Oleic Acid and Linoleic Acid Enhances the Biocontrol Potential of Metarhizium rileyi
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Strain and Media
2.2. Phenotype Assays
2.3. Fungal Virulence-Related Phenotypes
2.4. Bioassays for Fungal Virulence
2.5. CAT, POD, and SOD Activity Assays
2.6. Quantitative Real-Time Reverse-Transcription Polymerase Chain Reaction (RT-qPCR)
2.7. Statistical Analysis
3. Results
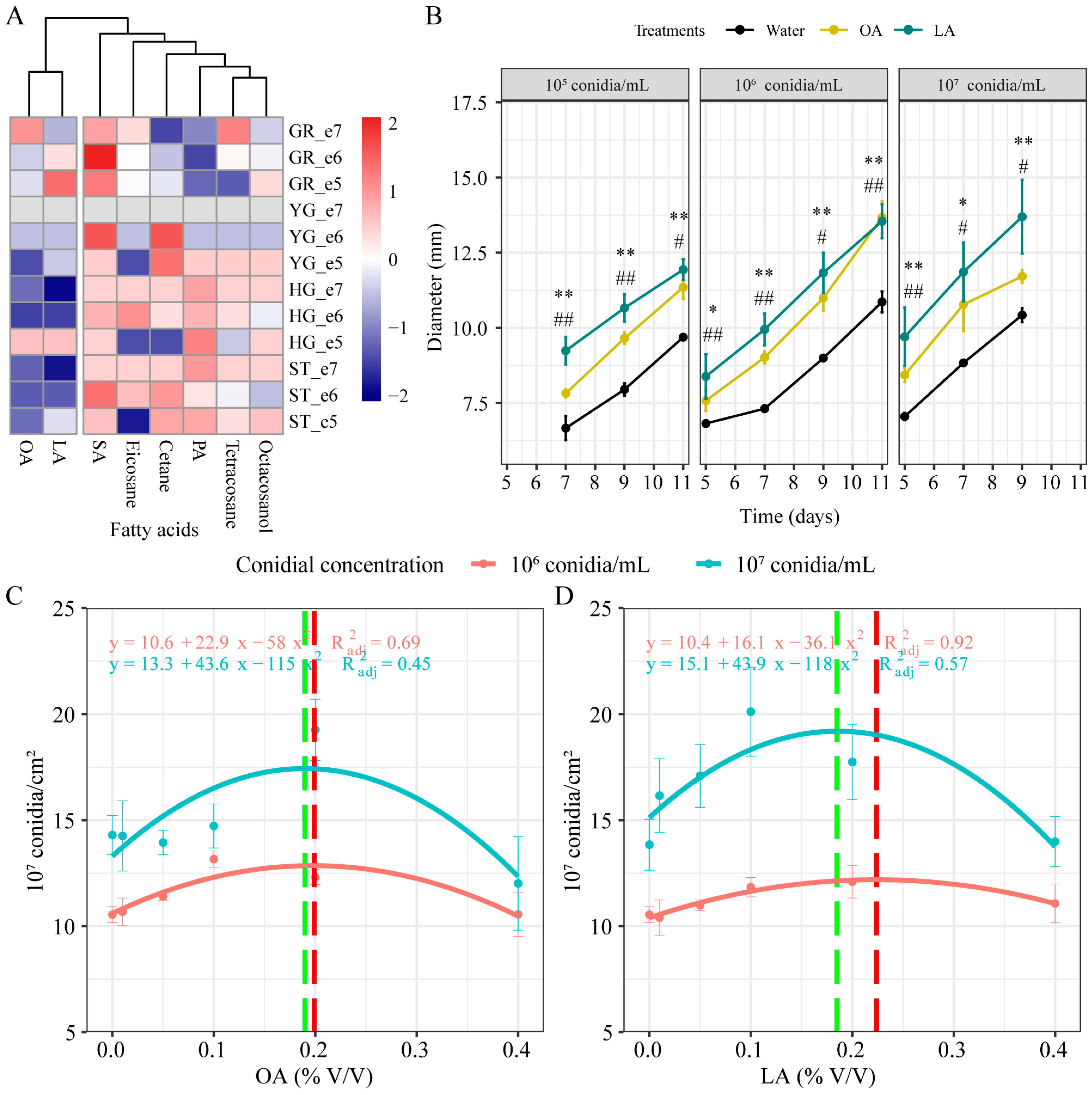
3.1. Effects of Lipids Addition on M. rileyi Growth and Development
3.2. Exogenous Oleic Acid and Linoleic Acid Enhanced the Growth and Development of M. rileyi
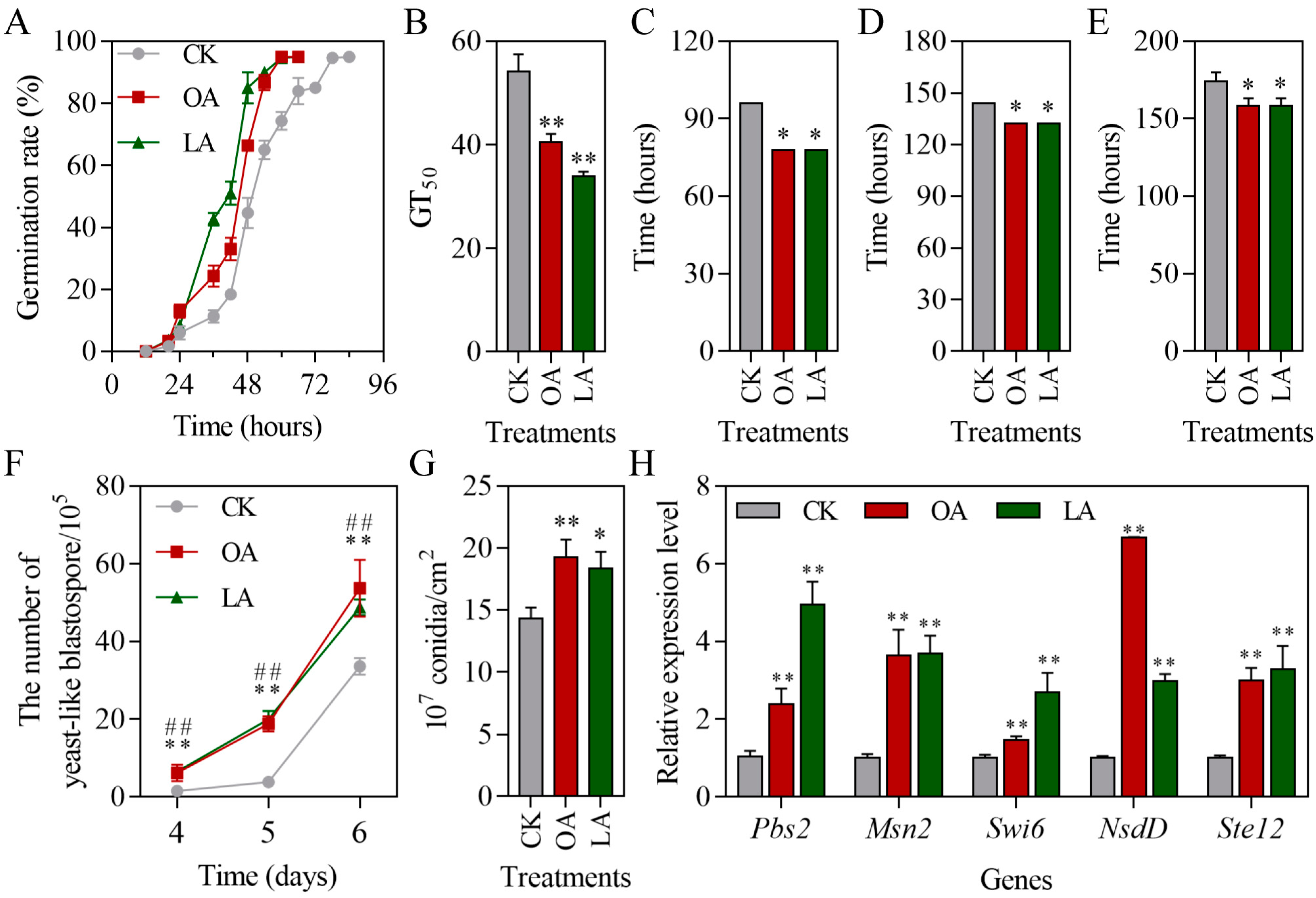
3.3. Exogenous Oleic Acid and Linoleic Acid Enhanced the Multiple Stress Tolerances of M. rileyi
3.4. Exogenous Oleic Acid and Linoleic Acid Protected Conidial Germination of M. rileyi and Improved Stress Tolerance
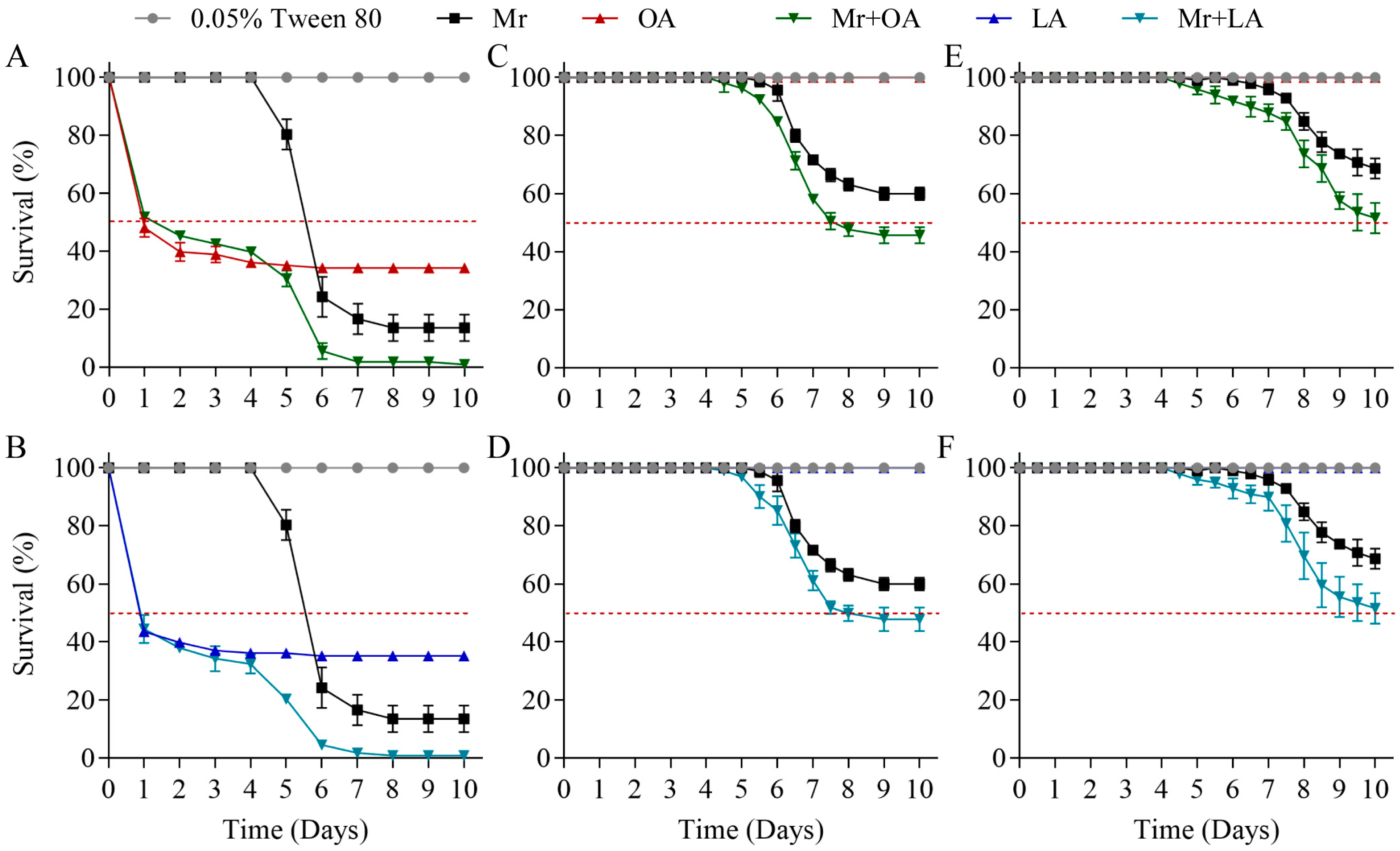
3.5. Exogenous Oleic Acid and Linoleic Acid Improved the Virulence of M. rileyi
3.6. Exogenous Oleic Acid and Linoleic Acid Increased Fungal Virulence-Related Phenotypes
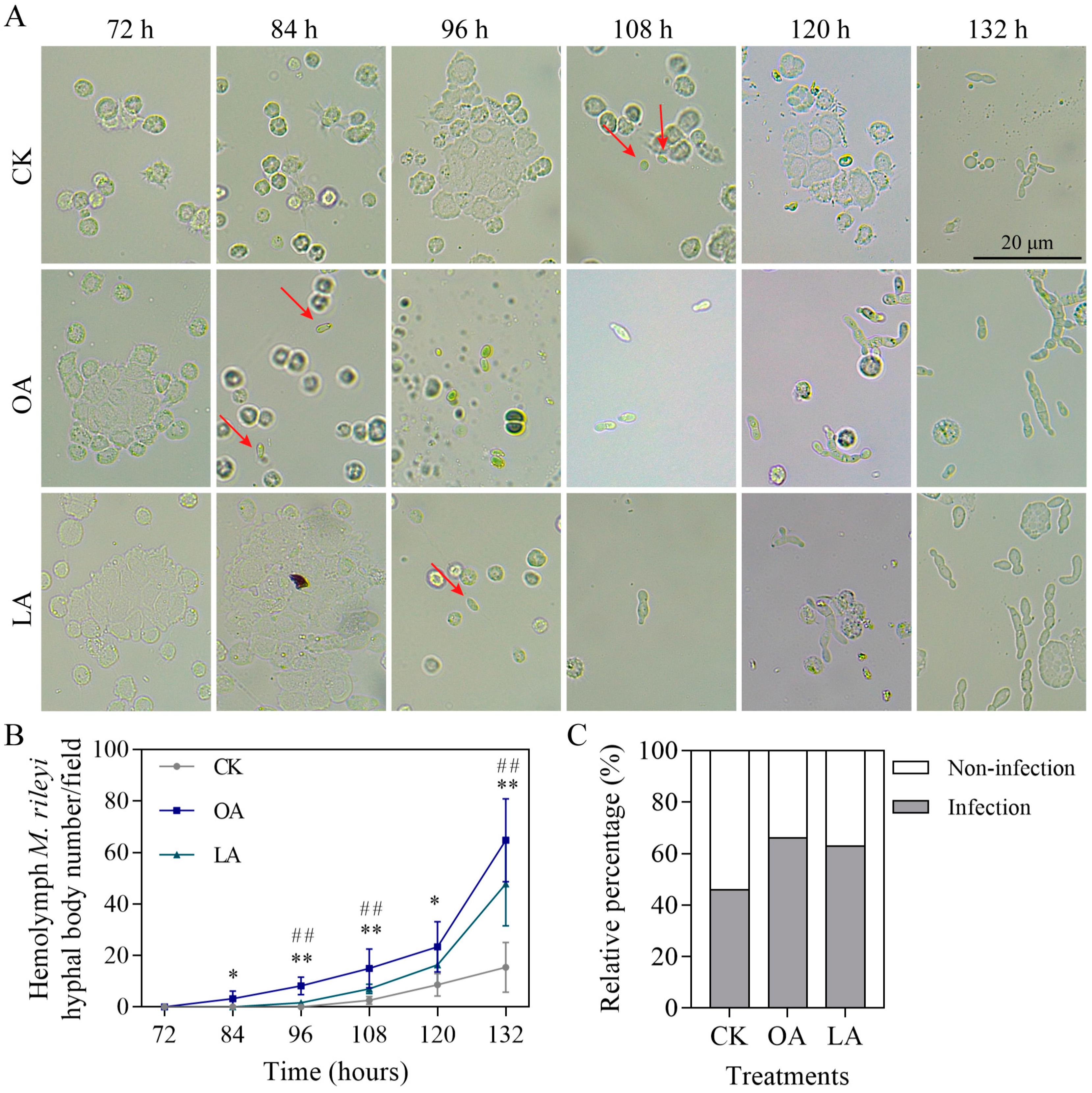
3.7. Exogenous Oleic Acid and Linoleic Acid Enhanced Cuticle Infection of M. rileyi
