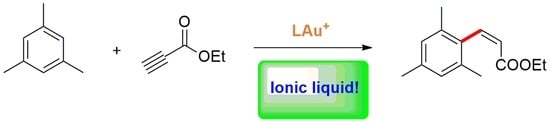
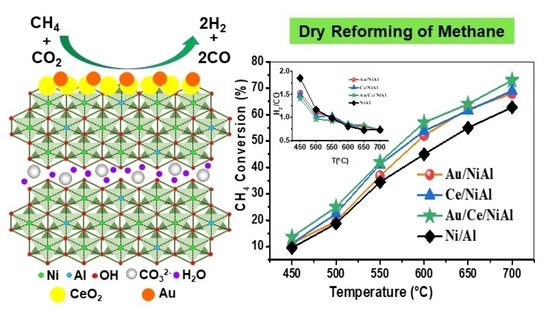
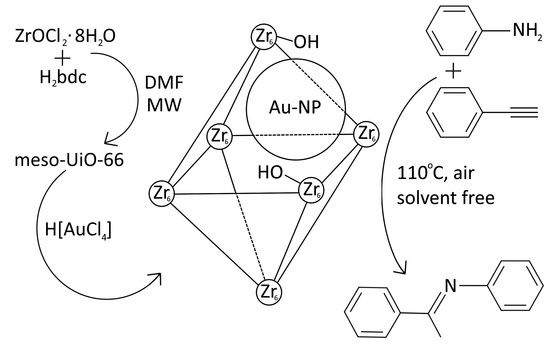
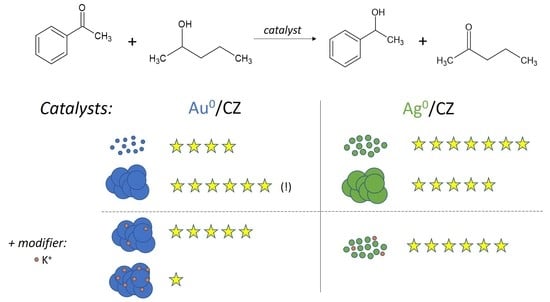
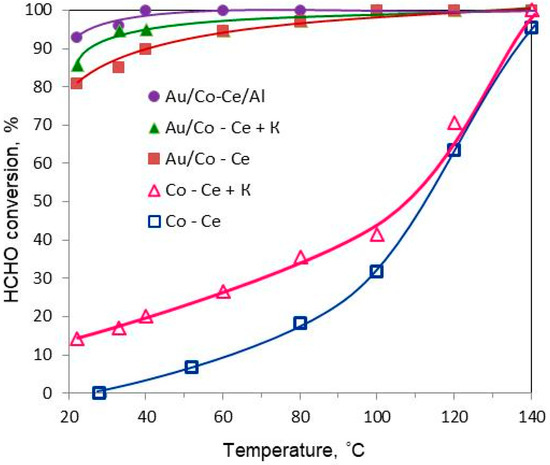
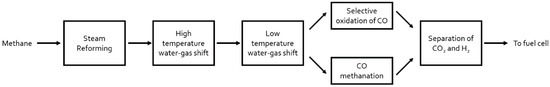
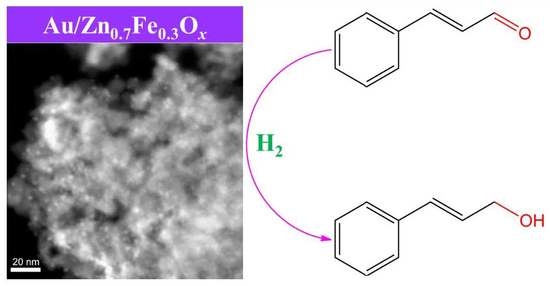
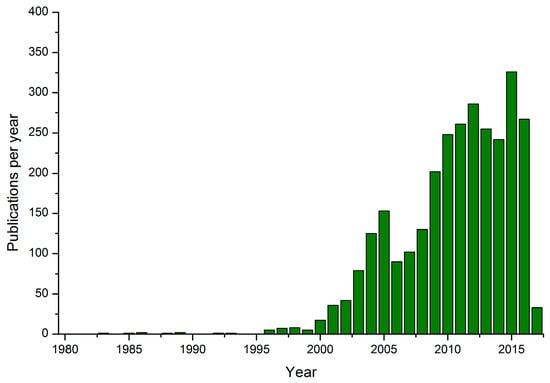
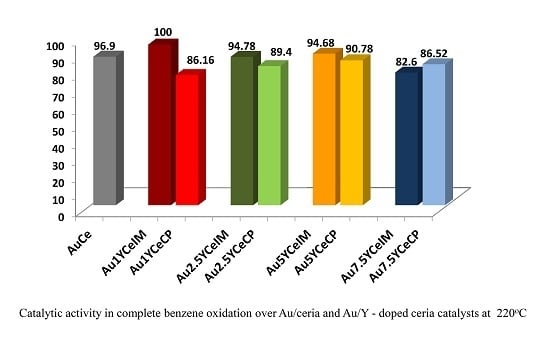
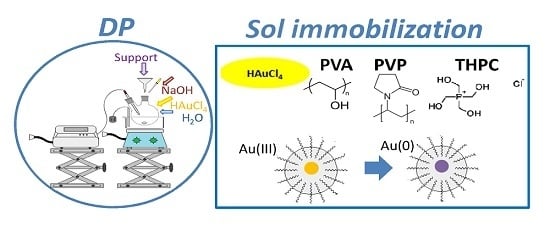
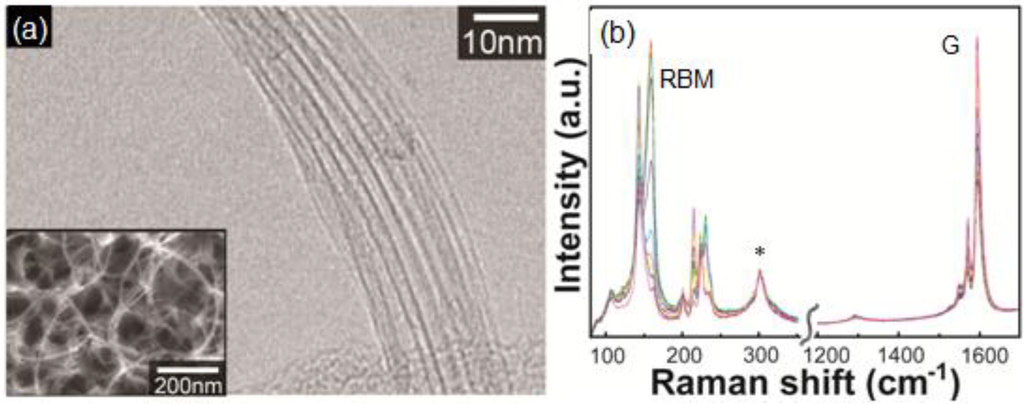
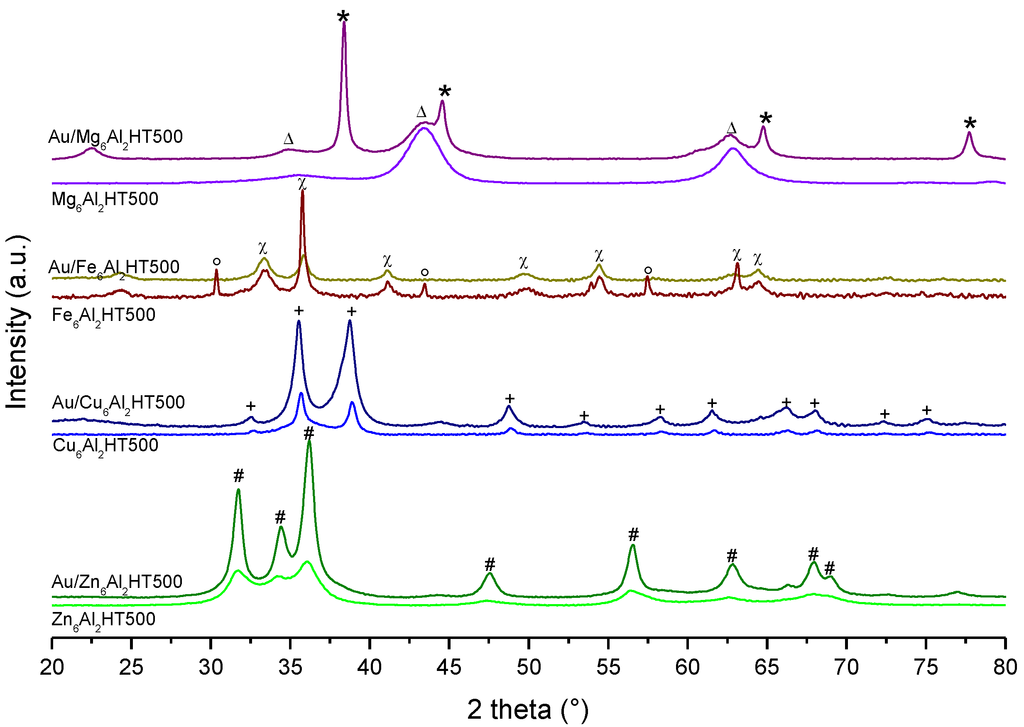
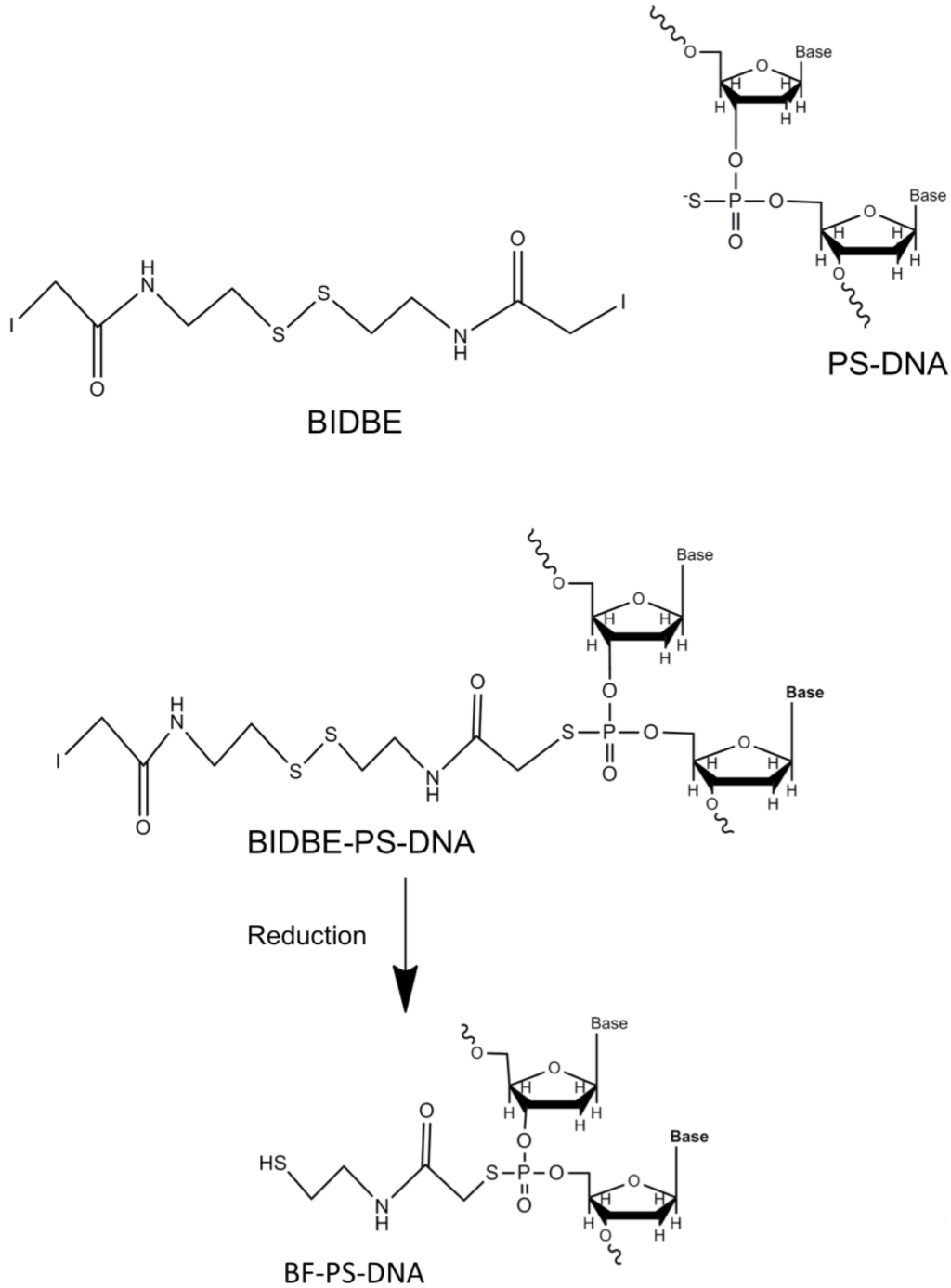
