Diagnosis and Management of Acute Respiratory Distress Syndrome in a Time of COVID-19
Abstract
:1. Introduction
1.1. Causes of ARDS
1.2. Clinical Pathophysiology of ARDS
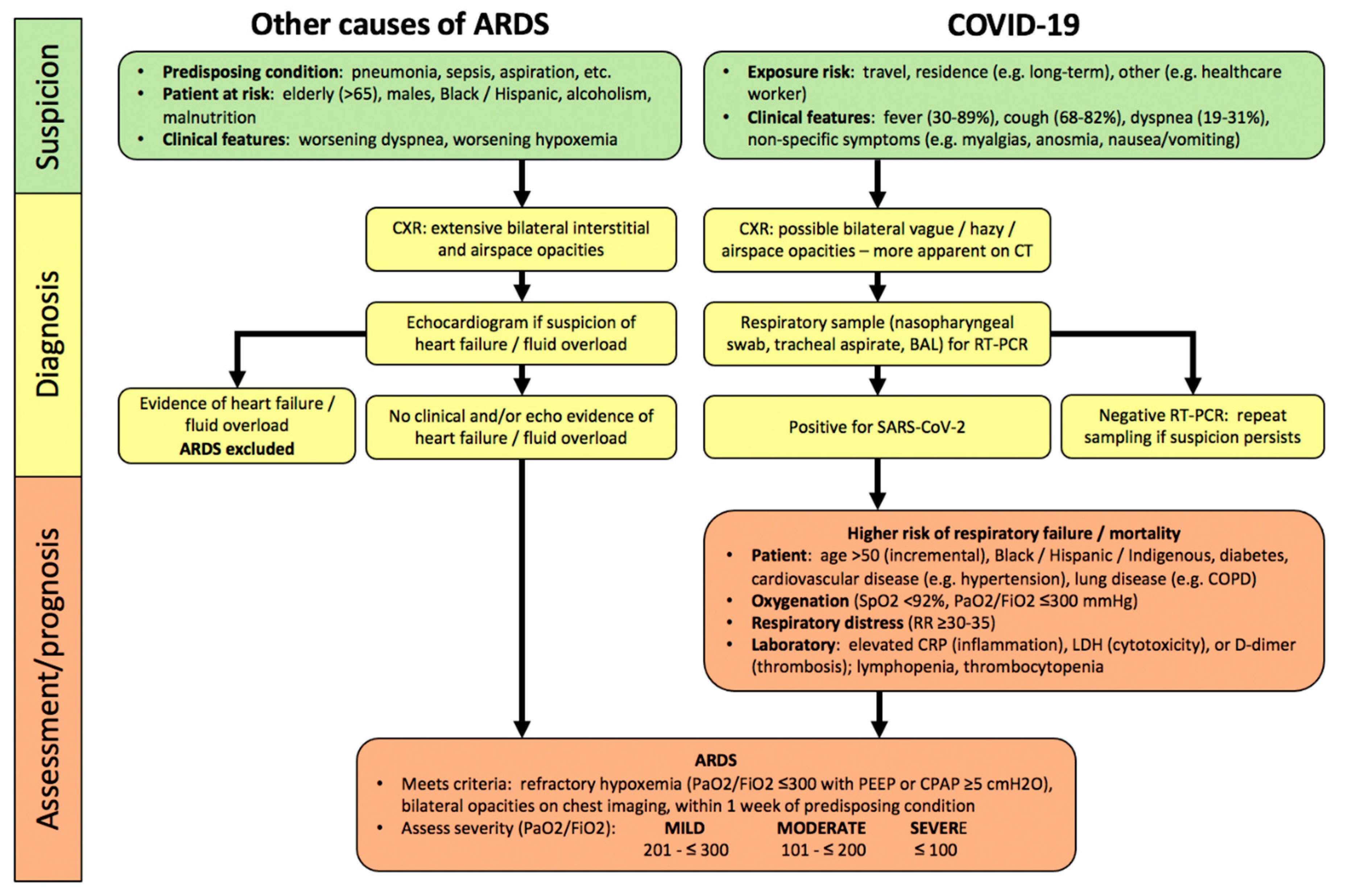
2. Diagnosis of ARDS
2.1. Clinical Assessment
2.2. Assessment of Severity
3. Management of Patients with ARDS
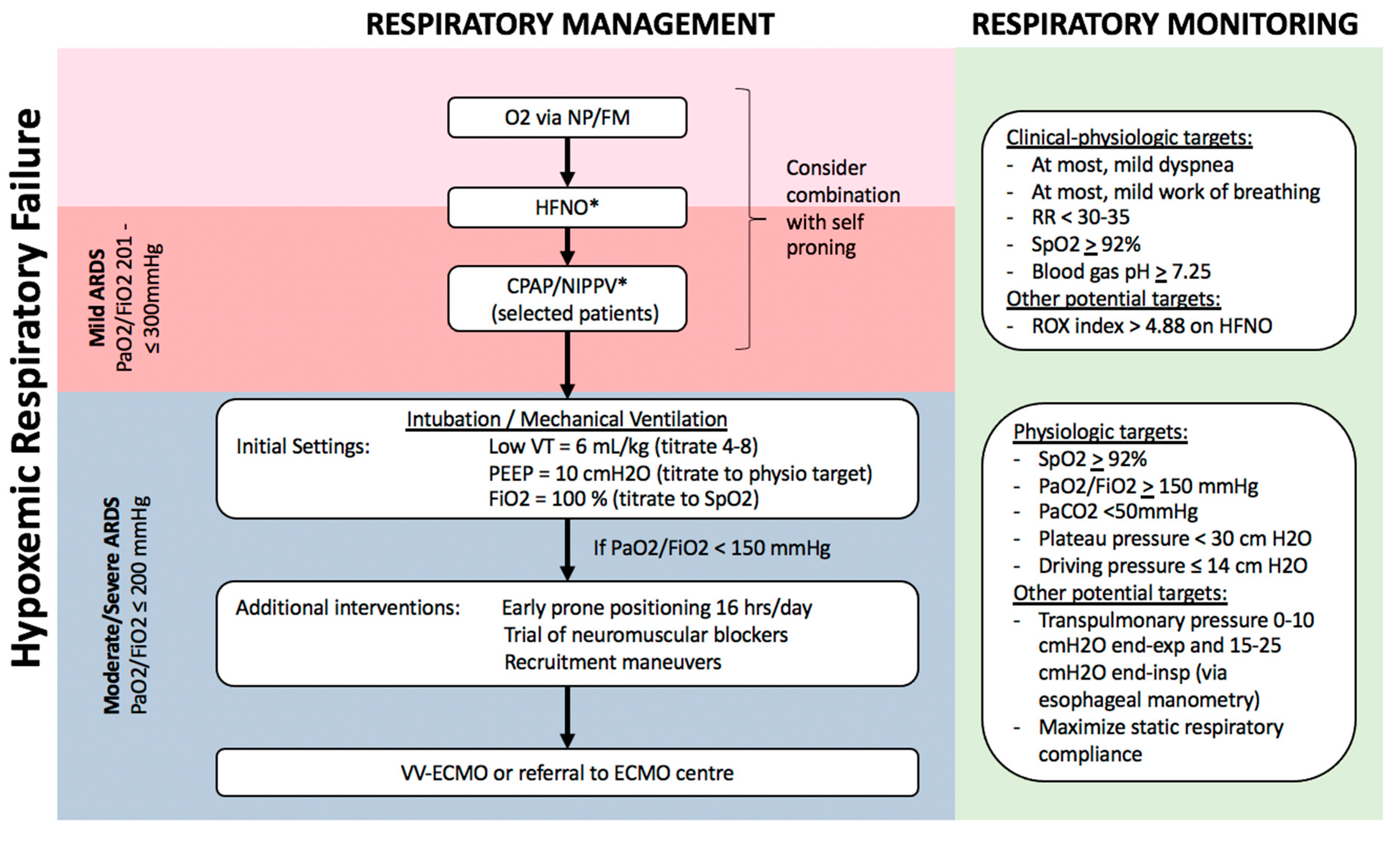
3.1. General Approach
3.2. Respiratory Support of Mild ARDS
3.2.1. High-Flow Nasal-Cannula O2 (HFNO)
3.2.2. Continuous Positive Airway Pressure (CPAP)/Non-Invasive Positive Pressure Ventilation (NIPPV)
3.2.3. Prone Positioning
3.3. Respiratory Support of Moderate–Severe ARDS
3.4. Medical Approaches to ARDS Therapy
4. Outcomes in Patients with ARDS
5. Future Management of Patients with ARDS
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar] [PubMed]
- Bellani, G.; Laffey, J.G.; Pham, T.; Fan, E.; Brochard, L.; Esteban, A.; Gattinoni, L.; Van Haren, F.; Larsson, A.; McAuley, D.F.; et al. Epidemiology, Patterns of Care, and Mortality for Patients with Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016, 315, 788–800. [Google Scholar] [CrossRef] [PubMed]
- Brun-Buisson, C.; Minelli, C.; Bertolini, G.; Brazzi, L.; Pimentel, J.; Lewandowski, K.; Bion, J.; Romand, J.-A.; Villar, J.; Thorsteinsson, A.; et al. Epidemiology and outcome of acute lung injury in European intensive care units. Intensive Care Med. 2004, 30, 51–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, A.; Tansey, C.M.; Tomlinson, G.; Diaz-Granados, N.; Matte, A.; Barr, A.; Mehta, S.; Mazer, C.D.; Guest, C.B.; Stewart, T.E.; et al. Two-Year Outcomes, Health Care Use, and Costs of Survivors of Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2006, 174, 538–544. [Google Scholar] [CrossRef]
- Pham, T.; Rubenfeld, G.D. Fifty Years of Research in ARDS.The Epidemiology of Acute Respiratory Distress Syndrome. A 50th Birthday Review. Am. J. Respir. Crit. Care Med. 2017, 195, 860–870. [Google Scholar] [CrossRef]
- De Prost, N.; Pham, T.; Carteaux, G.; Dessap, A.M.; Brun-Buisson, C.; Fan, E.; Bellani, G.; Laffey, J.G.; Mercat, A.; Brochard, L.; et al. Etiologies, diagnostic work-up and outcomes of acute respiratory distress syndrome with no common risk factor: A prospective multicenter study. Ann. Intensive Care 2017, 7, 69. [Google Scholar] [CrossRef] [Green Version]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Coronavirus Disease (COVID-19) Situation Reports [Internet]. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (accessed on 3 November 2020).
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Guan, W.-J.; Ni, Z.-Y.; Hu, Y.; Liang, W.-H.; Ou, C.-Q.; He, J.-X.; Liu, L.; Shan, H.; Lei, C.-L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Luo, L.; Shaver, C.M.; Zhao, Z.; Koyama, T.; Calfee, C.S.; Bastarache, J.A.; Ware, L.B. Clinical Predictors of Hospital Mortality Differ Between Direct and Indirect ARDS. Chest 2017, 151, 755–763. [Google Scholar] [CrossRef] [Green Version]
