BNCT Drug Development and Preclinical Testing
A special issue of Cells (ISSN 2073-4409).
Deadline for manuscript submissions: closed (15 April 2023) | Viewed by 18127
Special Issue Editors
2. EBG MedAustron GmbH, Marie Curie-Straße 5A-2700 Wiener Neustadt, Austria
3. Neutron Therapy Research Center, Okayama University, Okayama 700-8530, Japan
4. German Society for Boron Neutron Capture Therapy, 45122 Essen, Germany
Interests: radiation oncology; hadron therapy; boron neutron capture therapy (BNCT); neutrons; high-LET radiation; radiation biology; eye tumors; ophthalmic oncology
Special Issues, Collections and Topics in MDPI journals
Interests: cancer biology; imaging; BNCT; elemental imaging; activable X-ray nanodrugs; theranostic compounds
Special Issues, Collections and Topics in MDPI journals
Interests: radiation oncology; gastrointestinal cancer; nanotechnology
Special Issues, Collections and Topics in MDPI journals
Special Issue Information
Dear Colleagues,
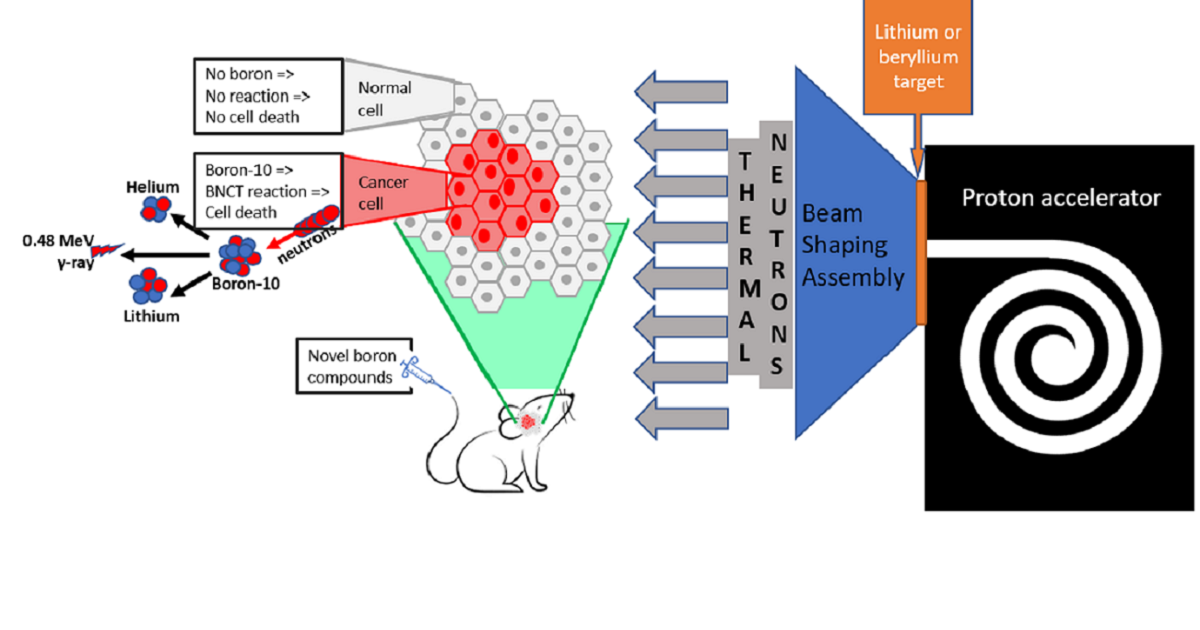
The appearance of hospital-based epithermal neutron sources has made boron neutron capture therapy (BNCT) a dedicated focus of innovative developments in radiation oncology. This binary treatment modality not only needs neutrons but also a carrier transporting B-10 selectively into cancer cells. This has fueled the quest for new compounds for BNCT and resurgence of interest in preclinical testing of these next-generation compounds.
This Special Issue of Cells is dedicated to collating results of recent preclinical research related to the development and testing of novel boron-containing therapeutic compounds and publishing a collection of intelligible and clear reviews of the most challenging aspects of drug development for BNCT. You are invited to submit your contributions in the form of original research articles, reviews, or shorter perspective articles.
Prof. Dr. Wolfgang Sauerwein
Prof. Dr. Lucie Sancey
Dr. Sunil Krishnan
Guest Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the special issue website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- Boron Neutron Capture Therapy
- BNCT
- boron-10
- drug development
- targeted therapy
- vectorized therapy
- radiation oncology
Benefits of Publishing in a Special Issue
- Ease of navigation: Grouping papers by topic helps scholars navigate broad scope journals more efficiently.
- Greater discoverability: Special Issues support the reach and impact of scientific research. Articles in Special Issues are more discoverable and cited more frequently.
- Expansion of research network: Special Issues facilitate connections among authors, fostering scientific collaborations.
- External promotion: Articles in Special Issues are often promoted through the journal's social media, increasing their visibility.
- Reprint: MDPI Books provides the opportunity to republish successful Special Issues in book format, both online and in print.
Further information on MDPI's Special Issue policies can be found here.
Related Special Issues
- Cell Biology for Boron Neutron Capture Therapy (BNCT) in Cells (4 articles)
- Biology of Boron Neutron Capture Therapy (BNCT) 2021 in Cells (4 articles)
- Biology of Boron Neutron Capture Therapy (BNCT) in Cells (10 articles)








