Efficacy of Artificial Intelligence-Assisted Discrimination of Oral Cancerous Lesions from Normal Mucosa Based on the Oral Mucosal Image: A Systematic Review and Meta-Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Selection Criteria
2.3. Data Extraction and Risk of Bias Assessment
2.4. Statistical Analysis and Outcome Measurements
3. Results
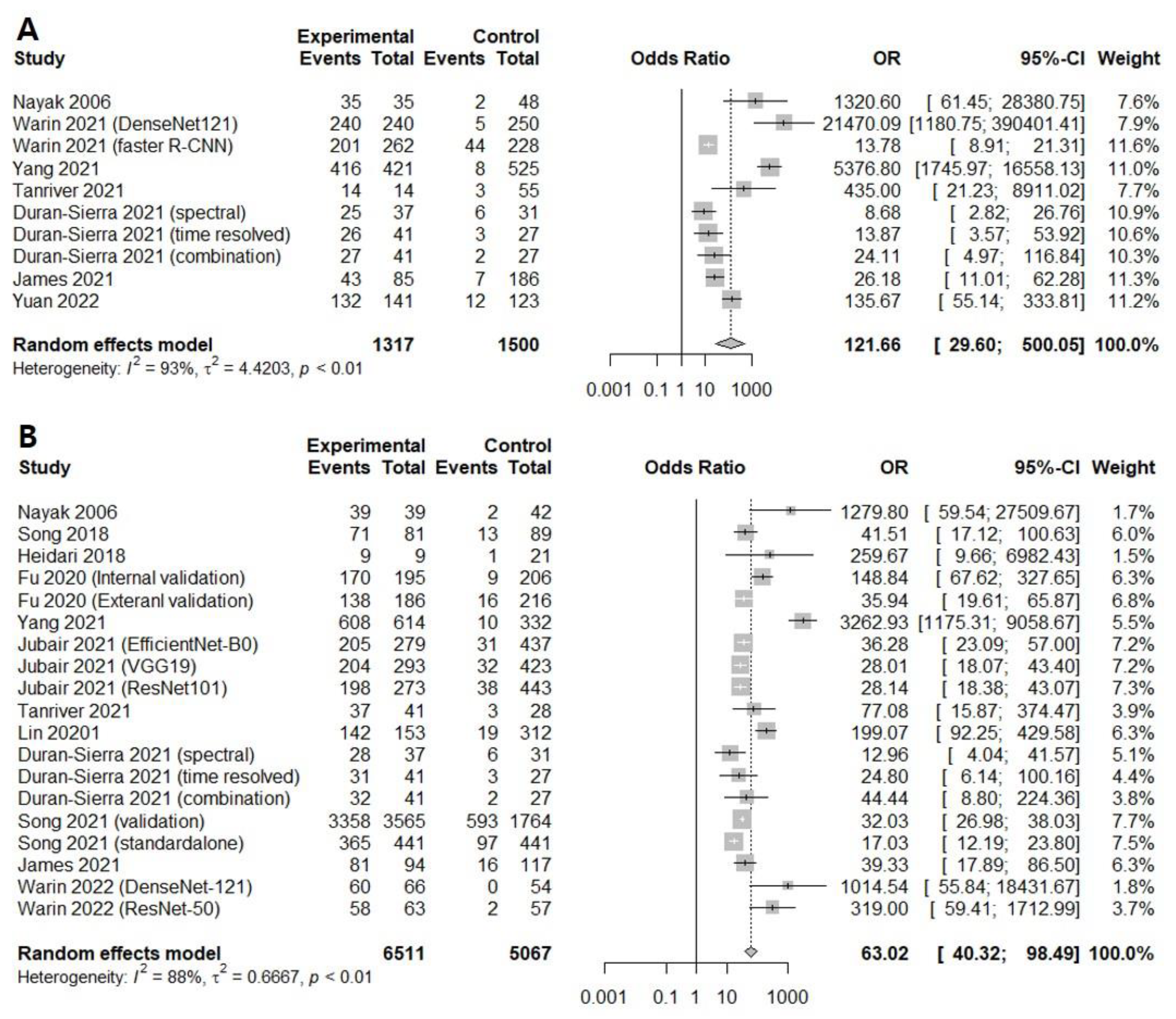
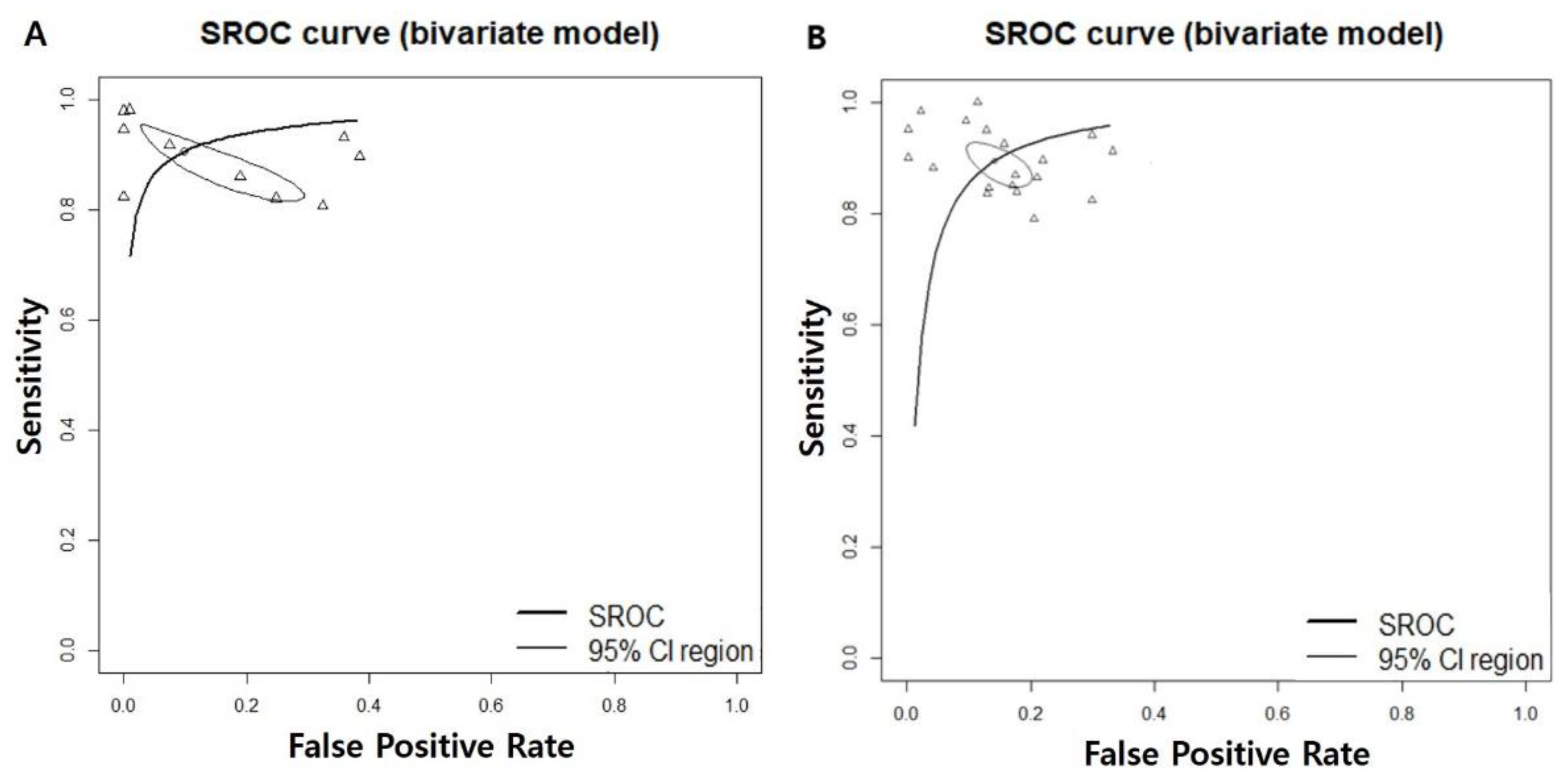
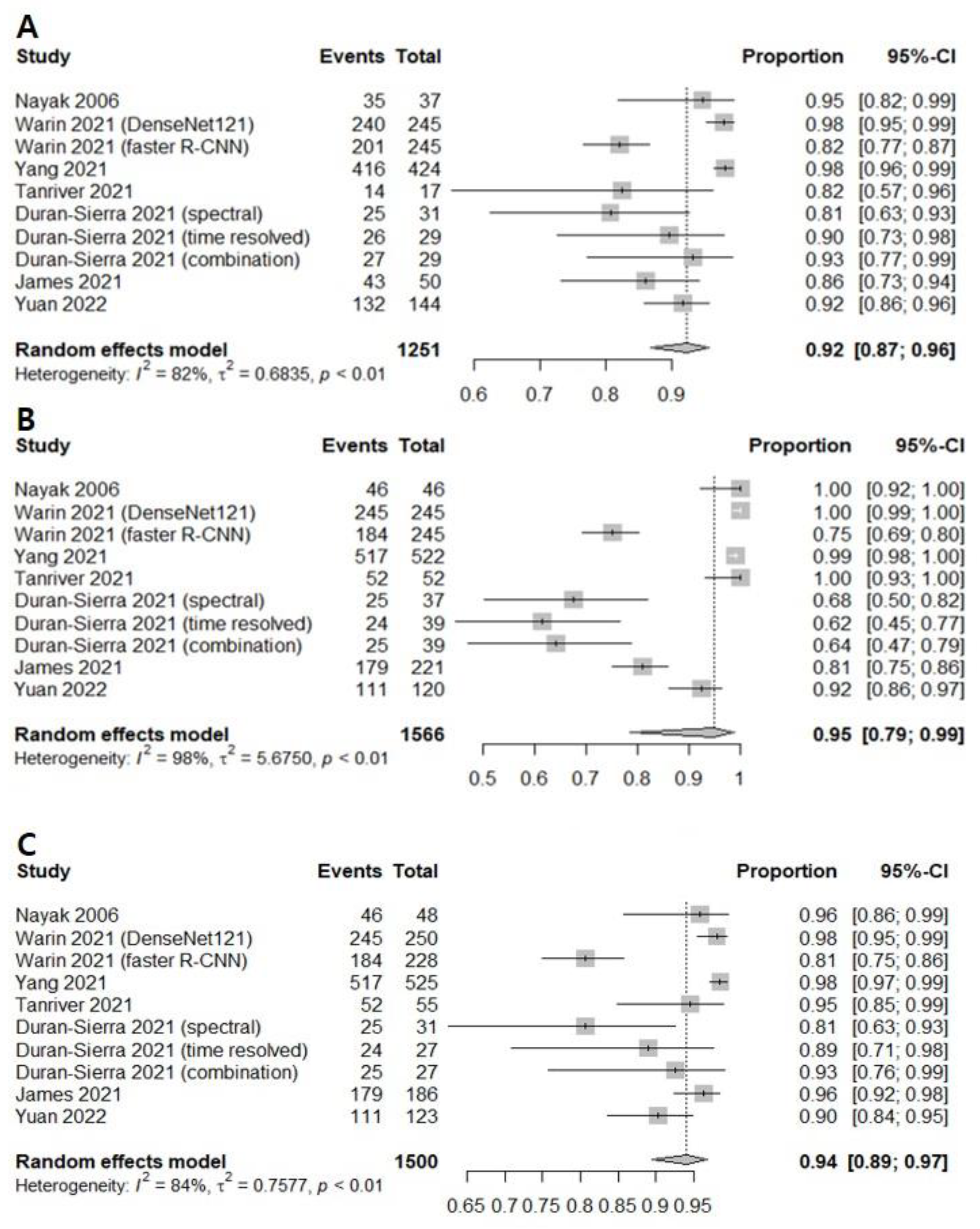
3.1. Diagnostic Accuracy of AI-Assisted Screening of Oral Mucosal Cancerous Lesions
3.2. Diagnostic Accuracy of AI-Assisted Screening of Oral Mucosal Precancerous and Cancerous Lesions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cunningham, M.J.; Johnson, J.T.; Myers, E.N.; Schramm, V.L., Jr.; Thearle, P.B. Cervical lymph node metastasis after local excision of early squamous cell carcinoma of the oral cavity. Am. J. Surg. 1986, 152, 361–366. [Google Scholar] [CrossRef]
- Messadi, D.V. Diagnostic aids for detection of oral precancerous conditions. Int. J. Oral Sci. 2013, 5, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Song, E.A.; Kim, S.W.; Hwang, S.H. Efficacy of toluidine blue in the diagnosis and screening of oral cancer and pre-cancer: A systematic review and meta-analysis. Clin. Otolaryngol. 2021, 46, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Awan, K.; Morgan, P.; Warnakulasuriya, S. Evaluation of an autofluorescence based imaging system (VELscope™) in the detection of oral potentially malignant disorders and benign keratoses. Oral Oncol. 2011, 47, 274–277. [Google Scholar] [CrossRef]
- Tsai, M.-T.; Lee, H.-C.; Lee, C.-K.; Yu, C.-H.; Chen, H.-M.; Chiang, C.-P.; Chang, C.-C.; Wang, Y.-M.; Yang, C. Effective indicators for diagnosis of oral cancer using optical coherence tomography. Opt. Express 2008, 16, 15847–15862. [Google Scholar]
- Fu, Q.; Chen, Y.; Li, Z.; Jing, Q.; Hu, C.; Liu, H.; Bao, J.; Hong, Y.; Shi, T.; Li, K. A deep learning algorithm for detection of oral cavity squamous cell carcinoma from photographic images: A retrospective study. eClinicalMedicine 2020, 27, 100558. [Google Scholar] [CrossRef]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef]