- Sheu, C.C.; Gong, M.N.; Zhai, R.; Chen, F.; Bajwa, E.K.; Clardy, P.F.; Gallagher, D.C.; Thompson, B.T.; Christiani, D.C. Clinical Characteristics and Outcomes of Sepsis-Related vs. Non-Sepsis-Related ARDS. Chest 2010, 138, 559–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardinal-Fernández, P.; Bajwa, E.K.; Dominguez-Calvo, A.; Menéndez, J.M.; Papazian, L.; Thompson, B.T. The Presence of Diffuse Alveolar Damage on Open Lung Biopsy Is Associated with Mortality in Patients with Acute Respiratory Distress Syndrome: A Systematic Review and Meta-Analysis. Chest 2016, 149, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Guerin, C.; Bayle, F.; Leray, V.; Debord, S.; Stoian, A.; Yonis, H.; Roudaut, J.; Bourdin, G.; Devouassoux-Shisheboran, M.; Bucher, E.; et al. Open lung biopsy in nonresolving ARDS frequently identifies diffuse alveolar damage regardless of the severity stage and may have implications for patient management. Intensive Care Med. 2015, 41, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Benzing, A.; Mols, G.; Brieschal, T.; Geiger, K. Hypoxic Pulmonary Vasoconstriction in Nonventilated Lung Areas Contributes to Differences in Hemodynamic and Gas Exchange Responses to Inhalation of Nitric Oxide. Anesthesiology 1997, 86, 1254–1261. [Google Scholar] [CrossRef]
- Nuckton, T.J.; Alonso, J.A.; Kallet, R.H.; Daniel, B.M.; Pittet, J.F.; Eisner, M.D. Matthay, M.A. Pulmonary Dead-Space Fraction as a Risk Factor for Death in the Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2002, 346, 1281–1286. [Google Scholar] [CrossRef]
- Tomashefski, J.F.; Davies, P.; Boggis, C.; Greene, R.; Zapol, W.M.; Reid, L.M. The pulmonary vascular lesions of the adult respiratory distress syndrome. Am. J. Pathol. 1983, 112, 112–126. [Google Scholar]
- Zapol, W.M.; Kobayashi, K.; Snider, M.T.; Greene, R.; Lover, M.B. Vascular Obstruction Causes Pulmonary Hypertension in Severe Acute Respiratory Failure. Chest 1977, 71, 306–307. [Google Scholar] [CrossRef]
- Brochard, L.; Slutsky, A.; Pesenti, A. Mechanical Ventilation to Minimize Progression of Lung Injury in Acute Respiratory Failure. Am. J. Respir. Crit. Care Med. 2016, 195, 438–442. [Google Scholar] [CrossRef]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Werlein, C.; Stark, H.; Tzankov, A.; Li, W.W.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef]
- Fox, S.E.; Akmatbekov, A.; Harbert, J.L.; Li, G.; Brown, J.Q.; Heide, R.S.V. Pulmonary and cardiac pathology in African American patients with COVID-19: An autopsy series from New Orleans. Lancet Respir. Med. 2020, 8, 681–686. [Google Scholar] [CrossRef]
- Barton, L.M.; Duval, E.J.; Stroberg, E.; Ghosh, S.; Mukhopadhyay, S. COVID-19 Autopsies, Oklahoma, USA. Am. J. Clin. Pathol. 2020, 153, 725–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaller, T.; Hirschbühl, K.; Burkhardt, K.; Braun, G.; Trepel, M.; Märkl, B.; Claus, R. Postmortem Examination of Patients with COVID-19. JAMA 2020, 323, 2518–2520. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, P.; Yanqiu, W.; Yue, H.; Wang, Y.; Hu, M.; Zhang, S.; Cao, T.; Yang, C.; Li, M.; et al. Histopathologic Changes and SARS-CoV-2 Immunostaining in the Lung of a Patient with COVID-19. Ann. Intern. Med. 2020, 172, 629–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haudebourg, A.F.; Perier, F.; Tuffet, S.; de Prost, N.; Razazi, K.; Mekontso Dessap, A.; Carteaux, G. Respiratory Mechanics of COVID-19 vs. Non-COVID-19 Associated Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2020, 202, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Fan, E.; Beitler, J.R.; Brochard, L.; Calfee, C.S.; Ferguson, N.D.; Slutsky, A.S.; Brodie, D. COVID-19-associated acute respiratory distress syndrome: Is a different approach to management warranted? Lancet Respir. Med. 2020, 8, 816–821. [Google Scholar] [CrossRef]
- Gattinoni, L.; Chiumello, D.; Caironi, P.; Busana, M.; Romitti, F.; Brazzi, L.; Camporota, L. COVID-19 pneumonia: Different respiratory treatments for different phenotypes? Intensive Care Med. 2020, 46, 1099–1102. [Google Scholar] [CrossRef]
