underwent activation induced by the TGF-β and the PDGF produced by the tumor cells, which increased their proliferation and IL-6 secretion. The IL-6 secreted by activated fibroblasts enhanced tumor-cell proliferation and chemoresistance. These results show that these breast cancer avatars possess an unexpected high level of complexity, which resembles that observed in vivo. As such, advanced co-cultures provide a pathologically relevant tractable system to study the role of the TME in breast cancer progression with a reductionist approach.
Full article
►▼
Show Figures
Open AccessArticle
Procaine Abrogates the Epithelial-Mesenchymal Transition Process through Modulating c-Met Phosphorylation in Hepatocellular Carcinoma
by
Min Hee Yang, Chakrabhavi Dhananjaya Mohan, Amudha Deivasigamani, Arunachalam Chinnathambi, Sulaiman Ali Alharbi, Kanchugarakoppal S. Rangappa, Sang Hoon Jung, Hyejin Ko, Kam Man Hui, Gautam Sethi and Kwang Seok Ahn
Cited by 7 | Viewed by 2149
Abstract
EMT is a critical cellular phenomenon that promotes tumor invasion and metastasis. Procaine is a local anesthetic agent used in oral surgeries and as an inhibitor of DNA methylation in some types of cancers. In this study, we have investigated whether procaine can
[...] Read more.
EMT is a critical cellular phenomenon that promotes tumor invasion and metastasis. Procaine is a local anesthetic agent used in oral surgeries and as an inhibitor of DNA methylation in some types of cancers. In this study, we have investigated whether procaine can inhibit the EMT process in HCC cells and the preclinical model. Procaine suppressed the expression of diverse mesenchymal markers but induced the levels of epithelial markers such as E-cadherin and occludin in HGF-stimulated cells. Procaine also significantly reduced the invasion and migration of HCC cells. Moreover, procaine inhibited HGF-induced c-Met and its downstream oncogenic pathways, such as PI3K/Akt/mTOR and MEK/ERK. Additionally, procaine decreased the tumor burden in the HCC mouse model and abrogated lung metastasis. Overall, our study suggests that procaine may inhibit the EMT process through the modulation of a c-Met signaling pathway.
Full article
►▼
Show Figures
Open AccessArticle
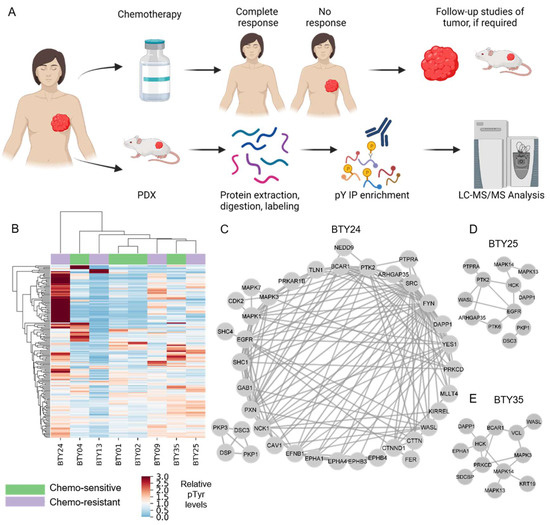
Identification of Src Family Kinases as Potential Therapeutic Targets for Chemotherapy-Resistant Triple Negative Breast Cancer
by
Ishwar N. Kohale, Jia Yu, Yongxian Zhuang, Xiaoyang Fan, Raven J. Reddy, Jason Sinnwell, Krishna R. Kalari, Judy C. Boughey, Jodi M. Carter, Matthew P. Goetz, Liewei Wang and Forest M. White
Cited by 7 | Viewed by 2499
Abstract
Neoadjuvant chemotherapy (NAC) remains the cornerstone of the treatment for triple negative breast cancer (TNBC), with the goal of complete eradication of disease. However, for patients with residual disease after NAC, recurrence and mortality rates are high and the identification of novel therapeutic
[...] Read more.
Neoadjuvant chemotherapy (NAC) remains the cornerstone of the treatment for triple negative breast cancer (TNBC), with the goal of complete eradication of disease. However, for patients with residual disease after NAC, recurrence and mortality rates are high and the identification of novel therapeutic targets is urgently needed. We quantified tyrosine phosphorylation (pTyr)-mediated signaling networks in chemotherapy sensitive (CS) and resistant (CR) TNBC patient-derived xenografts (PDX), to gain novel therapeutic insights. The antitumor activity of SFK inhibition was examined in vivo. Treated tumors were further subjected to phosphoproteomic and RNAseq analysis, to identify the mechanism of actions of the drug. We identified Src Family Kinases (SFKs) as potential therapeutic targets in CR TNBC PDXs. Treatment with dasatinib, an FDA approved SFK inhibitor, led to inhibition of tumor growth in vivo. Further analysis of post-treatment PDXs revealed multiple mechanisms of actions of the drug, confirming the multi-target inhibition of dasatinib. Analysis of pTyr in tumor specimens suggested a low prevalence of SFK-driven tumors, which may provide insight into prior clinical trial results demonstrating a lack of dasatinib antitumor activity in unselected breast cancer patients. Taken together, these results underscore the importance of pTyr characterization of tumors, in identifying new targets, as well as stratifying patients based on their activated signaling networks for therapeutic options. Our data provide a strong rationale for studying SFK inhibitors in biomarker-selected SFK-driven TNBC.