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, L.; Wang, J.; Zhang, X.; Yin, Y.; Li, R.; Lin, Y.; Deng, C.; Yang, K.; Liu, X.; Wang, Z. Pathogenicity of Metarhizium rileyi against Spodoptera litura larvae: Appressorium differentiation, proliferation in hemolymph, immune interaction, and reemergence of mycelium. Fungal Genet. Biol. 2021, 150, 103508. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, E.D.S.; Lima, A.R.D.; Pessoa, L.G.A.; Dias, P.M.; Assis, L.F. Virulência de Metarhizium rileyi (Ascomycota:Clavicipitaceae) a Spodoptera cosmioides (Lepidoptera: Noctuidae). Res. Soc. Dev. 2020, 9, 186973962. [Google Scholar] [CrossRef]
- Barros, S.; Pitta, R.; Lopes, R.; Almeida, E.; Ferreira, F. Susceptibility of Spodoptera frugiperda and Chrysodeixis includens (Lepidoptera: Noctuidae) to infections caused by Metarhizium rileyi. Pesqui. Agropecu. Trop. 2020, 50, e61713. [Google Scholar] [CrossRef]
- Devi, P.S.V.; Prasad, Y.G. Nomuraea Rileyi—A potential mycoinsecticide. In Biocontrol Potential and Its Exploitation in Sustainable Agriculture: Volume 2: Insect Pests; Upadhyay, R.K., Mukerji, K.G., Chamola, B.P., Eds.; Springer: Boston, MA, USA, 2001; pp. 23–38. [Google Scholar]
- Zhang, Z.; Sui, L.; Tian, Y.; Lu, Y.; Xia, X.; Liu, W.; Cheng, K.; Li, Q.; Shi, W. Metarhizium rileyi with broad-spectrum insecticidal ability confers resistance against phytopathogens and insect pests as a phytoendophyte. Pest Manag. Sci. 2024, 80, 3246–3257. [Google Scholar] [CrossRef]