- Marini, J.J.; Gattinoni, L. Management of COVID-19 Respiratory Distress. JAMA 2020, 323, 2329–2330. [Google Scholar] [CrossRef]
- Tobin, M.J.; Laghi, F.; Jubran, A. Why COVID-19 Silent Hypoxemia Is Baffling to Physicians. Am. J. Respir. Crit. Care Med. 2020, 202, 356–360. [Google Scholar] [CrossRef]
- Zilberberg, M.D.; Epstein, S.K. Acute Lung Injury in the Medical ICU. Am. J. Respir. Crit. Care Med. 1998, 157, 1159–1164. [Google Scholar] [CrossRef]
- Moss, M.; Mannino, D.M. Race and gender differences in acute respiratory distress syndrome deaths in the United States: An analysis of multiple-cause mortality data (1979–1996). Crit. Care Med. 2002, 30, 1679–1685. [Google Scholar] [CrossRef]
- Pham, T.; Neto, A.S.; Pelosi, P.; Laffey, J.G.; Haro, C.D.; Lorente, J.A.; Bellani, G.; Fan, E.; Brochard, L.J.; Pesenti, A.; et al. Outcomes of Patients Presenting with Mild Acute Respiratory Distress Syndrome: Insights from the LUNG SAFE Study. J. Am. Soc. Anesthesiol. 2019, 130, 263–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Ai, T.; Yang, Z.; Hou, H.; Zhan, C.; Chen, C.; Lv, W.; Tao, Q.; Sun, Z.; Xia, L. Correlation of Chest CT and RT-PCR Testing in Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases. Radiology 2020, 296, 32–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; Cohen, S.L.; et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized with COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef] [PubMed]
- Salameh, J.P.; Leeflang, M.M.; Hooft, L.; Islam, N.; McGrath, T.A.; Pol, C.B.; Frank, R.A.; Prager, R.; Hare, S.S.; Dennie, C.; et al. Thoracic imaging tests for the diagnosis of COVID-19. Cochrane Database Syst. Rev. 2020, 9. [Google Scholar] [CrossRef]
- Nin, N.; Muriel, A.; Peñuelas, O.; Brochard, L.; Lorente, J.A.; Ferguson, N.D.; Raymondos, K.; Rios, F.; Violi, D.A.; Thille, A.W.; et al. Severe hypercapnia and outcome of mechanically ventilated patients with moderate or severe acute respiratory distress syndrome. Intensive Care Med. 2017, 43, 200–208. [Google Scholar] [CrossRef]
- Kallet, R.H.; Lipnick, M.S.; Zhuo, H.; Pangilinan, L.P.; Gomez, A. Characteristics of Nonpulmonary Organ Dysfunction at Onset of ARDS Based on the Berlin Definition. Respir. Care 2019, 64, 493–501. [Google Scholar] [CrossRef]
- Alhazzani, W.; Møller, M.H.; Arabi, Y.M.; Loeb, M.; Gong, M.N.; Fan, E.; Oczkowski, S.; Levy, M.M.; Derde, L.; Dzierba, A.; et al. Surviving Sepsis Campaign: Guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19). Intensive Care Med. 2020, 46, 854–887. [Google Scholar] [CrossRef]
- Liang, W.; Liang, H.; Ou, L.; Chen, B.; Chen, A.; Li, C.; Li, Y.; Guan, W.; Sang, L.; Lu, J.; et al. Development and Validation of a Clinical Risk Score to Predict the Occurrence of Critical Illness in Hospitalized Patients with COVID-19. JAMA Intern. Med. 2020, 180, 1081–1089. [Google Scholar] [CrossRef]
- Wu, C.; Chen, X.; Cai, Y.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; Zhang, Y.; et al. Risk Factors Associated with Acute Respiratory Distress Syndrome and Death in Patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020, 180, 934–943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Clinical Management of Severe Acute Respiratory Infection When COVID-19 is Suspected [Internet]. 2020. Available online: https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected (accessed on 3 November 2020).
