PSMA in Prostate Cancer
A special issue of International Journal of Molecular Sciences (ISSN 1422-0067). This special issue belongs to the section "Molecular Oncology".
Deadline for manuscript submissions: closed (30 September 2021) | Viewed by 41111
Special Issue Editor
Interests: cancer biology; urologic oncology; tumor markers
Special Issues, Collections and Topics in MDPI journals
Special Issue Information
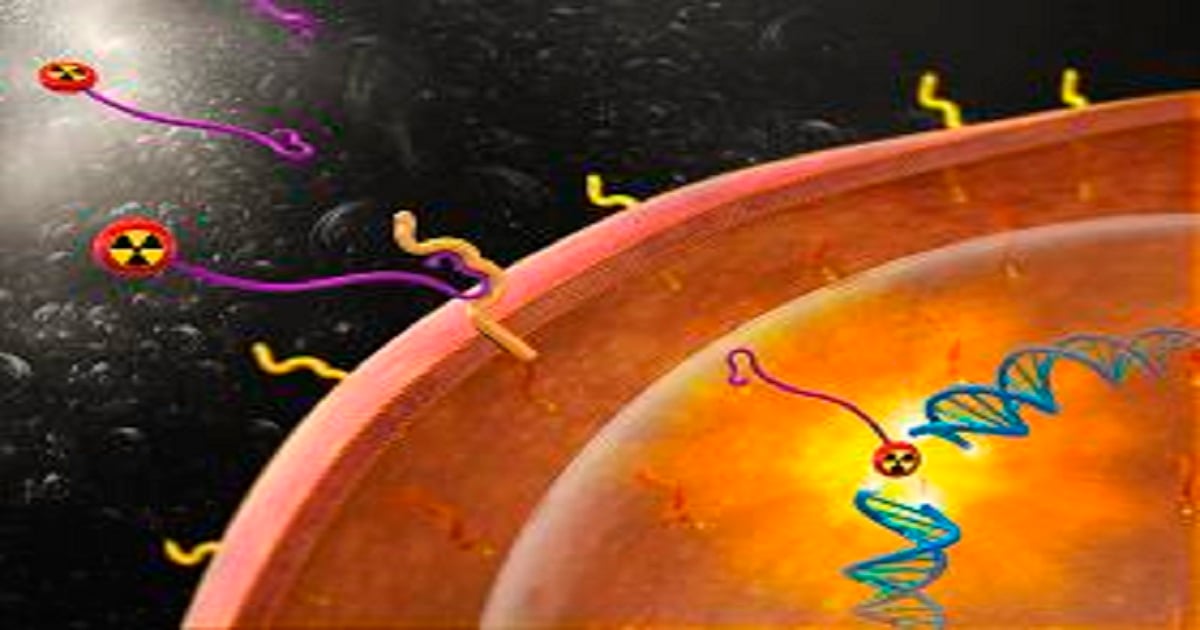
Dear Colleagues,
Prostate Cancer has the second highest mortality for men in Western Societies. Prostate Specific Membrane Antigen (PSMA) is a glycoprotein that is highly expressed in plasma membrane of prostate cancer especially in the most aggressive forms for prostate cancer. In the recent decade PSMA PET/CT has become widely used in diagnostics of patients with metastatic castration-resistant prostate cancer (mCRPC). PSMA PET/CT has a high detection rate even for patients with low values of prostate specific antigen (PSA). Staging with PSMA PET/CT change planned treatment for more than a quarter of the patients. Radioligands can bind to the extracellular part of PSMA. Thereby the radioligand is absorbed into the cancer cells and damage the DNA of the cells. PSMA radioligand therapy is a safe and effective treatment of patients with metastatic castration-resistant prostate cancer as documented in two published randomized controlled trials, the TheraP and VISION trials. This issue of International Journal of Molecular Sciences provides up-to-date information on the use of PSMA for diagnostics and treatment of patients with prostate cancer. The issue serves as a very useful resource tool for patients, physicians, and researchers dealing with the challenge of mCRPC.
This issue has a joint special issue (https://www.mdpi.com/journal/diagnostics/special_issues/PSMA_diag) with our sister journal /Diagnostics/ (ISSN 2075-4418, IF: 3.110, https://www.mdpi.com/journal/diagnostics). For papers focused on molecular science are encouraged to submit to IJMS, for others to Diagnostics.
Dr. Finn Edler von Eyben
Guest Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the special issue website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. International Journal of Molecular Sciences is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. There is an Article Processing Charge (APC) for publication in this open access journal. For details about the APC please see here. Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- prospective studies
- prostate cancer
- retrospective studies
- reviews
- tumor biology
- theranostics
Benefits of Publishing in a Special Issue
- Ease of navigation: Grouping papers by topic helps scholars navigate broad scope journals more efficiently.
- Greater discoverability: Special Issues support the reach and impact of scientific research. Articles in Special Issues are more discoverable and cited more frequently.
- Expansion of research network: Special Issues facilitate connections among authors, fostering scientific collaborations.
- External promotion: Articles in Special Issues are often promoted through the journal's social media, increasing their visibility.
- Reprint: MDPI Books provides the opportunity to republish successful Special Issues in book format, both online and in print.
Further information on MDPI's Special Issue policies can be found here.






