Mechanisms of TGF-β Signaling in Disease Progression
A special issue of Cells (ISSN 2073-4409). This special issue belongs to the section "Cell Signaling".
Deadline for manuscript submissions: closed (10 February 2023) | Viewed by 7944
Special Issue Editors
Interests: cytokine/growth factor signalling; cancer biology; exosomes; cancer immunology
Interests: brain tumor progression and signaling
Interests: cancer immunology-TGF-β; nanomedicine; magnetic nanomaterials
Special Issue Information
Dear Colleagues,
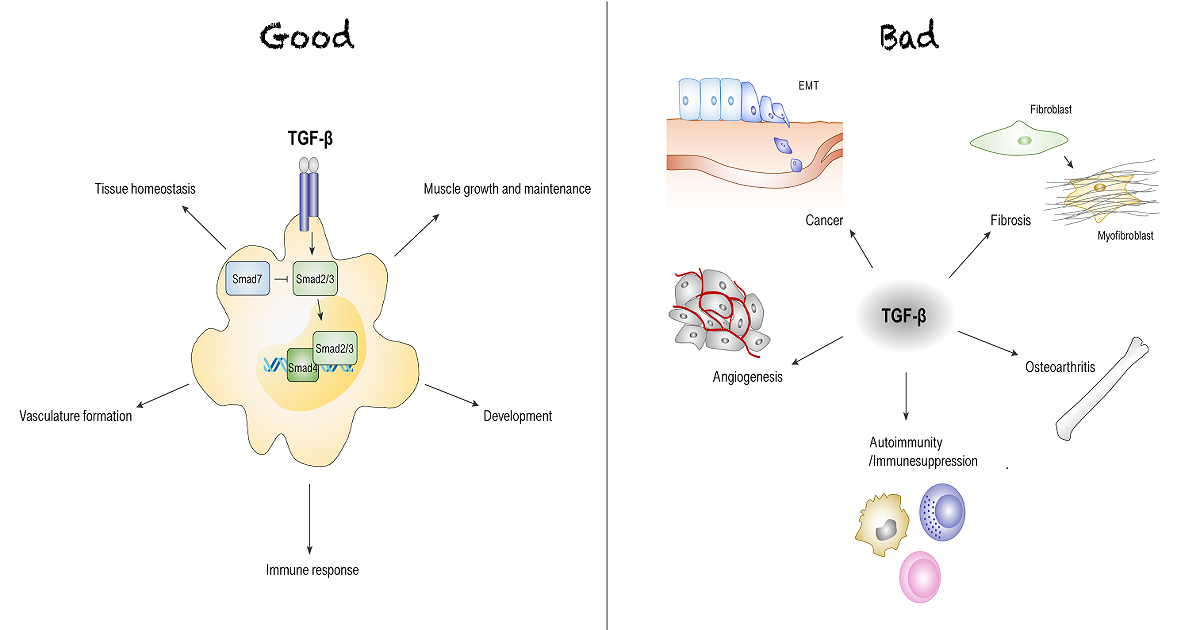
The Transforming Growth Factor-beta (TGF-β) superfamily is made up a of large number of secreted pleiotropic growth factors that regulate many processes involved in development, homeostasis of tissue function and repair, immune response and regulation, vasculature formation and muscle growth and maintenance. Due to its diverse range of functions, not surprisingly, dysregulation of signaling of the TGF-β superfamily results in a multitude of diseases including cancer, fibrosis, osteoarthritis, autoimmunity, rheumatoid arthritis, diabetes mellitus, multiple sclerosis, atherosclerosis, asthma, inflammatory bowel disease, glomerulonephritis, vascular disease, motor neuron disease and muscle atrophy and cachexia. However, the critical spatiotemporal mechanisms utilized by TGF-β to drive this multitude of pathological conditions and, importantly, how these mechanisms can be reversed therapeutically are not completely understood.
This Special Issue welcomes original research papers or focused reviews elucidating key and novel mechanistic regulation of TGF-β signaling involved in disease progression. It will also include articles that discuss the possible improvement of current therapeutic strategies targeting TGF-β signaling that are currently under investigation. This Special Issue aims to discover critical knowledge of TGF-β biology, which will underpin novel and improved therapeutic interventions targeting TGF-β signaling and activity.
Dr. Hong-Jian Zhu
Dr. Rodney Luwor
Dr. Jingyi Sheng
Dr. Lin Liu
Guest Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the special issue website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- TGF-β
- TGF-β signaling
- mechanistic regulation
- diseases
- treatment
Benefits of Publishing in a Special Issue
- Ease of navigation: Grouping papers by topic helps scholars navigate broad scope journals more efficiently.
- Greater discoverability: Special Issues support the reach and impact of scientific research. Articles in Special Issues are more discoverable and cited more frequently.
- Expansion of research network: Special Issues facilitate connections among authors, fostering scientific collaborations.
- External promotion: Articles in Special Issues are often promoted through the journal's social media, increasing their visibility.
- e-Book format: Special Issues with more than 10 articles can be published as dedicated e-books, ensuring wide and rapid dissemination.
Further information on MDPI's Special Issue policies can be found here.