- Faria, M.; Souza, D.A.; Sanches, M.M.; Schmidt, F.G.V.; Oliveira, C.M.; Benito, N.P.; Lopes, R.B. Evaluation of key parameters for developing a Metarhizium rileyi-based biopesticide against Spodoptera frugiperda (Lepidoptera: Noctuidae) in maize: Laboratory, greenhouse, and field trials. Pest Manag. Sci. 2022, 78, 1146–1154. [Google Scholar] [CrossRef]
- Barros, S.K.A.; de Almeida, E.G.; Ferreira, F.T.R.; Barreto, M.R.; Lopes, R.B.; Pitta, R.M. Field efficacy of Metarhizium rileyi applications against Spodoptera frugiperda (Lepidoptera: Noctuidae) in maize. Neotrop. Entomol. 2021, 50, 976–988. [Google Scholar] [CrossRef] [PubMed]
- Grijalba, E.P.; Espinel, C.; Cuartas, P.E.; Chaparro, M.L.; Villamizar, L.F. Metarhizium rileyi biopesticide to control Spodoptera frugiperda: Stability and insecticidal activity under glasshouse conditions. Fungal Biol. 2018, 122, 1069–1076. [Google Scholar] [CrossRef]
- Edelstein, J.; Lecuona, R.; Trumper, E. Selection of culture media and in vitro assessment of temperature-dependent development of Nomuraea rileyi. Neotrop. Entomol. 2004, 33, 737–742. [Google Scholar] [CrossRef]
- Sánchez-Rey, L.E.; Moreno-Sarmiento, N.; Grijalba-Bernal, E.P.; Quiroga-Cubides, G. Physiological response of Metarhizium rileyi with linoleic acid supplementation. Fungal Biol. 2024, 128, 1827–1835. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Xu, S.; Chen, L.; Zhan, T.; Zhang, X.; Liang, H.; Chen, B.; Peng, Y. Gut microbial diversity reveals differences in pathogenicity between Metarhizium rileyi and Beauveria bassiana during the early stage of infection in Spodoptera litura larvae. Microorganisms 2024, 12, 1129. [Google Scholar] [CrossRef]
- Shin, T.Y.; Lee, M.R.; Park, S.E.; Lee, S.J.; Kim, W.J.; Kim, J.S. Pathogenesis-related genes of entomopathogenic fungi. Arch. Insect Biochem. 2020, 105, e21747. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Xie, L.; Han, J.H.; Lee, S.Y. Influence of additives on the yield and pathogenicity of conidia produced by solid state cultivation of an Isaria javanica isolate. Mycobiology 2014, 42, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Paixão, F.R.S.; Muniz, E.R.; Barreto, L.P.; Bernardo, C.C.; Mascarin, G.M.; Luz, C.; Fernandes, É.K.K. Increased heat tolerance afforded by oil-based conidial formulations of Metarhizium anisopliae and Metarhizium robertsii. Biocontrol Sci. Technol. 2017, 27, 324–337. [Google Scholar] [CrossRef]
- Saeed, N.; Wakil, W.; Farooq, M.; Shakeel, M.; Arain, M.S.; Shakeel, Q. Evaluating the combination of Metarhizium anisopliae and an enhanced form of diatomaceous earth (Grain-Guard) for the environmentally friendly control of stored grain pests. Environ. Monit. Assess. 2020, 192, 210. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Sethiya, P.; Hu, X.; Guo, S.; Chen, Y.; Li, A.; Tan, K.; Wong, K.H. Transcription in fungal conidia before dormancy produces phenotypically variable conidia that maximize survival in different environments. Nat. Microbiol. 2021, 6, 1066–1081. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Tian, J.; Lou, Y.; Ran, J.; Mohamed, A.; Keyhani, N.O.; Jaronski, S.; Wang, G.; Chen, X.; Zang, L.-S.; et al. Efficacy of Metarhizium rileyi granules for the control of Spodoptera frugiperda and its synergistic effects with chemical pesticide, sex pheromone and parasitoid. Entomol. Gen. 2023, 6, 1211–1219. [Google Scholar] [CrossRef]
- Rangel, D.E.N.; Acheampong, M.A.; Bignayan, H.G.; Golez, H.G.; Roberts, D.W. Conidial mass production of entomopathogenic fungi and tolerance of their mass-produced conidia to UV-B radiation and heat. Fungal Biol. 2023, 127, 1524–1533. [Google Scholar] [CrossRef]
- Kim, J.S.; Je, Y.H.; Roh, J.Y. Production of thermotolerant entomopathogenic Isaria fumosorosea SFP-198 conidia in corn-corn oil mixture. J. Ind. Microbiol. Biot. 2010, 37, 419–423. [Google Scholar] [CrossRef]
- Mishra, S.; Kumar, P.; Malik, A. Preparation, characterization, and insecticidal activity evaluation of three different formulations of Beauveria bassiana against Musca domestica. Parasitol. Res. 2013, 112, 3485–3495. [Google Scholar] [CrossRef] [PubMed]