- Gulshan, V.; Peng, L.; Coram, M.; Stumpe, M.C.; Wu, D.; Narayanaswamy, A.; Venugopalan, S.; Widner, K.; Madams, T.; Cuadros, J.; et al. Development and Validation of a Deep Learning Algorithm for Detection of Diabetic Retinopathy in Retinal Fundus Photographs. JAMA 2016, 316, 2402–2410. [Google Scholar] [CrossRef]
- Esteva, A.; Kuprel, B.; Novoa, R.A.; Ko, J.; Swetter, S.M.; Blau, H.M.; Thrun, S. Dermatologist-level classification of skin cancer with deep neural networks. Nature 2017, 542, 115–118. [Google Scholar] [CrossRef]
- Varshni, D.; Thakral, K.; Agarwal, L.; Nijhawan, R.; Mittal, A. Pneumonia detection using CNN based feature extraction. In Proceedings of the 2019 IEEE International Conference on Electrical, Computer and Communication Technologies (ICECCT), Coimbatore, India, 20–22 February 2019; pp. 1–7. [Google Scholar]
- Ilhan, B.; Guneri, P.; Wilder-Smith, P. The contribution of artificial intelligence to reducing the diagnostic delay in oral cancer. Oral Oncol. 2021, 116, 105254. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, S.W.; Kim, S.H.; Jung, J.H.; Hwang, S.H. Usefulness of imaging studies for diagnosing and localizing cerebrospinal fluid rhinorrhea: A systematic review and meta-analysis. Int. Forum. Allergy Rhinol. 2022, 12, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Nayak, G.; Kamath, S.; Pai, K.M.; Sarkar, A.; Ray, S.; Kurien, J.; D’Almeida, L.; Krishnanand, B.; Santhosh, C.; Kartha, V. Principal component analysis and artificial neural network analysis of oral tissue fluorescence spectra: Classification of normal premalignant and malignant pathological conditions. Biopolym. Orig. Res. Biomol. 2006, 82, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Heidari, A.E.; Sunny, S.P.; James, B.L.; Lam, T.M.; Tran, A.V.; Yu, J.; Ramanjinappa, R.D.; Uma, K.; Birur, P.; Suresh, A. Optical coherence tomography as an oral cancer screening adjunct in a low resource settings. IEEE J. Sel. Top. Quantum Electron. 2018, 25, 7202008. [Google Scholar] [CrossRef] [Green Version]