- Haimovich, A.D.; Ravindra, N.G.; Stoytchev, S.; Young, H.P.; Wilson, F.P.; van Dijk, D.; Schulz, W.L.; Taylor, R.A. Development and Validation of the Quick COVID-19 Severity Index: A Prognostic Tool for Early Clinical Decompensation. Ann. Emerg. Med. 2020, 76, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Yang, P.; Xie, Y.; Woodruff, H.C.; Rao, X.; Guiot, J.; Frix, A.; Louis, R.; Moutschen, M.; Li, J.; et al. Development of a Clinical Decision Support System for Severity Risk Prediction and Triage of COVID-19 Patients at Hospital Admission: An International Multicenter Study. Eur. Respir. J. 2020, 56, 2001104. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, G.; Tonetti, T.; Protti, A.; Langer, T.; Girardis, M.; Bellani, G.; Laffey, J.; Carrafiello, G.; Carsana, L.; Rizzuto, C.; et al. Pathophysiology of COVID-19-associated acute respiratory distress syndrome: A multicentre prospective observational study. Lancet Respir. Med. 2020. [Google Scholar] [CrossRef]
- Vincent, J.L. Give your patient a fast hug (at least) once a day. Crit. Care Med. 2005, 33, 1225–1229. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Crit. Care Med. 2017, 45, 486–552. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R.; et al. Baseline Characteristics and Outcomes of 1591 Patients Infected with SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA 2020, 323, 1574–1581. [Google Scholar] [CrossRef] [Green Version]
- O’Driscoll, B.R.; Howard, L.S.; Earis, J.; Mak, V. BTS guideline for oxygen use in adults in healthcare and emergency settings. Thorax 2017, 72 (Suppl. 1), 1–90. [Google Scholar] [CrossRef] [Green Version]
- Chu, D.K.; Kim, L.H.Y.; Young, P.J.; Zamiri, N.; Almenawer, S.A.; Jaeschke, R.; Szczeklik, W.; Schünemann, H.J.; Neary, J.D.; Alhazzani, W. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): A systematic review and meta-analysis. Lancet 2018, 391, 1693–1705. [Google Scholar] [CrossRef]
- Munshi, L.; Ferguson, N.D. Evolving Issues in Oxygen Therapy in Acute Care Medicine. JAMA 2020, 323, 607–608. [Google Scholar] [CrossRef]
- Barrot, L.; Asfar, P.; Mauny, F.; Winiszewski, H.; Montini, F.; Badie, J.; Quenot, J.; Pili-Floury, S.; Bouhemad, B.; Louis, G.; et al. Liberal or Conservative Oxygen Therapy for Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2020, 382, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Girardis, M.; Busani, S.; Damiani, E.; Donati, A.; Rinaldi, L.; Marudi, A.; Morelli, A.; Antonelli, M.; Singer, M. Effect of Conservative vs Conventional Oxygen Therapy on Mortality Among Patients in an Intensive Care Unit: The Oxygen-ICU Randomized Clinical Trial. JAMA 2016, 316, 1583. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, M.E.; Anderson, B.; Christie, J.D.; Hopkins, R.O.; Lanken, P.N. Can We Optimize Long-Term Outcomes in Acute Respiratory Distress Syndrome by Targeting Normoxemia? Ann. Am. Thorac. Soc. 2014, 11, 613–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siemieniuk, R.A.C.; Chu, D.K.; Kim, L.H.Y.; Güell-Rous, M.R.; Alhazzani, W.; Soccal, P.M.; Karanicolas, P.J.; Farhoumand, P.D.; Siemeniuk, J.L.K.; Satia, I.; et al. Oxygen therapy for acutely ill medical patients: A clinical practice guideline. BMJ 2018, 363, k4169. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Basmaji, J.; Muttalib, F.; Granton, D.; Chaudhuri, D.; Chetan, D.; Hu, M.; Fernando, S.M.; Honarmand, K.; Bakaa, L.; et al. High-flow nasal cannula for acute hypoxemic respiratory failure in patients with COVID-19: Systematic reviews of effectiveness and its risks of aerosolization, dispersion, and infection transmission. Can. J. Anaesth. 2020, 67, 1217–1248. [Google Scholar] [CrossRef]
- Nicholson, T.W.; Talbot, N.P.; Nickol, A.; Chadwick, A.J.; Lawton, O. Respiratory failure and non-invasive respiratory support during the covid-19 pandemic: An update for re-deployed hospital doctors and primary care physicians. BMJ 2020, 369, m2446. [Google Scholar] [CrossRef]
- Winck, J.C.; Ambrosino, N. COVID-19 pandemic and non invasive respiratory management: Every Goliath needs a David. An evidence based evaluation of problems. Pulmonology 2020, 26, 213–220. [Google Scholar] [CrossRef]
- Frat, J.P.; Thille, A.W.; Mercat, A.; Girault, C.; Ragot, S.; Perbet, S.; Prat, G.; Boulain, T.; Morawiec, E.; Cottereau, A.; et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N. Engl. J. Med. 2015, 372, 2185–2196. [Google Scholar] [CrossRef] [Green Version]
- Messika, J.; Ahmed, K.B.; Gaudry, S.; Miguel-Montanes, R.; Rafat, C.; Sztrymf, B.; Dreyfuss, D.; Ricard, J. Use of High-Flow Nasal Cannula Oxygen Therapy in Subjects with ARDS: A 1-Year Observational Study. Respir. Care 2015, 60, 162–169. [Google Scholar] [CrossRef] [Green Version]
- Ferreyro, B.L.; Angriman, F.; Munshi, L.; Del Sorbo, L.; Ferguson, N.D.; Rochwerg, B.; Ryu, M.J.; Saskin, R.; Wunsch, H.; da Costa, B.R.; et al. Association of Noninvasive Oxygenation Strategies with All-Cause Mortality in Adults with Acute Hypoxemic Respiratory Failure: A Systematic Review and Meta-analysis. JAMA 2020, 324, 57. [Google Scholar] [CrossRef]
