Analysis by TeloView® Technology Predicts the Response of Hodgkin’s Lymphoma to First-Line ABVD Therapy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Information
2.2. Tissue Specimens
2.3. H&E and CD30 IHC
2.4. Quantitative 3D Telomere Co-Immuno-FISH Assay
2.5. 3D Image Acquisition and Processing
2.6. TeloView® Analysis
- (a)
- Nuclear volume: Nuclear volume within one 5 μm thin nuclear section of H-cells or RS-cells is calculated according to the 3D nuclear DAPI staining, as previously described [52]. Contrary to whole cell preparations (cells or cell lines), where the nuclei can be visualized with their entire volumes and z-stack analysis along the z-direction over 15 μm allows the calculation of the entire nuclear volume, in tissue sections the nuclear volume analysis is limited to 5 μm nuclear segments (as used as a standard for histopathologic diagnosis) along the z-direction. Deparaffinized tissue slides of 10 and 15 μm thickness are technically unsatisfactory for Q-FISH analysis. Thus, the nuclear volume represents about 30–50% of the total nuclear volume of H-cells (with a nuclear diameter of about 10–15 μm) and about 15–25% of the total nuclear volume of RS-cells (diameter of two up to several nuclei measures about 20–40 μm).
- (b)
- Telomere number: The sum of all very small, small, mid-sized, and large telomeres and aggregates identified within one 5 μm thin segmental nuclear section of an H-cell or RS-cell.
- (c)
- Telomere intensity: The sum of intensities of all very small, small, mid-sized, and large telomeres and aggregates identified within one 5 μm thin segmental nuclear section of an H-cell or RS-cell (viz. ∑ 2 × 15,000 units > ∑ 7 × 4000 units).
- (d)
- Mean telomere intensity: Mean telomere relative fluorescent intensity (size) of all telomeres within a given segmental volume.
- (e)
- Telomere size: Telomeres with a relative fluorescent intensity (x-axis) ranging from 0 to 5000 units are classified as very small (t-stumps), with an intensity ranging from 5000 to 15,000 units as small, with an intensity from 15,000 to 30,000 units as mid-sized, and with an intensity > 30,000 units as large [45].
- (f)
- Telomere aggregates: Telomere aggregates are defined as clusters of telomeres that are found in close association and cannot be further resolved as separate entities at an optical resolution limit of 200 nm [53].
2.7. Statistical Analysis
3. Results
3.1. Cohort Clinical Data and Outcome
3.2. Target cHL Tumor Cell Identification and Confirmation
3.3. 3D Telomere Profiling of cHL Patients with RR Versus CR for Minimally 5 Years
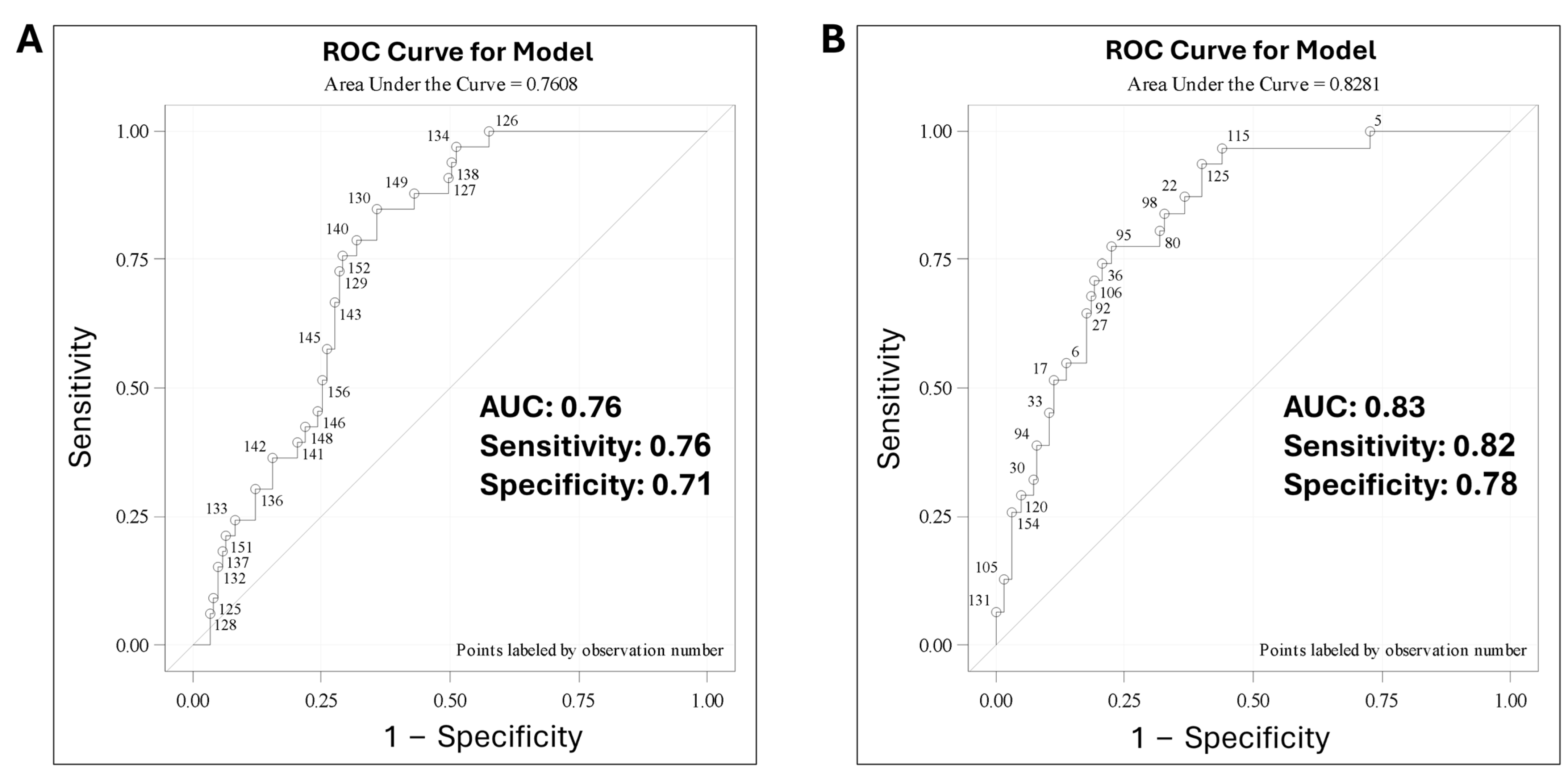
3.4. Regression Analysis and Predictive Modeling
3.5. Predictive Modeling
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Piris, M.A.; Medeiros, L.J.; Chang, K.-C. Hodgkin lymphoma: A review of pathological features and recent advances in pathogenesis. Pathology 2020, 52, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Bienz, M.; Ramdani, S.; Knecht, H. Molecular pathogenesis of Hodgkin lymphoma: Past, present, future. Int. J. Mol. Sci. 2020, 21, 6623. [Google Scholar] [CrossRef] [PubMed]