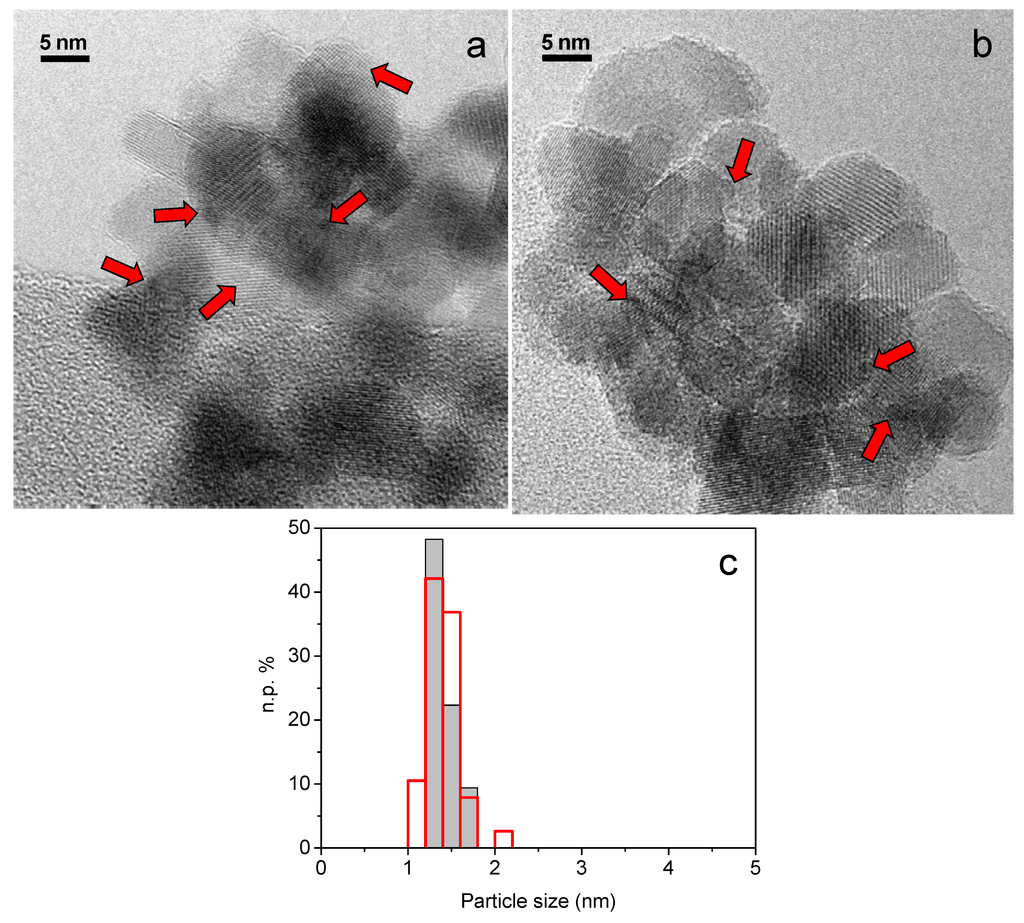
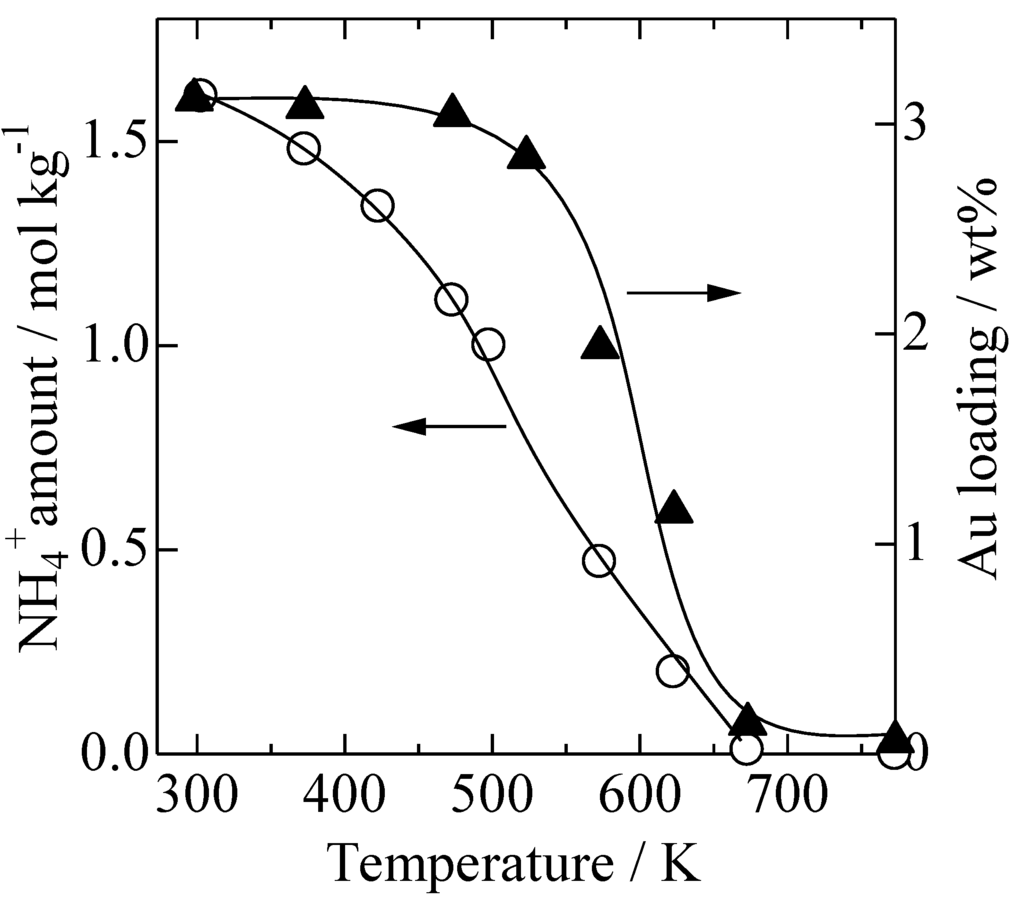
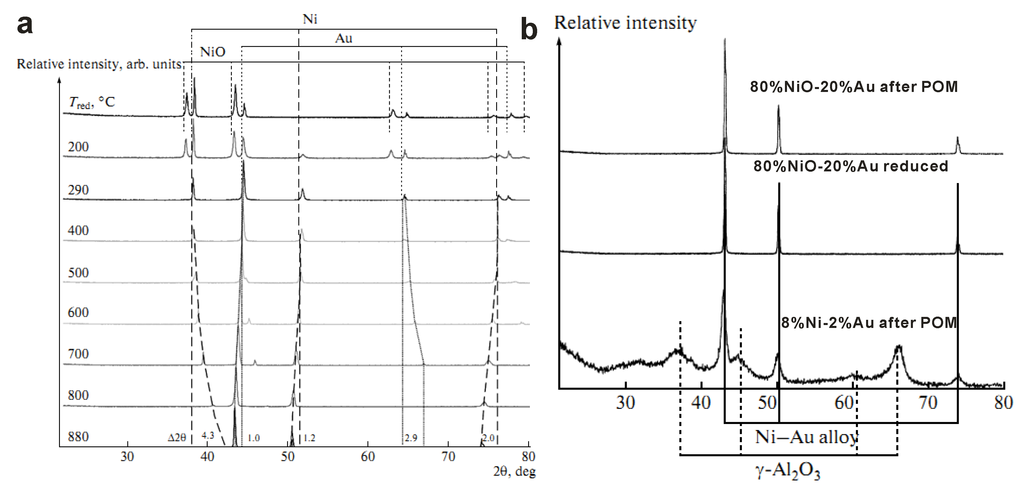
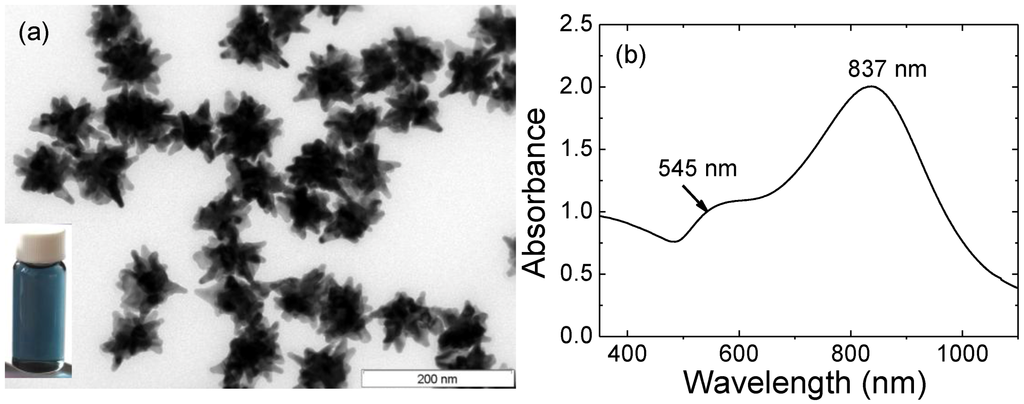
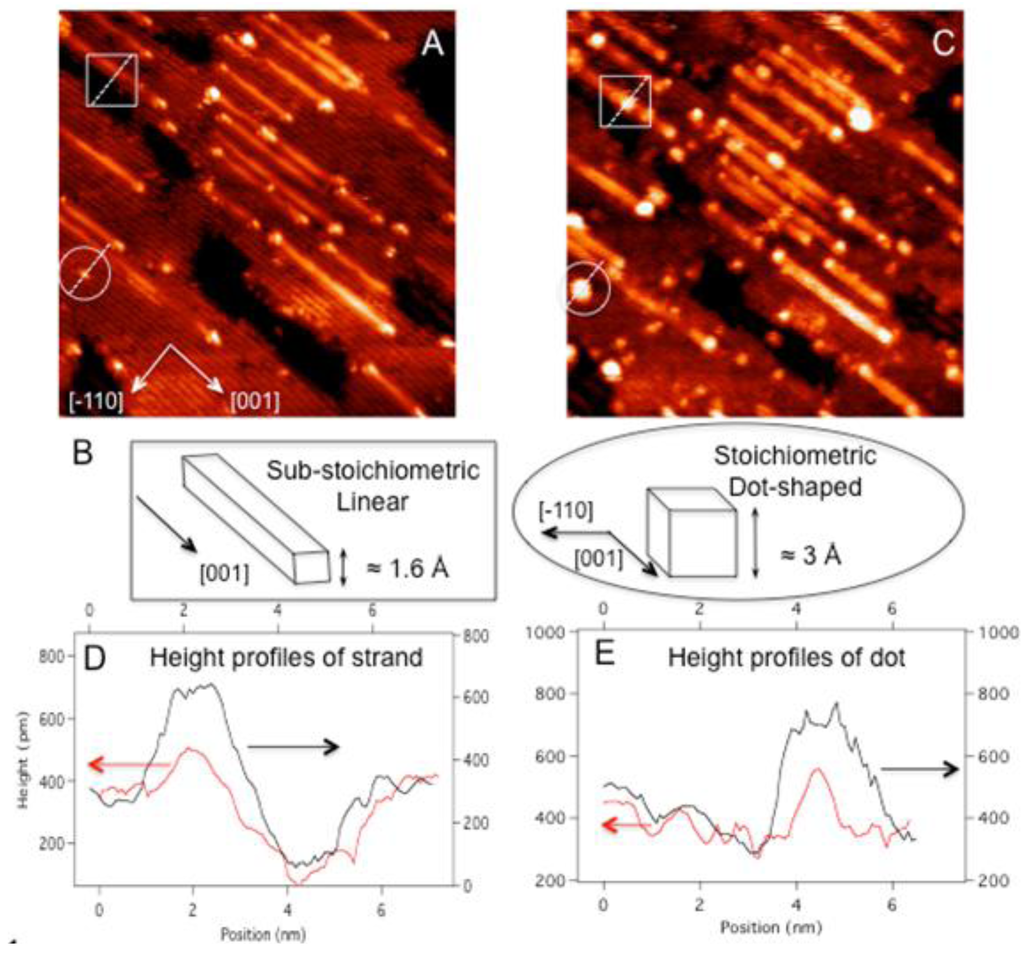
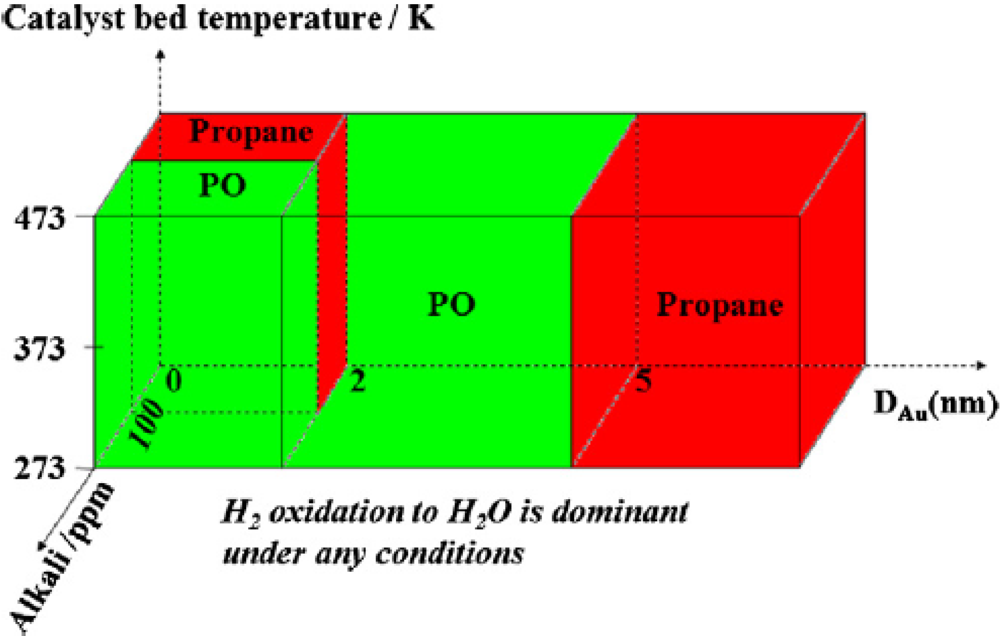
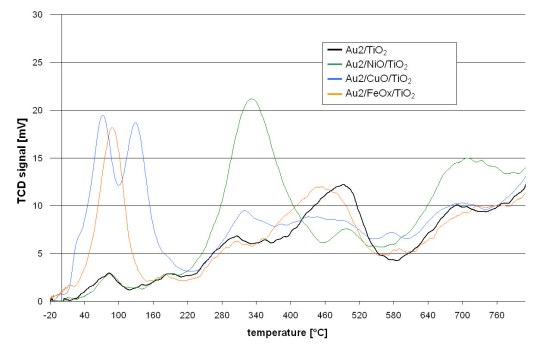
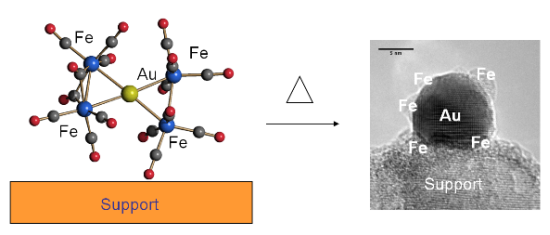
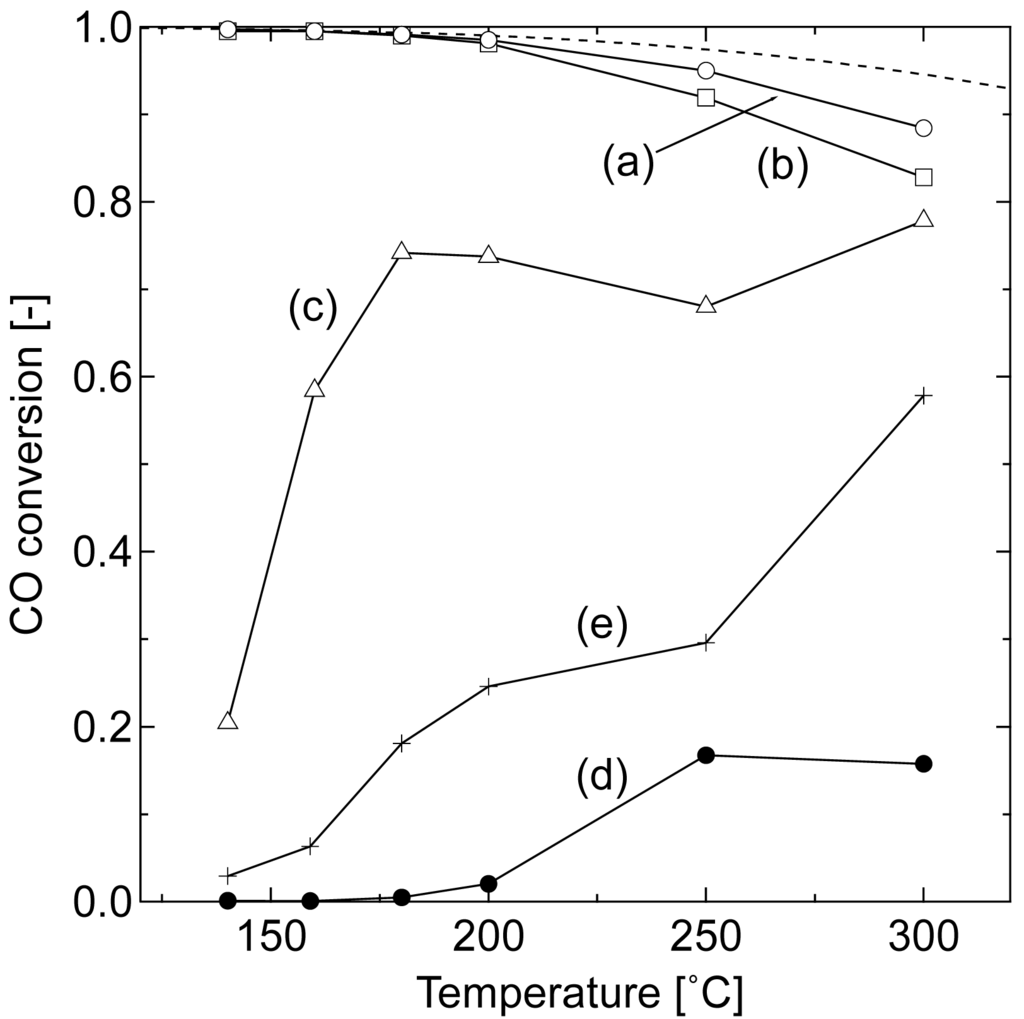
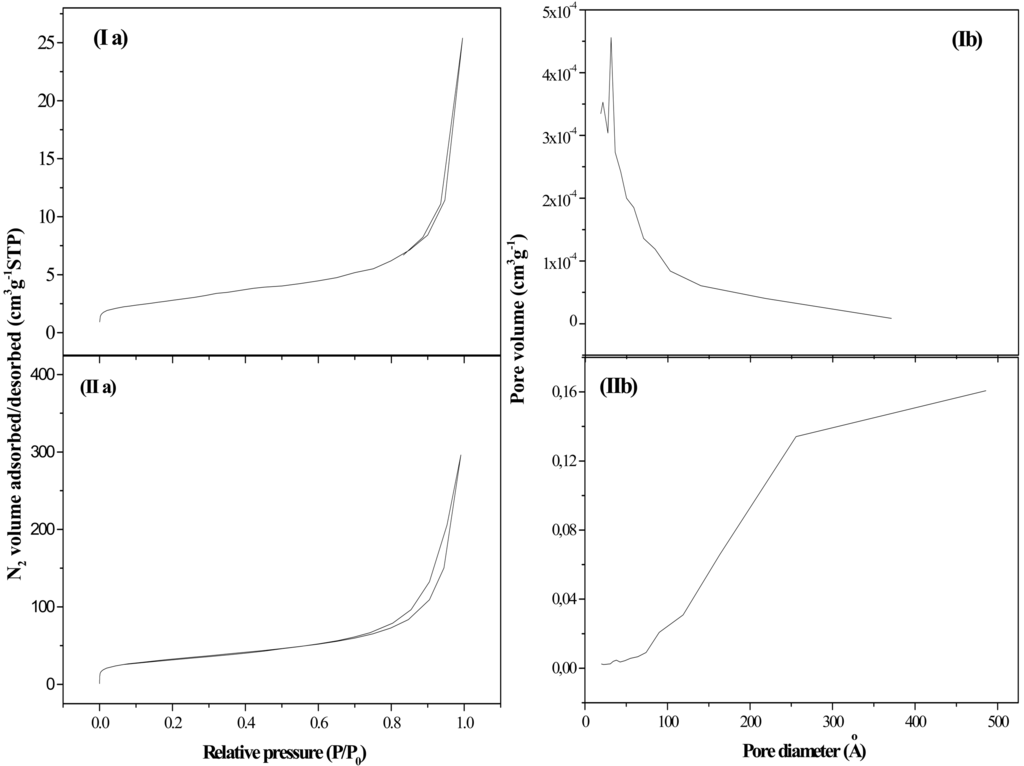
Gold Catalysts
A topical collection in Catalysts (ISSN 2073-4344).
Viewed by 93496Editors