- Song, B.; Sunny, S.; Uthoff, R.D.; Patrick, S.; Suresh, A.; Kolur, T.; Keerthi, G.; Anbarani, A.; Wilder-Smith, P.; Kuriakose, M.A. Automatic classification of dual-modalilty, smartphone-based oral dysplasia and malignancy images using deep learning. Biomed. Opt. Express 2018, 9, 5318–5329. [Google Scholar] [CrossRef] [PubMed]
- Duran-Sierra, E.; Cheng, S.; Cuenca, R.; Ahmed, B.; Ji, J.; Yakovlev, V.V.; Martinez, M.; Al-Khalil, M.; Al-Enazi, H.; Cheng, Y.-S.L. Machine-Learning Assisted Discrimination of Precancerous and Cancerous from Healthy Oral Tissue Based on Multispectral Autofluorescence Lifetime Imaging Endoscopy. Cancers 2021, 13, 4751. [Google Scholar] [CrossRef]
- James, B.L.; Sunny, S.P.; Heidari, A.E.; Ramanjinappa, R.D.; Lam, T.; Tran, A.V.; Kankanala, S.; Sil, S.; Tiwari, V.; Patrick, S. Validation of a Point-of-Care Optical Coherence Tomography Device with Machine Learning Algorithm for Detection of Oral Potentially Malignant and Malignant Lesions. Cancers 2021, 13, 3583. [Google Scholar] [CrossRef]
- Jubair, F.; Al-karadsheh, O.; Malamos, D.; Al Mahdi, S.; Saad, Y.; Hassona, Y. A novel lightweight deep convolutional neural network for early detection of oral cancer. Oral Dis. 2022, 28, 1123–1130. [Google Scholar] [CrossRef]
- Lin, H.; Chen, H.; Weng, L.; Shao, J.; Lin, J. Automatic detection of oral cancer in smartphone-based images using deep learning for early diagnosis. J. Biomed. Opt. 2021, 26, 086007. [Google Scholar] [CrossRef]
- Song, B.; Sunny, S.; Li, S.; Gurushanth, K.; Mendonca, P.; Mukhia, N.; Patrick, S.; Gurudath, S.; Raghavan, S.; Imchen, T.; et al. Mobile-based oral cancer classification for point-of-care screening. J. Biomed. Opt. 2021, 26, 065003. [Google Scholar] [CrossRef]
- Tanriver, G.; Soluk Tekkesin, M.; Ergen, O. Automated Detection and Classification of Oral Lesions Using Deep Learning to Detect Oral Potentially Malignant Disorders. Cancers 2021, 13, 2766. [Google Scholar] [CrossRef]