- Weniger, M.A.; Küppers, R. Molecular biology of Hodgkin lymphoma. Leukemia 2021, 35, 968–981. [Google Scholar] [CrossRef] [PubMed]
- Canellos, G.P.; Anderson, J.R.; Propert, K.J.; Nissen, N.; Cooper, M.R.; Henderson, E.S.; Green, M.R.; Gottlieb, A.; Peterson, B.A. Chemotherapy of advanced Hodgkin’s disease with MOPP, ABVD, or MOPP alternating with ABVD. N. Engl. J. Med. 1992, 327, 1478–1484. [Google Scholar] [CrossRef]
- Kuruvilla, J. Standard therapy of advanced Hodgkin lymphoma. ASH Educ. Program Book 2009, 2009, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Gordon, L.I.; Hong, F.; Fisher, R.I.; Bartlett, N.L.; Connors, J.M.; Gascoyne, R.D.; Wagner, H.; Stiff, P.J.; Cheson, B.D.; Gospodarowicz, M.; et al. Randomized phase III trial of ABVD versus Stanford V with or without radiation therapy in locally extensive and advanced-stage Hodgkin lymphoma: An intergroup study coordinated by the Eastern Cooperative Oncology Group (E2496). J. Clin. Oncol. 2013, 31, 684. [Google Scholar] [CrossRef] [PubMed]
- Merli, F.; Luminari, S.; Gobbi, P.G.; Cascavilla, N.; Mammi, C.; Ilariucci, F.; Stelitano, C.; Musso, M.; Baldini, L.; Galimberti, S.; et al. Long-term results of the HD2000 trial comparing ABVD versus BEACOPP versus COPP-EBV-CAD in untreated patients with advanced Hodgkin lymphoma: A study by Fondazione Italiana Linfomi. J. Clin. Oncol. 2016, 34, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Carde, P.; Karrasch, M.; Fortpied, C.; Brice, P.; Khaled, H.; Casasnovas, O.; Caillot, D.; Gaillard, I.; Bologna, S.; Ferme, C.; et al. Eight cycles of ABVD versus four cycles of BEACOPPescalated plus four cycles of BEACOPPbaseline in stage III to IV, international prognostic score ≥ 3, high-risk Hodgkin lymphoma: First results of the phase III EORTC 20012 Intergroup Trial. J. Clin. Oncol. 2016, 34, 2028–2036. [Google Scholar] [CrossRef]
- Viviani, S.; Zinzani, P.L.; Rambaldi, A.; Brusamolino, E.; Levis, A.; Bonfante, V.; Vitolo, U.; Pulsoni, A.; Liberati, A.M.; Specchia, G.; et al. ABVD versus BEACOPP for Hodgkin’s lymphoma when high-dose salvage is planned. N. Engl. J. Med. 2011, 365, 203–212. [Google Scholar] [CrossRef]
- Johnson, P.W. Response-adapted frontline therapy for Hodgkin lymphoma: Are we there yet? Hematol. 2014 Am. Soc. Hematol. Educ. Program Book 2016, 2016, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Younes, A.; Gopal, A.K.; Smith, S.E.; Ansell, S.M.; Rosenblatt, J.D.; Savage, K.J.; Ramchandren, R.; Bartlett, N.L.; Cheson, B.D.; De Vos, S.; et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J. Clin. Oncol. 2012, 30, 2183. [Google Scholar] [CrossRef] [PubMed]
- Gopal, A.K.; Chen, R.; Smith, S.E.; Ansell, S.M.; Rosenblatt, J.D.; Savage, K.J.; Connors, J.M.; Engert, A.; Larsen, E.K.; Chi, X.; et al. Durable remissions in a pivotal phase 2 study of brentuximab vedotin in relapsed or refractory Hodgkin lymphoma. Blood J. Am. Soc. Hematol. 2015, 125, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Ansell, S.M.; Lesokhin, A.M.; Borrello, I.; Halwani, A.; Scott, E.C.; Gutierrez, M.; Schuster, S.J.; Millenson, M.M.; Cattry, D.; Freeman, G.J.; et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N. Engl. J. Med. 2015, 372, 311–319. [Google Scholar] [CrossRef]