Interests: supported noble metals; nanostructured and mesoporous oxides; inorganic perovskites; catalytic applications in soot oxidation and NOx SCR of exhaust gases emitted from stationary and mobile sources; VOC oxidation; dry/steam hydrocarbon reforming; CO2 hydrogenation to CH4 and light olefines
Special Issues, Collections and Topics in MDPI journals
Interests: synthesis, characterization and catalytic activity of supported catalysts; catalysts for environmental protection and energy production; catalysts in the petrochemical industry and refinery; gold based mono and bimetallic catalysts; photocatalytic oxidation and water splitting; hydrogen purification for fuel cell applications
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
After the first successful edition of the Topical Collection of Gold Catalysts, launched in 2016 with several interesting and highly cited papers, we decided together with the Editorial Board of Catalysts to renew this Collection, considering that interest in the catalysis of gold still remains very high, with several new fields of applications, mainly in reactions targeting environmental protection and sustainable energy topics, currently very popular and strictly related to the ongoing energy transition toward a zero-carbon economy, which is attracting the interest of the whole research and industrial community.
We thus invite all researchers working in all fields of gold catalysts to submit high-quality papers in this area, and also to serve as scholars in this field.
A brief but not complete list of the topics of this new Topical Collection could include:
- Recent progress and novel trends in the basic research of gold;
