Cellular Senescence in Aging and Aging-Related Diseases
A special issue of Cells (ISSN 2073-4409). This special issue belongs to the section "Cellular Aging".
Deadline for manuscript submissions: closed (15 November 2022) | Viewed by 28644
Special Issue Editors
Interests: aging; senescence; cell adhesion; fibrosis; neurodegeneration
Special Issue Information
Dear Colleagues,
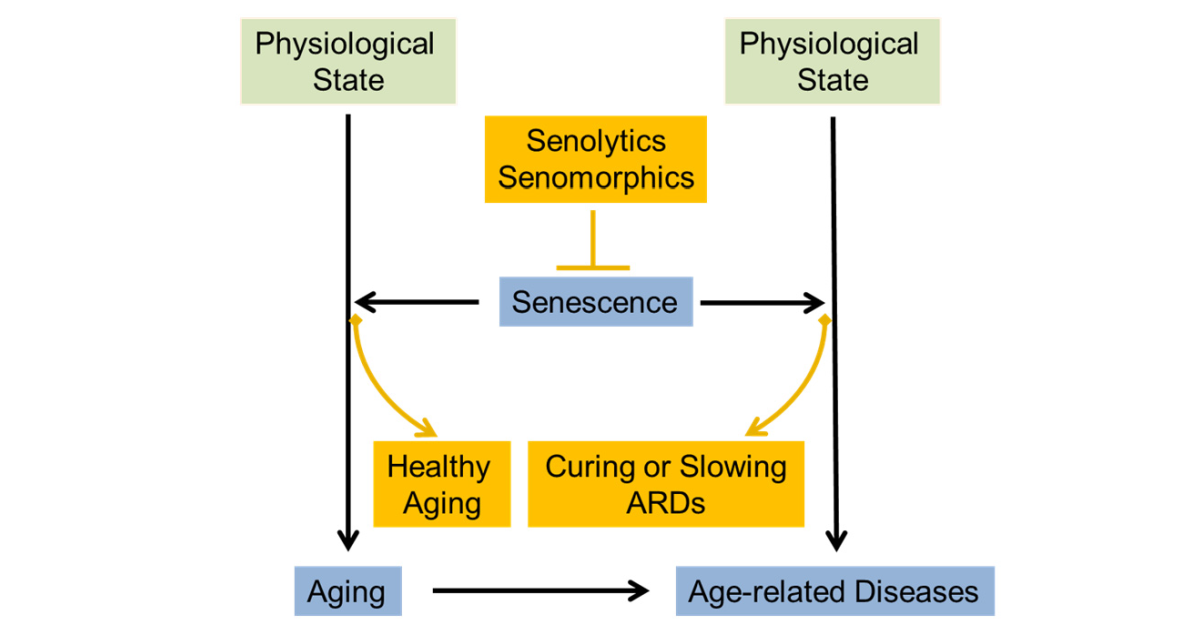
Diverse types of stress evoke cellular senescence, stress-induced premature senescence and replicative senescence, characterized by several features: a cell cycle arrest, increased senescence-associated beta-galactosidase activity, typical morphological changes and senescence-associated secretory phenotype (SASP). Hallmarks of aging include senescence; in this line, studies unraveling a causal effect of senescence on aging at the organism level are increasing. During the events of organismal aging, senescence results in both beneficial and detrimental consequences. Accumulated evidence indicates dysregulated senescence as a pathogenic mechanism in aging-related diseases (ARDs) such as cancer, diabetes, atherosclerosis, arthritis, fibrosis and neurodegenerative diseases. A couple of senolytics targeting these ARDs are currently under clinical trial. Senomorphics can also ameliorate ARDs by regulating the SASP components such as the pro-inflammatory cytokines and chemokines, and growth factors.
This Special Issue recruits a wide range of studies that examine senescence regarding the issues from the basic mechanisms to the translational applications to clinics. In particular, we welcome the studies that address unresolved questions, for instance, the necessity of simple senescence biomarkers, context-dependent heterogeneity of senescence, single-cell level analysis and novel senolytics and senomorphics.
Prof. Dr. Eung-Gook Kim
Dr. Jaehong Kim
Guest Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the special issue website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- senescence
- SASP
- aging
- age-related disease
- biomarker
- senolytics
- senomorphics
Benefits of Publishing in a Special Issue
- Ease of navigation: Grouping papers by topic helps scholars navigate broad scope journals more efficiently.
- Greater discoverability: Special Issues support the reach and impact of scientific research. Articles in Special Issues are more discoverable and cited more frequently.
- Expansion of research network: Special Issues facilitate connections among authors, fostering scientific collaborations.
- External promotion: Articles in Special Issues are often promoted through the journal's social media, increasing their visibility.
- e-Book format: Special Issues with more than 10 articles can be published as dedicated e-books, ensuring wide and rapid dissemination.
Further information on MDPI's Special Issue policies can be found here.