- Chen, R.; Zinzani, P.L.; Fanale, M.A.; Armand, P.; Johnson, N.A.; Brice, P.; Radford, J.; Ribrag, V.; Molin, D.; Vassilakopoulos, T.P.; et al. Phase II study of the efficacy and safety of pembrolizumab for relapsed/refractory classic Hodgkin lymphoma. J. Clin. Oncol. 2017, 35, 2125. [Google Scholar] [CrossRef] [PubMed]
- Kuruvilla, J.; Ramchandren, R.; Santoro, A.; Paszkiewicz-Kozik, E.; Gasiorowski, R.; Johnson, N.A.; Fogliatto, L.M.; Goncalves, I.; de Oliveira, J.S.R.; Buccheri, V.; et al. Pembrolizumab versus brentuximab vedotin in relapsed or refractory classical Hodgkin lymphoma (KEYNOTE-204): An interim analysis of a multicentre, randomised, open-label, phase 3 study. Lancet Oncol. 2021, 22, 512–524. [Google Scholar] [CrossRef] [PubMed]
- Ansell, S.M.; Radford, J.; Connors, J.M.; Długosz-Danecka, M.; Kim, W.-S.; Gallamini, A.; Ramchandren, R.; Friedberg, J.W.; Advani, R.; Hutchings, M.; et al. Overall survival with brentuximab vedotin in stage III or IV Hodgkin’s lymphoma. N. Engl. J. Med. 2022, 387, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Evens, A.M.; Connors, J.M.; Younes, A.; Ansell, S.M.; Kim, W.S.; Radford, J.; Feldman, T.; Tuscano, J.; Savage, K.J.; Oki, Y.; et al. Older patients (aged ≥60 years) with previously untreated advanced-stage classical Hodgkin lymphoma: A detailed analysis from the phase III ECHELON-1 study. Haematologica 2022, 107, 1086. [Google Scholar] [CrossRef]
- Huntington, S.F.; von Keudell, G.; Davidoff, A.J.; Gross, C.P.; Prasad, S.A. Cost-effectiveness analysis of brentuximab vedotin with chemotherapy in newly diagnosed stage III and IV Hodgkin lymphoma. J. Clin. Oncol. 2018, 36, 3307. [Google Scholar] [CrossRef]
- Raymakers, A.; Costa, S.; Cameron, D.; Regier, D.A. Cost-effectiveness of brentuximab vedotin in advanced stage Hodgkin’s lymphoma: A probabilistic analysis. BMC Cancer 2020, 20, 992. [Google Scholar] [CrossRef]
- Coleman, W.B.; Tsongalis, G.J. Multiple mechanisms account for genomic instability and molecular mutation in neoplastic transformation. Clin. Chem. 1995, 41, 644–657. [Google Scholar] [CrossRef] [PubMed]
- Pihan, G.A.; Purohit, A.; Wallace, J.; Knecht, H.; Woda, B.; Quesenberry, P.; Doxsey, S.J. Centrosome defects and genetic instability in malignant tumors. Cancer Res. 1998, 58, 3974–3985. [Google Scholar] [PubMed]
- Blackburn, E.H. Switching and signaling at the telomere. Cell 2001, 106, 661–673. [Google Scholar] [CrossRef] [PubMed]
- Maser, R.S.; DePinho, R.A. Connecting chromosomes, crisis, and cancer. Science 2002, 297, 565–569. [Google Scholar] [CrossRef] [PubMed]
- De Lange, T. Telomere-Related Genome Instability in Cancer; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2005; pp. 197–204. [Google Scholar]
- Xu, L.; Blackburn, E.H. Human cancer cells harbor T-stumps, a distinct class of extremely short telomeres. Mol. Cell 2007, 28, 315–327. [Google Scholar] [CrossRef]