- Patel, M.; Gangemi, A.; Marron, R.; Chowdhury, J.; Yousef, I.; Zheng, M.; Mills, N.; Tragesser, L.; Giurintano, L.; Gupta, R.; et al. Retrospective analysis of high flow nasal therapy in COVID-19-related moderate-to-severe hypoxaemic respiratory failure. BMJ Open Respir. Res. 2020, 7, e000650. [Google Scholar] [CrossRef] [PubMed]
- Rochwerg, B.; Granton, D.; Wang, D.X.; Helviz, Y.; Einav, S.; Frat, J.P.; Mekontso-Dessap, A.; Schreiber, A.; Azoulay, E.; Mercat, A.; et al. High flow nasal cannula compared with conventional oxygen therapy for acute hypoxemic respiratory failure: A systematic review and meta-analysis. Intensive Care Med. 2019, 45, 563–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sartini, C.; Tresoldi, M.; Scarpellini, P.; Tettamanti, A.; Carcò, F.; Landoni, G.; Zangrillo, A. Respiratory Parameters in Patients with COVID-19 After Using Noninvasive Ventilation in the Prone Position Outside the Intensive Care Unit. JAMA 2020, 323, 2338. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhao, W.; Li, J.; Shu, W.; Duan, J. The experience of high-flow nasal cannula in hospitalized patients with 2019 novel coronavirus-infected pneumonia in two hospitals of Chongqing, China. Ann. Intensive Care 2020, 10, 37. [Google Scholar] [CrossRef] [Green Version]
- Arentz, M.; Yim, E.; Klaff, L.; Lokhandwala, S.; Riedo, F.X.; Chong, M.; Lee, M. Characteristics and Outcomes of 21 Critically Ill Patients with COVID-19 in Washington State. JAMA 2020, 323, 1612. [Google Scholar] [CrossRef] [Green Version]
- Mitra, A.R.; Fergusson, N.A.; Lloyd-Smith, E.; Wormsbecker, A.; Foster, D.; Karpov, A.; Crowe, A.; Haljan, G.; Chittock, D.R.; Kanji, H.D.; et al. Baseline characteristics and outcomes of patients with COVID-19 admitted to intensive care units in Vancouver, Canada: A case series. CMAJ 2020, 192, 694–701. [Google Scholar] [CrossRef]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef] [Green Version]
- Bellani, G.; Laffey, J.G.; Pham, T.; Madotto, F.; Fan, E.; Brochard, L.; Esteban, A.; Gattinoni, L.; Bumbasirevic, V.; Piquilloud, L.; et al. Noninvasive Ventilation of Patients with Acute Respiratory Distress Syndrome. Insights from the LUNG SAFE Study. Am. J. Respir. Crit. Care Med. 2016, 195, 67–77. [Google Scholar] [CrossRef] [Green Version]
- Rochwerg, B.; Brochard, L.; Elliott, M.W.; Hess, D.; Hill, N.S.; Nava, S.; Navalesi, P.; Antonelli, M.; Brozek, J.; Conti, G.; et al. Official ERS/ATS clinical practice guidelines: Noninvasive ventilation for acute respiratory failure. Eur. Respir. J. 2017, 50, 1602426. [Google Scholar] [CrossRef]
- Patel, B.K.; Kress, J.P.; Hall, J.B. Alternatives to Invasive Ventilation in the COVID-19 Pandemic. JAMA 2020, 324, 43. [Google Scholar] [CrossRef]
- Antonelli, M.; Conti, G.; Moro, M.; Esquinas, A.; Gonzalez-Diaz, G.; Confalonieri, M.; Pelaia, P.; Prinicipi, T.; Gregoretti, C.; Beltrame, F.; et al. Predictors of failure of noninvasive positive pressure ventilation in patients with acute hypoxemic respiratory failure: A multi-center study. Intensive Care Med. 2001, 27, 1718–1728. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, T.D.; Sanches, P.R.; de Morais, L.C.; Scarin, F.C.; Silva, E.; Barbas, C.S.V. Performance of noninvasive ventilation in acute respiratory failure in critically ill patients: A prospective, observational, cohort study. BMC Pulm. Med. 2015, 15, 144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffiths, M.J.D.; McAuley, D.F.; Perkins, G.D.; Barrett, N.; Blackwood, B.; Boyle, A.; Chee, N.; Connolly, B.; Dark, P.; Finney, S.; et al. Guidelines on the management of acute respiratory distress syndrome. BMJ Open Respir. Res. 2019, 6. [Google Scholar] [CrossRef] [Green Version]
- Argenziano, M.G.; Bruce, S.L.; Slater, C.L.; Tiao, J.R.; Baldwin, M.R.; Barr, R.G.; Chang, B.P.; Chau, K.H.; Choi, J.J.; Gavin, N.; et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: Retrospective case series. BMJ 2020, 369, m1996. [Google Scholar] [CrossRef] [PubMed]
- Bhatraju, P.K.; Ghassemieh, B.J.; Nichols, M.; Kim, R.; Jerome, K.R.; Nalla, A.K.; Greninger, A.L.; Pipvath, S.; Wurfel, M.M.; Evans, L.; et al. Covid-19 in Critically Ill Patients in the Seattle Region—Case Series. N. Engl. J. Med. 2020, 382, 2012–2022. [Google Scholar] [CrossRef] [PubMed]
- Oranger, M.; Gonzalez-Bermejo, J.; Dacosta-Noble, P.; Llontop, C.; Guerder, A.; Trosini-Desert, V.; Faure, M.; Raux, M.; Decavele, A.; Morélot-Panzini, C.; et al. Continuous positive airway pressure to avoid intubation in SARS-CoV-2 pneumonia: A two-period retrospective case-control study. Eur. Respir. J. 2020. [Google Scholar] [CrossRef]