- New synthetic approaches of gold catalyst;
- Characterization and structure–activity relationship of supported mono and multimetallic catalysts;
- Investigations on the stability of gold catalysts under reaction conditions;
- Gold catalysts for sensors and pollution control devices,
- Gold catalysts for hydrogen production and purification;
- Gold catalysts for photocatalytic, photoelectrocatalytic, and thermophotocatalytic applications;
- Au-plasmon enhanced heterogeneous catalysis;
- Bimetallic and alloy Au-based catalysts;
- Photocatalytic applications of gold in homogeneous phase;
- Density Functional Theory (DFT) calculations and gold catalysis.
Dr. Leonarda Liotta
Prof. Dr. Salvatore Scirè
Collection Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 250 words) can be sent to the Editorial Office for assessment.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Catalysts is an international peer-reviewed open access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2200 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- Preparation methods
- Au oxidation state
- Au-support interaction
- Structural and electronic characterization
- CO oxidation and PROX
- Reduction of NOx and COx
- Selective hydrogenation and oxidation
- VOC and soot oxidation
- Hydrodechlorination reactions
- Stability under reaction conditions: Effect of CO2 and water added to the gas feed
- Au-based photocatalysts
Related Special Issues
- Gold Catalysts in Catalysts (16 articles - displayed below)
- New Trends in Gold Catalysts in Catalysts (9 articles - displayed below)