- Mai, S. Initiation of telomere-mediated chromosomal rearrangements in cancer. J. Cell. Biochem. 2010, 109, 1095–1102. [Google Scholar] [CrossRef]
- Lajoie, V.; Lemieux, B.; Sawan, B.; Lichtensztejn, D.; Lichtensztejn, Z.; Wellinger, R.; Mai, S.; Knecht, H. LMP1 mediates multinuclearity through downregulation of shelterin proteins and formation of telomeric aggregates. Blood J. Am. Soc. Hematol. 2015, 125, 2101–2110. [Google Scholar] [CrossRef] [PubMed]
- Knecht, H.; Sawan, B.; Lichtensztejn, D.; Lemieux, B.; Wellinger, R.; Mai, S. The 3D nuclear organization of telomeres marks the transition from Hodgkin to Reed–Sternberg cells. Leukemia 2009, 23, 565–573. [Google Scholar] [CrossRef]
- Guffei, A.; Sarkar, R.; Klewes, L.; Righolt, C.; Knecht, H.; Mai, S. Dynamic chromosomal rearrangements in Hodgkin’s lymphoma are due to ongoing three-dimensional nuclear remodeling and breakage-bridge-fusion cycles. Haematologica 2010, 95, 2038. [Google Scholar] [CrossRef] [PubMed]
- Re, D.; Zander, T.; Diehl, V.; Wolf, J. Genetic instability in Hodgkin’s lymphoma. Ann. Oncol. 2002, 13, 19–22. [Google Scholar] [CrossRef]
- Knecht, H.; Kongruttanachok, N.; Sawan, B.; Brossard, J.; Prévost, S.; Turcotte, E.; Lichtensztejn, Z.; Lichtensztejn, D.; Mai, S. Three-dimensional telomere signatures of Hodgkin-and Reed-Sternberg cells at diagnosis identify patients with poor response to conventional chemotherapy. Transl. Oncol. 2012, 5, 269–277. [Google Scholar] [CrossRef]
- Knecht, H.; Righolt, C.; Mai, S. Genomic instability: The driving force behind refractory/relapsing Hodgkin’s lymphoma. Cancers 2013, 5, 714–725. [Google Scholar] [CrossRef] [PubMed]
- Roemer, M.G.; Advani, R.H.; Ligon, A.H.; Natkunam, Y.; Redd, R.A.; Homer, H.; Connelly, C.F.; Sun, H.H.; Daadi, S.E.; Freeman, G.J.; et al. PD-L1 and PD-L2 genetic alterations define classical Hodgkin lymphoma and predict outcome. J. Clin. Oncol. 2016, 34, 2690. [Google Scholar] [CrossRef] [PubMed]
- Cuceu, C.; Hempel, W.M.; Sabatier, L.; Bosq, J.; Carde, P.; M’kacher, R. Chromosomal instability in Hodgkin lymphoma: An in-depth review and perspectives. Cancers 2018, 10, 91. [Google Scholar] [CrossRef] [PubMed]
- Gadji, M.; Fortin, D.; Tsanaclis, A.-M.; Garini, Y.; Katzir, N.; Wienburg, Y.; Yan, J.; Klewes, L.; Klonisch, T.; Drouin, R.; et al. Three-dimensional nuclear telomere architecture is associated with differential time to progression and overall survival in glioblastoma patients. Neoplasia 2010, 12, 183–191. [Google Scholar] [CrossRef]
- Gadji, M.; Vallente, R.; Klewes, L.; Righolt, C.; Wark, L.; Kongruttanachok, N.; Knecht, H.; Mai, S. Nuclear remodeling as a mechanism for genomic instability in cancer. Adv. Cancer Res. 2011, 112, 77–126. [Google Scholar] [PubMed]
- Gadji, M.; Adebayo Awe, J.; Rodrigues, P.; Kumar, R.; Houston, D.S.; Klewes, L.; Dièye, T.N.; Rego, E.M.; Passetto, R.F.; de Oliveira, F.M.; et al. Profiling three-dimensional nuclear telomeric architecture of myelodysplastic syndromes and acute myeloid leukemia defines patient subgroups. Clin. Cancer Res. 2012, 18, 3293–3304. [Google Scholar] [CrossRef] [PubMed]