- Warin, K.; Limprasert, W.; Suebnukarn, S.; Jinaporntham, S.; Jantana, P. Automatic classification and detection of oral cancer in photographic images using deep learning algorithms. J. Oral Pathol. Med. 2021, 50, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Shang, J.; Liu, C.; Zhang, J.; Liang, Y. Identification of oral precancerous and cancerous tissue by swept source optical coherence tomography. Lasers Surg. Med. 2022, 54, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Warin, K.; Limprasert, W.; Suebnukarn, S.; Jinaporntham, S.; Jantana, P. Performance of deep convolutional neural network for classification and detection of oral potentially malignant disorders in photographic images. Int. J. Oral Maxillofac. Surg. 2022, 51, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Cheng, L.; Yang, J.; Yin, B.; Fan, X.; Yang, J.; Li, S.; Zhong, J.; Huang, X. Noninvasive oral cancer screening based on local residual adaptation network using optical coherence tomography. Med. Biol. Eng. Comput. 2022, 60, 1363–1375. [Google Scholar] [CrossRef]
- Scott, S.E.; Grunfeld, E.A.; McGurk, M. Patient’s delay in oral cancer: A systematic review. Community Dent. Oral Epidemiol. 2006, 34, 337–343. [Google Scholar] [CrossRef]
- Epstein, J.B.; Güneri, P.; Boyacioglu, H.; Abt, E. The limitations of the clinical oral examination in detecting dysplastic oral lesions and oral squamous cell carcinoma. J. Am. Dent. Assoc. 2012, 143, 1332–1342. [Google Scholar] [CrossRef]
- Camalan, S.; Mahmood, H.; Binol, H.; Araujo, A.L.D.; Santos-Silva, A.R.; Vargas, P.A.; Lopes, M.A.; Khurram, S.A.; Gurcan, M.N. Convolutional Neural Network-Based Clinical Predictors of Oral Dysplasia: Class Activation Map Analysis of Deep Learning Results. Cancers 2021, 13, 1291. [Google Scholar] [CrossRef]
- Mintz, Y.; Brodie, R. Introduction to artificial intelligence in medicine. Minim. Invasive Ther. Allied Technol. 2019, 28, 73–81. [Google Scholar] [CrossRef]