- Bhimraj, A.; Morgan, R.L.; Shumaker, A.H.; Lavergne, V.; Baden, L.; Cheng, V.C.; Edwards, K.M.; Gandhi, R.; Muller, W.J.; O’Horo, J.C.; et al. Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Guérin, C.; Reignier, J.; Richard, J.C.; Beuret, P.; Gacouin, A.; Boulain, T.; Mercier, E.; Badet, M.; Mercat, A.; Baudin, O.; et al. Prone positioning in severe acute respiratory distress syndrome. N. Engl. J. Med. 2013, 368, 2159–2168. [Google Scholar] [CrossRef]
- Ding, L.; Wang, L.; Ma, W.; He, H. Efficacy and safety of early prone positioning combined with HFNC or NIV in moderate to severe ARDS: A multi-center prospective cohort study. Crit. Care 2020, 24, 28. [Google Scholar] [CrossRef] [Green Version]
- Caputo, N.D.; Strayer, R.J.; Levitan, R. Early Self-Proning in Awake, Non-intubated Patients in the Emergency Department: A Single ED’s Experience During the COVID-19 Pandemic. Acad. Emerg. Med. 2020, 27, 375–378. [Google Scholar] [CrossRef]
- Chad, T.; Sampson, C. Prone positioning in conscious patients on medical wards: A review of the evidence and its relevance to patients with COVID-19 infection. Clin. Med. 2020, 20, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Coppo, A.; Bellani, G.; Winterton, D.; Pierro, M.D.; Soria, A.; Faverio, P.; Cairo, M.; Mori, S.; Messinesi, G.; Contro, E.; et al. Feasibility and physiological effects of prone positioning in non-intubated patients with acute respiratory failure due to COVID-19 (PRON-COVID): A prospective cohort study. Lancet Respir. Med. 2020, 8, 765–774. [Google Scholar] [CrossRef]
- Elharrar, X.; Trigui, Y.; Dols, A.M.; Touchon, F.; Martinez, S.; Prud’homme, E.; Papazian, L. Use of Prone Positioning in Nonintubated Patients with COVID-19 and Hypoxemic Acute Respiratory Failure. JAMA 2020, 323, 2336–2338. [Google Scholar] [CrossRef] [PubMed]
- Retucci, M.; Aliberti, S.; Ceruti, C.; Santambrogio, M.; Tammaro, S.; Cuccarini, F.; Carai, C.; Grasselli, G.; Oneta, A.M.; Saderi, L.; et al. Prone and Lateral Positioning in Spontaneously Breathing Patients with COVID-19 Pneumonia Undergoing Noninvasive Helmet CPAP Treatment. Chest 2020. [Google Scholar] [CrossRef]
- Thompson, A.E.; Ranard, B.L.; Wei, Y.; Jelic, S. Prone Positioning in Awake, Nonintubated Patients with COVID-19 Hypoxemic Respiratory Failure. JAMA Intern. Med. 2020, 180, 1537. [Google Scholar] [CrossRef] [PubMed]
- Taboada, M.; González, M.; Álvarez, A.; González, I.; García, J.; Eiras, M.; Diaz Vieito, M.; Naveira, A.; Otero, P.; Campaña, O.; et al. Effectiveness of prone positioning in non-intubated ICU patients with moderate to severe ARDS by COVID-19. Anesth Analg 2020. [Google Scholar] [CrossRef]
- Cruces, P.; Retamal, J.; Hurtado, D.E.; Erranz, B.; Iturrieta, P.; González, C.; Diaz, F. A physiological approach to understand the role of respiratory effort in the progression of lung injury in SARS-CoV-2 infection. Crit. Care 2020, 24, 494. [Google Scholar] [CrossRef]
- Koeckerling, D.; Barker, J.; Mudalige, N.L.; Oyefeso, O.; Pan, D.; Pareek, M.; Thompson, J.P.; Ng, G.A. Awake prone positioning in COVID-19. Thorax 2020, 75, 833–834. [Google Scholar] [CrossRef]
- Fan, E.; Del Sorbo, L.; Goligher, E.C.; Hodgson, C.L.; Munshi, L.; Walkey, A.J.; Adhikari, N.K.J.; Amato, M.B.P.; Branson, R.; Brower, R.G.; et al. An Official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine Clinical Practice Guideline: Mechanical Ventilation in Adult Patients with Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2017, 195, 1253–1263. [Google Scholar] [CrossRef]
- Slutsky, A.S.; Ranieri, V.M. Ventilator-Induced Lung Injury. N. Engl. J. Med. 2013, 369, 2126–2136. [Google Scholar] [CrossRef] [Green Version]
- Brower, R.G.; Matthay, M.A.; Morris, A.; Schoenfeld, D.; Thompson, B.T.; Wheeler, A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N. Engl. J. Med. 2000, 342, 1301–1308. [Google Scholar] [PubMed]
- Amato, M.B.P.; Meade, M.O.; Slutsky, A.S.; Brochard, L.; Costa, E.L.V.; Schoenfeld, D.A.; Stewart, T.E.; Briel, M.; Talmor, D.; Mercat, A.; et al. Driving Pressure and Survival in the Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2015, 372, 747–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talmor, D.; Sarge, T.; Malhotra, A.; O’Donnell, C.R.; Ritz, R.; Lisbon, A.; Novack, V.; Loring, S.H. Mechanical Ventilation Guided by Esophageal Pressure in Acute Lung Injury. N. Engl. J. Med. 2008, 359, 2095–2104. [Google Scholar] [CrossRef] [Green Version]
- Beitler, J.R.; Sarge, T.; Banner-Goodspeed, V.M.; Gong, M.N.; Cook, D.; Novack, V.; Loring, S.H.; Talmor, D. Effect of Titrating Positive End-Expiratory Pressure (PEEP) with an Esophageal Pressure–Guided Strategy vs an Empirical High PEEP-FiO2 Strategy on Death and Days Free from Mechanical Ventilation Among Patients with Acute Respiratory Distress Syndrome: A Randomized Clinical Trial. JAMA 2019, 321, 846–857. [Google Scholar] [PubMed] [Green Version]
- Moss, M.; Huang, D.T.; Brower, R.G.; Ferguson, N.D.; Ginde, A.A.; Gong, M.N.; Grissom, C.K.; Gundel, S.; Hayden, D.; Hite, R.D.; et al. Early Neuromuscular Blockade in the Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2019, 380, 1997–2008. [Google Scholar]