- Klewes, L.; Vallente, R.; Dupas, E.; Brand, C.; Grün, D.; Guffei, A.; Sathitruangsak, C.; Awe, J.A.; Kuzyk, A.; Lichtensztejn, D.; et al. Three-dimensional nuclear telomere organization in multiple myeloma. Transl. Oncol. 2013, 6, 749-IN36. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Pozzo, A.; Yu, P.L.I.; LaL, S.; Asbaghi, Y.; Sisdelli, L.; Tammur, P.; Tamm, A.; Punab, M.; Klewes, L.; Louis, S.; et al. Telomere architecture correlates with aggressiveness in multiple myeloma. Cancers 2021, 13, 1969. [Google Scholar] [CrossRef]
- Bienz, M.N.; Petrogiannis-Haliotis, T.; Pehr, K.; Benlimame, N.; Mai, S.; Knecht, H. Three-Dimensional Telomeric Fingerprint of Mycosis Fungoides and/or Sézary Syndrome: A Pilot Study. J. Investig. Dermatol. 2021, 141, 1598–1601. [Google Scholar] [CrossRef]
- Wark, L.; Danescu, A.; Natarajan, S.; Zhu, X.; Cheng, S.-Y.; Hombach-Klonisch, S.; Mai, S.; Klonisch, T. Three-dimensional telomere dynamics in follicular thyroid cancer. Thyroid 2014, 24, 296–304. [Google Scholar] [CrossRef]
- Caria, P.; Dettori, T.; Frau, D.V.; Lichtenzstejn, D.; Pani, F.; Vanni, R.; Mai, S. Characterizing the three-dimensional organization of telomeres in papillary thyroid carcinoma cells. J. Cell. Physiol. 2019, 234, 5175–5185. [Google Scholar] [CrossRef] [PubMed]
- Drachenberg, D.; Awe, J.A.; Rangel Pozzo, A.; Saranchuk, J.; Mai, S. Advancing risk assessment of intermediate risk prostate cancer patients. Cancers 2019, 11, 855. [Google Scholar] [CrossRef]
- Rangel-Pozzo, A.; Sisdelli, L.; Cordioli, M.I.V.; Vaisman, F.; Caria, P.; Mai, S.; Cerutti, J.M. Genetic landscape of papillary thyroid carcinoma and nuclear architecture: An overview comparing pediatric and adult populations. Cancers 2020, 12, 3146. [Google Scholar] [CrossRef] [PubMed]
- Knecht, H.; Sawan, B.; Lichtensztejn, Z.; Lichtensztejn, D.; Mai, S. 3D Telomere FISH defines LMP1-expressing Reed–Sternberg cells as end-stage cells with telomere-poor ‘ghost’ nuclei and very short telomeres. Lab. Investig. 2010, 90, 611–619. [Google Scholar] [CrossRef]
- Knecht, H.; Johnson, N.A.; Haliotis, T.; Lichtensztejn, D.; Mai, S. Disruption of direct 3D telomere–TRF2 interaction through two molecularly disparate mechanisms is a hallmark of primary Hodgkin and Reed–Sternberg cells. Lab. Investig. 2017, 97, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Contu, F.; Rangel-Pozzo, A.; Trokajlo, P.; Wark, L.; Klewes, L.; Johnson, N.A.; Petrogiannis-Haliotis, T.; Gartner, J.G.; Garini, Y.; Vanni, R.; et al. Distinct 3D structural patterns of lamin A/C expression in Hodgkin and Reed-Sternberg cells. Cancers 2018, 10, 286. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, L.; Schuster, D.; Herz, H. Generalized approach for accelerated maximum likelihood based image restoration applied to three-dimensional fluorescence microscopy. J. Microsc. 2001, 204, 99–107. [Google Scholar] [CrossRef]