- Pavlova, I.; Williams, M.; El-Naggar, A.; Richards-Kortum, R.; Gillenwater, A. Understanding the biological basis of autofluorescence imaging for oral cancer detection: High-resolution fluorescence microscopy in viable tissue. Clin. Cancer Res. 2008, 14, 2396–2404. [Google Scholar] [CrossRef] [Green Version]
- Skala, M.C.; Riching, K.M.; Gendron-Fitzpatrick, A.; Eickhoff, J.; Eliceiri, K.W.; White, J.G.; Ramanujam, N. In vivo multiphoton microscopy of NADH and FAD redox states, fluorescence lifetimes, and cellular morphology in precancerous epithelia. Proc. Natl. Acad. Sci. USA 2007, 104, 19494–19499. [Google Scholar] [CrossRef] [Green Version]
- Mehrotra, R.; Singh, M.; Thomas, S.; Nair, P.; Pandya, S.; Nigam, N.S.; Shukla, P. A cross-sectional study evaluating chemiluminescence and autofluorescence in the detection of clinically innocuous precancerous and cancerous oral lesions. J. Am. Dent. Assoc. 2010, 141, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Farah, C.S.; McIntosh, L.; Georgiou, A.; McCullough, M.J. Efficacy of tissue autofluorescence imaging (VELScope) in the visualization of oral mucosal lesions. Head Neck 2012, 34, 856–862. [Google Scholar] [CrossRef] [PubMed]
- Lagarto, J.L.; Villa, F.; Tisa, S.; Zappa, F.; Shcheslavskiy, V.; Pavone, F.S.; Cicchi, R. Real-time multispectral fluorescence lifetime imaging using Single Photon Avalanche Diode arrays. Sci. Rep. 2020, 10, 8116. [Google Scholar] [CrossRef]
- Uthoff, R.D.; Song, B.; Sunny, S.; Patrick, S.; Suresh, A.; Kolur, T.; Keerthi, G.; Spires, O.; Anbarani, A.; Wilder-Smith, P. Point-of-care, smartphone-based, dual-modality, dual-view, oral cancer screening device with neural network classification for low-resource communities. PLoS ONE 2018, 13, e0207493. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A.; et al. Optical coherence tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Reference | Risk of Bias | Concerns about Application | |||||
|---|---|---|---|---|---|---|---|