- Papazian, L.; Aubron, C.; Brochard, L.; Chiche, J.D.; Combes, A.; Dreyfuss, D.; Forel, J.; Guerin, C.; Jaber, S.; Mekontso-Dessap, A.; et al. Formal guidelines: Management of acute respiratory distress syndrome. Ann. Intensive Care 2019, 9, 69. [Google Scholar] [CrossRef] [Green Version]
- Munshi, L.; Walkey, A.; Goligher, E.; Pham, T.; Uleryk, E.M.; Fan, E. Venovenous extracorporeal membrane oxygenation for acute respiratory distress syndrome: A systematic review and meta-analysis. Lancet Respir. Med. 2019, 7, 163–172. [Google Scholar] [CrossRef]
- Schmidt, M.; Hajage, D.; Lebreton, G.; Monsel, A.; Voiriot, G.; Levy, D.; Baron, E.; Beurton, A.; Chommeloux, J.; Meng, P.; et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome associated with COVID-19: A retrospective cohort study. Lancet Respir. Med. 2020, 8, 1121–1131. [Google Scholar] [CrossRef]
- Wiedemann, H.P.; Wheeler, A.P.; Bernard, G.R.; Thompson, T.; Hayden, D.; de Boisblanc, B.; Connors, A.F., Jr.; Hite, D.R.; Harabin, A.L. Comparison of Two Fluid-Management Strategies in Acute Lung Injury. N. Engl. J. Med. 2016, 354, 2564–2575. [Google Scholar] [CrossRef] [Green Version]
- Annane, D.; Pastores, S.M.; Rochwerg, B.; Arlt, W.; Balk, R.A.; Beishuizen, A.; Briegel, J.; Carcillo, J.; Christ-Crain, M.; Cooper, M.S.; et al. Guidelines for the Diagnosis and Management of Critical Illness-Related Corticosteroid Insufficiency (CIRCI) in Critically Ill Patients (Part I): Society of Critical Care Medicine (SCCM) and European Society of Intensive Care Medicine (ESICM) 2017. Crit. Care Med. 2017, 45, 2078–2088. [Google Scholar] [CrossRef]
- Ye, Z.; Rochwerg, B.; Wang, Y.; Adhikari, N.K.; Murthy, S.; Lamontagne, F.; Fowler, R.A.; Qiu, H.; Li, W.; Ling, S.; et al. Treatment of patients with nonsevere and severe coronavirus disease 2019: An evidence-based guideline. CMAJ 2020, 192, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; Elmahi, E.; et al. Dexamethasone in Hospitalized Patients with Covid-19—Preliminary Report. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef]
- Ye, Z.; Wang, Y.; Colunga-Lozano, L.E.; Prasad, M.; Tangamornsuksan, W.; Rochwerg, B.; Yao, L.; Motaghi, S.; Couban, R.J.; Ghadimi, M.; et al. Efficacy and safety of corticosteroids in COVID-19 based on evidence for COVID-19, other coronavirus infections, influenza, community-acquired pneumonia and acute respiratory distress syndrome: A systematic review and meta-analysis. CMAJ 2020, 192, E756–E767. [Google Scholar] [CrossRef] [PubMed]
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the Treatment of Covid-19—Final Report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef]
- Paranjpe, I.; Fuster, V.; Lala, A.; Russak, A.J.; Glicksberg, B.S.; Levin, M.A.; Charney, A.W.; Narula, J.; Fayad, Z.A.; Bagiella, E.; et al. Association of Treatment Dose Anticoagulation with In-Hospital Survival Among Hospitalized Patients with COVID-19. J. Am. Coll. Cardiol. 2020, 76, 122–124. [Google Scholar] [CrossRef] [PubMed]
- Simonovich, V.A.; Burgos Pratx, L.D.; Scibona, P.; Beruto, M.V.; Vallone, M.G.; Vázquez, C.; Savoy, N.; Giunta, D.H.; Pérez, L.G.; Sánchez, M.D.L.; et al. A Randomized Trial of Convalescent Plasma in Covid-19 Severe Pneumonia. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef]
- Entrenas Castillo, M.; Entrenas Costa, L.M.; Vaquero Barrios, J.M.; Alcalá Díaz, J.F.; López Miranda, J.; Bouillon, R.; Quesada Gomez, J.M. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study. J. Steroid. Biochem. Mol. Biol. 2020, 203. [Google Scholar] [CrossRef]
- Cao, B.; Wang, Y.; Wen, D.; Liu, W.; Wang, J.; Fan, G.; Ruan, L.; Song, B.; Cai, Y.; Wei, M.; et al. A Trial of Lopinavir–Ritonavir in Adults Hospitalized with Severe Covid-19. N. Engl. J. Med. 2020, 382, 1787–1799. [Google Scholar] [CrossRef]
- Horby, P.; Mafham, M.; Linsell, L.; Bell, J.L.; Staplin, N.; Emberson, J.R.; Wiselka, M.; Ustianowski, A.; Elmahi, E.; Prudon, B.; et al. Effect of Hydroxychloroquine in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef]
- Hodge, C.; Marra, F.; Marzolini, C.; Boyle, A.; Gibbons, S.; Siccardi, M.; Burger, D.; Back, D.; Khoo, S. Drug interactions: A review of the unseen danger of experimental COVID-19 therapies. J. Antimicrob. Chemother. 2020. [Google Scholar] [CrossRef]
- Neff, T.A.; Stocker, R.; Frey, H.-R.; Stein, S.; Russi, E.W. Long-term Assessment of Lung Function in Survivors of Severe ARDS. Chest 2003, 123, 845–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herridge, M.S.; Tansey, C.M.; Matté, A.; Tomlinson, G.; Diaz-Granados, N.; Cooper, A.; Guest, C.B.; Mazer, C.D.; Mehta, S.; Stewart, T.E.; et al. Functional Disability 5 Years after Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2011, 364, 1293–1304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabrera-Benitez, N.E.; Laffey, J.G.; Parotto, M.; Spieth, P.M.; Villar, J.; Zhang, H.; Slutsky, A.S. Mechanical Ventilation–associated Lung Fibrosis in Acute Respiratory Distress Syndrome A Significant Contributor to Poor Outcome. Anesthesiology 2014, 121, 189–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- George, P.M.; Wells, A.U.; Jenkins, R.G. Pulmonary fibrosis and COVID-19: The potential role for antifibrotic therapy. Lancet Respir. Med. 2020, 8, 807–815. [Google Scholar] [CrossRef]