- Chuang, T.C.Y.; Moshir, S.; Garini, Y.; Chuang, A.Y.-C.; Young, I.T.; Vermolen, B.; Doel, R.v.D.; Mougey, V.; Perrin, M.; Braun, M.; et al. The three-dimensional organization of telomeres in the nucleus of mammalian cells. BMC Biol. 2004, 2, 12. [Google Scholar] [CrossRef]
- Vermolen, B.; Garini, Y.; Mai, S.; Mougey, V.; Fest, T.; Chuang, T.C.; Chuang, A.Y.; Wark, L.; Young, I.T. Characterizing the three-dimensional organization of telomeres. Cytom. Part A J. Int. Soc. Anal. Cytol. 2005, 67, 144–150. [Google Scholar] [CrossRef]
- Poon, S.S.; Martens, U.M.; Ward, R.K.; Lansdorp, P.M. Telomere length measurements using digital fluorescence microscopy. Cytom. J. Int. Soc. Anal. Cytol. 1999, 36, 267–278. [Google Scholar] [CrossRef]
- Mai, S.; Garini, Y. The significance of telomeric aggregates in the interphase nuclei of tumor cells. J. Cell. Biochem. 2006, 97, 904–915. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, R.; Guffei, A.; Vermolen, B.J.; Garini, Y.; Mai, S. Alterations of centromere positions in nuclei of immortalized and malignant mouse lymphocytes. Cytom. Part A J. Int. Soc. Anal. Cytol. 2007, 71, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Bax, B.E. Biomarkers in Rare Diseases 2.0. Int. J. Mol. Sci. 2022, 23, 4582. [Google Scholar] [CrossRef]
- Felman, P.; Bryon, P.; Gentilhomme, O.; Magaud, J.P.; Manel, A.M.; Coiffier, B.; Lenoir, G. Burkitt’s lymphoma. Distinction of subgroups by morphometric analysis of the characteristics of 55 cell lines. Anal. Quant. Cytol. Histol. 1985, 7, 275–282. [Google Scholar] [PubMed]
- El Hussein, S.; Chen, P.; Medeiros, L.J.; Wistuba, I.I.; Jaffray, D.; Wu, J.; Khoury, J.D. Artificial intelligence strategy integrating morphologic and architectural biomarkers provides robust diagnostic accuracy for disease progression in chronic lymphocytic leukemia. J. Pathol. 2022, 256, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Hayflick, L.; Moorhead, P.S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961, 25, 585–621. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, A.G.; Ouellette, M.; Frolkis, M.; Holt, S.E.; Chiu, C.-P.; Morin, G.B.; Harley, C.B.; Shay, J.W.; Lichtsteiner, S.; Wright, W.E. Extension of life-span by introduction of telomerase into normal human cells. Science 1998, 279, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Soudet, J.; Jolivet, P.; Teixeira, M.T. Elucidation of the DNA end-replication problem in Saccharomyces cerevisiae. Mol. Cell 2014, 53, 954–964. [Google Scholar] [CrossRef]
- Blackburn, E.H.; Epel, E.S.; Lin, J. Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science 2015, 350, 1193–1198. [Google Scholar] [CrossRef]
- Carlund, O.; Thörn, E.; Osterman, P.; Fors, M.; Dernstedt, A.; Forsell, M.N.E.; Erlanson, M.; Landfors, M.; Degerman, S.; Hultdin, M. Semimethylation is a feature of diffuse large B-cell lymphoma, and subgroups with poor prognosis are characterized by global hypomethylation and short telomere length. Clin. Epigenetics 2024, 16, 68. [Google Scholar] [CrossRef]