| Patient Selection | Index Test | Reference Standard | Flow and Timing | Patient Selection | Index Test | Reference Standard | |
| Nayak 2006 [13] | Unclear | Low | Unclear | Unclear | Low | Low | Low |
| Heidari 2018 [14] | Low | Low | Low | Low | Low | Low | Low |
| Song 2018 [15] | Low | Low | Low | Low | Low | Low | Low |
| Fu 2020 [6] | high | Low | Low | Low | Low | Low | Low |
| Duran-Sierra 2021 [16] | Unclear | Low | Unclear | Unclear | Low | Low | Low |
| James 2021 [17] | Low | Low | Unclear | Low | Low | Low | Low |
| Jubair 2021 [18] | Unclear | Low | Low | Low | Low | Low | Low |
| Lin 2021 [19] | Unclear | Low | Unclear | Low | Low | Low | Low |
| Song 2021 [20] | Low | Low | Low | Low | Low | Low | Low |
| Tanriver 2021 [21] | Low | Low | Low | Low | Low | Low | Low |
| Warin 2021 [22] | Low | Low | Low | Low | Low | Low | Low |
| Yang 2021 [23] | Low | Low | Low | Low | Low | Low | Low |
| Warin 2022 [24] | Low | Low | Low | Unclear | Low | Low | Low |
| Yuan 2022 [25] | Low | Low | Low | Low | Low | Low | Low |
| Subgroup | Study (n) | DOR [95% CIs] | Sensitivity [95% CIs] | Specificity [95% CIs] | NPV [95% CIs] | AUC |
|---|---|---|---|---|---|---|
| 7 | 121.6609 [29.5996; 500.0534]; I2 = 93.5% | 0.9232 [0.8686; 0.9562]; I2 = 81.9% | 0.9494 [0.7850; 0.9897]; I2 = 98.3% | 0.9405 [0.8947; 0.9671]; I2 = 83.6% | 0.948 | |
| Image tool | ||||||
| Autofluorescence | 2 | 25.9083 [ 6.3059; 106.4464]; I2 = 68.0% | 0.8972 [0.8262; 0.9413]; I2 = 63.5% | 0.8213 [0.4430; 0.9637]; 94.0% | 0.9041 [0.8263; 0.9492]; 23.9% | |
| Optical coherense tomography | 3 | 261.9981 [14.7102; 4666.3521]; I2 = 96.3% | 0.9419 [0.8544; 0.9781]; I2 = 84.4% | 0.9461 [0.7931; 0.9877]; 94.6% | 0.9625 [0.9106; 0.9848]; 81.9% | |
| Photographic image | 2 | 431.6524 [ 4.0037; 46537.4743]; I2 = 93.0% | 0.9149 [0.7475; 0.9750]; I2 = 87.4% | 0.9983 [0.2906; 1.0000]; 94.9% | 0.9381 [0.8109; 0.9816]; 87.5% | |
| 0.2332 | 0.5910 | 0.2907 | 0.2291 | |||
| Subgroup | Study (n) | DOR [95% CIs] | Sensitivity [95% CIs] | Specificity [95% CIs] | NPV [95% CIs] | AUC |