- Calfee, C.S.; Delucchi, K.; Parsons, P.E.; Thompson, B.T.; Ware, L.B.; Matthay, M.A. Latent Class Analysis of ARDS Subphenotypes: Analysis of Data from Two Randomized Controlled Trials. Lancet Respir. Med. 2014, 2, 611–620. [Google Scholar] [CrossRef] [Green Version]
- Calfee, C.S.; Delucchi, K.L.; Sinha, P.; Matthay, M.A.; Hackett, J.; Shankar-Hari, M.; McDowell, C.; Laffey, J.G.; O’Kane, C.M.; McAuley, D.F.; et al. ARDS Subphenotypes and Differential Response to Simvastatin: Secondary Analysis of a Randomized Controlled Trial. Lancet Respir. Med. 2018, 6, 691–698. [Google Scholar] [CrossRef] [Green Version]
- Famous, K.R.; Delucchi, K.; Ware, L.B.; Kangelaris, K.N.; Liu, K.D.; Thompson, B.T.; Calfee, C.S. Acute Respiratory Distress Syndrome Subphenotypes Respond Differently to Randomized Fluid Management Strategy. Am. J. Respir. Crit. Care Med. 2017, 195, 331–338. [Google Scholar] [CrossRef]
- Shankar-Hari, M.; Fan, E.; Ferguson, N.D. Acute respiratory distress syndrome (ARDS) phenotyping. Intensive Care Med. 2019, 45, 516–519. [Google Scholar] [CrossRef] [Green Version]
- Levi, M.; Thachil, J.; Iba, T.; Levy, J.H. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020, 7, 438–440. [Google Scholar] [CrossRef]
| Pathology | ARDS | COVID-ARDS |
|---|---|---|
| Diffuse Alveolar Damage (DAD) | Early/Exudative: - interstitial/alveolar edema - “hyaline” membranes - neutrophil infiltration - AEC desquamation - pulmonary microvascular thrombosis Late/Fibroproliferative: - alveolar/interstitial fibrosis - type II AEC hyperplasia | Similar to ARDS except: - paucity of neutrophils - interstitial/alveolar lymphocytic infiltration - possibly increased pulmonary microvascular thrombi relative to other causes |
| Other features | - organizing pneumonia (fibrosis) - alveolar haemorrhage - viral pneumonia | - occasional viral cytopathic changes (multinucleated syncytial cells, atypical enlarged AEC) - viral inclusions in AEC |
| Intervention | ARDS | COVID-ARDS |
|---|---|---|
| Fluid management | ||
| Conservative fluid strategy | Weak recommendation post initial resuscitation (SCCM [48], FICM-ICS [75]) | Weak recommendation (SSC [40]) |
| Anti-inflammatory therapy | ||
| Steroid | Weak recommendation - Methylprednisolone 1–2 mg/kg/d with 14 d taper (FICM-ICS [75], SCCM-ESICM [102] | Recommended - Dexamethasone 6 mg/d for 10 d (WHO [33], IDSA [79], CMAJ [103]) |
| Other (Physiologic/Biologic) | Not recommended - β2-agonists - Exogenous surfactant - Anti-IL1β - Statins | Not recommended - Hydroxychloroquine/chloroquine - Lopanivir/ritonavir |
| Experimental | Current trials - Anti-tissue factor antibody fragment - MAPK inhibitor - Stem cell therapies - Complement inhibitor - JAK inhibitor | Current trials- Convalescent human plasma - Intravenous Immunoglobulin - IL-6 inhibitor (e.g., tocilizumab) - IL-1 inhibitor (e.g., anakinra) - Anti-GM-CSF (e.g., mavrilimumab) - Anticoagulants (e.g., Low molecular weight heparin) - Fibrinolytics (e.g., tPA) - 25-OH vitamin D |
| Anti-microbials | ||
| Antibiotics | Strong recommendation - If ARDS due to pneumonia or sepsis (SCCM [48]) - If evidence of ventilator-associated pneumonia (SCCM [48]) | Weak recommendation - In patients requiring MV (SSC [40], IDSA [79]) - If concomitant bacterial pneumonia (SSC [40], IDSA [79])) |
| Antivirals | Specific viral targeted therapy indicated - If viral infection identified (e.g., influenza, RSV) | Specific viral targeted therapy indicated - If evidence of concomitant viral pneumonia (e.g., influenza, RSV) SARS-CoV-2 targeted therapy - Remdesivir (IDSA [79]) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kassirian, S.; Taneja, R.; Mehta, S. Diagnosis and Management of Acute Respiratory Distress Syndrome in a Time of COVID-19. Diagnostics 2020, 10, 1053. https://doi.org/10.3390/diagnostics10121053
Kassirian S, Taneja R, Mehta S. Diagnosis and Management of Acute Respiratory Distress Syndrome in a Time of COVID-19. Diagnostics. 2020; 10(12):1053. https://doi.org/10.3390/diagnostics10121053
Chicago/Turabian StyleKassirian, Shayan, Ravi Taneja, and Sanjay Mehta. 2020. "Diagnosis and Management of Acute Respiratory Distress Syndrome in a Time of COVID-19" Diagnostics 10, no. 12: 1053. https://doi.org/10.3390/diagnostics10121053
APA StyleKassirian, S., Taneja, R., & Mehta, S. (2020). Diagnosis and Management of Acute Respiratory Distress Syndrome in a Time of COVID-19. Diagnostics, 10(12), 1053. https://doi.org/10.3390/diagnostics10121053