- Montero-Conde, C.; Leandro-García, L.J.; Martínez-Montes, Á.M.; Martínez, P.; Moya, F.J.; Letón, R.; Gil, E.; Martínez-Puente, N.; Guadalix, S.; Currás-Freixes, M.; et al. Comprehensive molecular analysis of immortalization hallmarks in thyroid cancer reveals new prognostic markers. Clin. Transl. Med. 2022, 12, e1001. [Google Scholar] [CrossRef] [PubMed]
- Hasenclever, D.; Diehl, V.; Armitage, J.O.; Assouline, D.; Björkholm, M.; Brusamolino, E.; Canellos, G.P.; Carde, P.; Crowther, D.; Cunningham, D.; et al. A prognostic score for advanced Hodgkin’s disease. N. Engl. J. Med. 1998, 339, 1506–1514. [Google Scholar] [CrossRef] [PubMed]
- Moccia, A.A.; Donaldson, J.; Chhanabhai, M.; Hoskins, P.J.; Klasa, R.J.; Savage, K.J.; Shenkier, T.N.; Slack, G.W.; Skinnider, B.; Gascoyne, R.D.; et al. International Prognostic Score in advanced-stage Hodgkin’s lymphoma: Altered utility in the modern era. J. Clin. Oncol. 2012, 30, 3383–3388. [Google Scholar] [CrossRef]
- Gautam, S.; Yeola, S.; Nahar, A.; Sarpong, E.M.; Prescott, J.; Yang, X.; Sineshaw, H.M. Unfavorable early-stage Hodgkin lymphoma: Assessment of patient characteristics in a real-world setting. Future Oncol. 2023, 19, 1249–1259. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Burger, I.A.; Zhang, Z.; Drill, E.N.; Migliacci, J.C.; Ng, A.; LaCasce, A.; Wall, D.; Witzig, T.E.; Ristow, K.; et al. Definition of bulky disease in early stage Hodgkin lymphoma in computed tomography era: Prognostic significance of measurements in the coronal and transverse planes. Haematologica 2016, 101, 1237. [Google Scholar] [CrossRef] [PubMed]
- Mohite, A.; Rangarajan, V.; Goda, J.; Chugh, S.; Agrawal, A.; Sengar, M. Metabolic tumor parameters complement clinicopathological factors in prognosticating advanced stage Hodgkin Lymphoma. Asia Ocean. J. Nucl. Med. Biol. 2023, 11, 111. [Google Scholar]
- Wang, Q.; Qin, Y.; Zhou, S.; He, X.; Yang, J.; Kang, S.; Liu, P.; Yang, S.; Zhang, C.; Gui, L.; et al. Prognostic value of pretreatment serum beta-2 microglobulin level in advanced classical Hodgkin lymphoma treated in the modern era. Oncotarget 2016, 7, 72219. [Google Scholar] [CrossRef] [PubMed]
- Primerano, S.; Burnelli, R.; Carraro, E.; Pillon, M.; Elia, C.; Farruggia, P.; Sala, A.; Vinti, L.; Buffardi, S.; Basso, G.; et al. Kinetics of circulating plasma cell-free dna in paediatric classical Hodgkin lymphoma. J. Cancer 2016, 7, 364. [Google Scholar] [CrossRef]
- Maco, M.; Kupcova, K.; Herman, V.; Kozak, T.; Mocikova, H.; Havranek, O. Circulating tumor DNA in Hodgkin lymphoma. Ann. Hematol. 2022, 101, 2393–2403. [Google Scholar] [CrossRef]
- Driessen, J.; Kersten, M.J.; Visser, L.; Berg, A.v.D.; Tonino, S.H.; Zijlstra, J.M.; Lugtenburg, P.J.; Morschhauser, F.; Hutchings, M.; Amorim, S.; et al. Prognostic value of TARC and quantitative PET parameters in relapsed or refractory Hodgkin lymphoma patients treated with brentuximab vedotin and DHAP. Leukemia 2022, 36, 2853–2862. [Google Scholar] [CrossRef]
- Ansell, S.M. Hodgkin lymphoma: 2023 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2022, 97, 1478–1488. [Google Scholar] [CrossRef] [PubMed]