|---|---|---|---|---|---|---|
| 12 | 63.0193 [40.3234; 98.4896]; I2 = 88.2% | 0.9094 [0.8725; 0.9364]; I2 = 92.3% | 0.8848 [0.8400; 0.9183]; I2 = 93.8% | 0.9169 [0.8815; 0.9424]; I2 = 92.8% | 0.943 | |
| Image tool | ||||||
| Autofluorescence | 4 | 27.6313 [17.2272; 44.3186]; I2 = 69.3% | 0.8562 [0.8002; 0.8985]; I2 = 69.6% | 0.8356 [0.7591; 0.8913]; 86.8% | 0.8405 [0.7487; 0.9031]; 91.1% | |
| Optical coherense tomography | 3 | 324.3335 [10.2511; 10261.6006]; I2 = 95.6% | 0.9424 [0.8000; 0.9853]; I2 = 88.3% | 0.9653 [0.8737; 0.9911]; 79.8% | 0.9399 [0.8565; 0.9762]; 75.7% | |
| Photographic image | 5 | 66.8107 [38.0216; 117.3983]; I2 = 81.7% | 0.9123 [0.8683; 0.9426]; I2 = 79.5% | 0.8779 [0.8322; 0.9125]; 87.4% | 0.9311 [0.9196; 0.9410]; 0.0% | |
| 0.0312 | 0.1120 | 0.0659 | 0.0073 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-S.; Kim, B.G.; Hwang, S.H. Efficacy of Artificial Intelligence-Assisted Discrimination of Oral Cancerous Lesions from Normal Mucosa Based on the Oral Mucosal Image: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 3499. https://doi.org/10.3390/cancers14143499
Kim J-S, Kim BG, Hwang SH. Efficacy of Artificial Intelligence-Assisted Discrimination of Oral Cancerous Lesions from Normal Mucosa Based on the Oral Mucosal Image: A Systematic Review and Meta-Analysis. Cancers. 2022; 14(14):3499. https://doi.org/10.3390/cancers14143499
Chicago/Turabian StyleKim, Ji-Sun, Byung Guk Kim, and Se Hwan Hwang. 2022. "Efficacy of Artificial Intelligence-Assisted Discrimination of Oral Cancerous Lesions from Normal Mucosa Based on the Oral Mucosal Image: A Systematic Review and Meta-Analysis" Cancers 14, no. 14: 3499. https://doi.org/10.3390/cancers14143499
APA StyleKim, J.-S., Kim, B. G., & Hwang, S. H. (2022). Efficacy of Artificial Intelligence-Assisted Discrimination of Oral Cancerous Lesions from Normal Mucosa Based on the Oral Mucosal Image: A Systematic Review and Meta-Analysis. Cancers, 14(14), 3499. https://doi.org/10.3390/cancers14143499