Full article
►▼
Show Figures
Open AccessArticle
Expression of SARS-CoV-2-Related Surface Proteins in Non-Small-Cell Lung Cancer Patients and the Influence of Standard of Care Therapy
by
Christophe Deben, Maxim Le Compte, Vasiliki Siozopoulou, Hilde Lambrechts, Christophe Hermans, Ho Wa Lau, Manon Huizing, Kevin Lamote, Jeroen M. H. Hendriks, Peter Van Dam, Patrick Pauwels, Evelien L. J. Smits, Marc Peeters and Filip Lardon
Cited by 4 | Viewed by 2131
Abstract
In this study, we aimed to study the expression of SARS-CoV-2-related surface proteins in non-small-cell lung cancer (NSCLC) cells and identify clinicopathological characteristics that are related to increased membranous (m)ACE2 protein expression and soluble (s)ACE2 levels, with a particular focus on standard of
[...] Read more.
In this study, we aimed to study the expression of SARS-CoV-2-related surface proteins in non-small-cell lung cancer (NSCLC) cells and identify clinicopathological characteristics that are related to increased membranous (m)ACE2 protein expression and soluble (s)ACE2 levels, with a particular focus on standard of care (SOC) therapies. ACE2 (
n = 107), TMPRSS2, and FURIN (
n = 38) protein expression was determined by immunohistochemical (IHC) analysis in NSCLC patients. sACE2 levels (
n = 64) were determined in the serum of lung cancer patients collected before, during, or after treatment with SOC therapies. Finally, the TCGA lung adenocarcinoma (LUAD) database was consulted to study the expression of ACE2 in EGFR- and KRAS-mutant samples and ACE2 expression was correlated with EGFR/HER, RAS, BRAF, ROS1, ALK, and MET mRNA expression. Membranous (m)ACE2 was found to be co-expressed with mFURIN and/or mTMPRSS2 in 16% of the NSCLC samples and limited to the adenocarcinoma subtype. TMPRSS2 showed predominantly atypical cytoplasmic expression. mACE2 and sACE2 were more frequently expressed in mutant EGFR patients, but not mutant-KRAS patients. A significant difference was observed in sACE2 for patients treated with targeted therapies, but not for chemo- and immunotherapy. In the TCGA LUAD cohort, ACE2 expression was significantly higher in EGFR-mutant patients and significantly lower in KRAS-mutant patients. Finally, ACE2 expression was positively correlated with ERBB2-4 and ROS1 expression and inversely correlated with KRAS, NRAS, HRAS, and MET mRNA expression. We identified a role for EGFR pathway activation in the expression of mACE2 in NSCLC cells, associated with increased sACE2 levels in patients. Therefore, it is of great interest to study SARS-CoV-2-infected EGFR-mutated NSCLC patients in greater depth in order to obtain a better understanding of how mACE2, sACE2, and SOC TKIs can affect the course of COVID-19.
Full article
►▼
Show Figures
Open AccessArticle
The AKT1E17K Allele Promotes Breast Cancer in Mice
by
Donatella Malanga, Carmelo Laudanna, Teresa Mirante, Fabiana Colelli, Simona Migliozzi, Pietro Zoppoli, Gianluca Santamaria, Luca Roberto, Carmela De Marco, Marzia Scarfò, Donatella Montanaro, Orlando Paciello, Serenella Papparella, Chiara Mignogna, Alfonso Baldi and Giuseppe Viglietto
Cited by 1 | Viewed by 2089
Abstract
The gain-of-function mutation in the pleckstrin homology domain of AKT1 (AKT1E17K) occurs in lung and breast cancer. Through the use of human cellular models and of a AKT1E17K transgenic Cre-inducible murine strain (R26-AKT1E17K mice), we have demonstrated that AKT1E17K is a bona fide
[...] Read more.
The gain-of-function mutation in the pleckstrin homology domain of AKT1 (AKT1E17K) occurs in lung and breast cancer. Through the use of human cellular models and of a AKT1E17K transgenic Cre-inducible murine strain (R26-AKT1E17K mice), we have demonstrated that AKT1E17K is a bona fide oncogene for lung epithelial cells. However, the role of AKT1E17K in breast cancer remains to be determined. Here, we report the generation and the characterization of a MMTV-CRE; R26-AKT1E17K mouse strain that expresses the mutant AKT1E17K allele in the mammary epithelium. We observed that AKT1E17K stimulates the development of mammary tumors classified as ductal adenocarcinoma of medium–high grade and presented a variety of proliferative alterations classified as adenosis with low-to-high grade dysplasia in the mammary epithelium. A subsequent immunohistochemical characterization suggested they were PR
−/HER2
−/ER
+, basal-like and CK8
−/CK10
−/CK5
+/CK14
+. We also observed that, in parallel with an increased proliferation rate, tumors expressing mutant AKT1E17K presented an activation of the GSK3/cyclin D1 pathway in the mammary epithelium and cluster significantly with the human basal-like tumors. In conclusion, we demonstrate AKT1E17K is a bona fide oncogene that can initiate tumors at high efficiency in murine mammary epithelium in vivo.
Full article
►▼
Show Figures