- Caputo, V.; Ciardiello, F.; Della Corte, C.M.; Martini, G.; Troiani, T.; Napolitano, S. Diagnostic value of liquid biopsy in the era of precision medicine: 10 years of clinical evidence in cancer. Explor. Target. Anti-Tumor Ther. 2023, 4, 102. [Google Scholar] [CrossRef] [PubMed]

| Inclusion Criteria | Exclusion Criteria |
|---|---|
| First time diagnosed with cHL a | Age < 18 and >80 years old |
| Availability of lymph node diagnostic tissue | |
| Availability of demographic and clinical follow up data | |
| First line treatment with ABVD b |
| Gender | Age Groups | Disease Stage (Costwold) | Response to ABVD | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | ≥50 Years | <50 Years | I | II | III | IV | Remission ≥5 Years | Refractory or Relapse within 12 Months | |
| n = 156 | 79 | 77 | 126 | 30 | 23 | 70 | 32 | 31 | 125 | 31 |
| A—Remission > 5 Years (n-125) | B—Refractory/Relapse ≤ 12 Month (n = 31) | <HA + RSA> vs. <HB + RSB> | HA vs. HB | RSA vs. RSB | |||
|---|---|---|---|---|---|---|---|
| Telomere Parameter | HA | RSA | HB | RSB | p-Value | p-Value | p-Value |
| Number of telomeres | 36 (17.8; 0.29) | 55 (36; 0.6) | 31 (14, 0.48) | 47 (31; 1.1) | 0.04 * | 0.000014 * | 0.0007 * |
| Number of Aggregates | 4 (2.9; 0.05) | 7 (5.5; 0.09) | 3 (2.5; 0.08) | 6 (4.8; 0.16) | 0.034 * | 0.0026 * | 0.03 * |
| Average telomere length | 2926 (1185; 39.5) | 2599 (924; 15.4) | 2788 (1132; 18.9) | 2457 (871; 29) | 0.0029 * | 0.012 * | 0.0003 * |
| Nuclear Volume (μ3) | 469.31 (400.81.8; 6.68) | 718.10 (686.11; 11.43) | 440.08 (330.29; 11.00) | 702.00 (574.54; 19.15) | 0.017 * | 0.4 | 0.5 |
| A/C Ratio | 3 (1.2; 0.02) | 3.6 (1.5; 0.026) | 2.8 (1.1; 0.04) | 3.4 (1.4; 0.049) | 0.078 | 0.42 | 0.08 |
| Distribution of telomeres | 3813 (777; 25.8) | 4876 (1450.7; 24) | 3684 (775; 25.8) | 4740 (1428.4; 47.6) | 0.085 | 0.00016 * | 0.1 |
| % of t-stumps | 42 (14; 0.21) | 57 (21, 0.33) | 49 (17; 0.27) | 84 (27; 39) | 0.0012 * | 0.000011 * | 0.000077 * |
| Confidence Test | TeloView Parameters Only | TeloView with Clinical Risk Factors |
|---|---|---|
| Likelihood Ratio | 0.0003 | <0.0001 |
| Score | 0.0009 | 0.0003 |
| Wald | 0.0024 | 0.0011 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knecht, H.; Johnson, N.; Bienz, M.N.; Brousset, P.; Memeo, L.; Shifrin, Y.; Alikhah, A.; Louis, S.F.; Mai, S. Analysis by TeloView® Technology Predicts the Response of Hodgkin’s Lymphoma to First-Line ABVD Therapy. Cancers 2024, 16, 2816. https://doi.org/10.3390/cancers16162816
Knecht H, Johnson N, Bienz MN, Brousset P, Memeo L, Shifrin Y, Alikhah A, Louis SF, Mai S. Analysis by TeloView® Technology Predicts the Response of Hodgkin’s Lymphoma to First-Line ABVD Therapy. Cancers. 2024; 16(16):2816. https://doi.org/10.3390/cancers16162816
Chicago/Turabian StyleKnecht, Hans, Nathalie Johnson, Marc N. Bienz, Pierre Brousset, Lorenzo Memeo, Yulia Shifrin, Asieh Alikhah, Sherif F. Louis, and Sabine Mai. 2024. "Analysis by TeloView® Technology Predicts the Response of Hodgkin’s Lymphoma to First-Line ABVD Therapy" Cancers 16, no. 16: 2816. https://doi.org/10.3390/cancers16162816