- Nitbani, F.O.; Tjitda, P.J.P.; Nurohmah, B.A.; Wogo, H.E. Preparation of fatty acid and monoglyceride from vegetable oil. J. Oleo Sci. 2020, 69, 277–295. [Google Scholar] [CrossRef]
- Rush, T.A.; Puech-Pagès, V.; Bascaules, A.; Jargeat, P.; Maillet, F.; Haouy, A.; Maës, A.Q.; Carriel, C.C.; Khokhani, D.; Keller-Pearson, M.; et al. Lipo-chitooligosaccharides as regulatory signals of fungal growth and development. Nat. Commun. 2020, 11, 3897. [Google Scholar] [CrossRef]
- Xue, R.; Du, G.; Chen, C.; Chen, B.; Peng, Y. Oleic acid improves the conidial production and quality of Metarhizium rileyi as a biocontrol agent. Biocontrol Sci. Technol. 2023, 33, 758–771. [Google Scholar] [CrossRef]
- Jasińska, A.; Różalska, S.; Rusetskaya, V.; Słaba, M.; Bernat, P. Microplastic-induced oxidative stress in metolachlor-degrading filamentous fungus Trichoderma harzianum. Int. J. Mol. Sci. 2022, 23, 12978. [Google Scholar] [CrossRef]
- Peng, Y.; Chen, B. Role of cell membrane homeostasis in the pathogenicity of pathogenic filamentous fungi. Virulence 2024, 15, 2299183. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.J.; Wang, J.J.; Lin, H.Y.; Ding, J.L.; Feng, M.G.; Ying, S.H. HapX, an indispensable bzip transcription factor for iron acquisition, regulates infection initiation by orchestrating conidial oleic acid homeostasis and cytomembrane functionality in mycopathogen Beauveria bassiana. mSystems 2020, 5, e00695-20. [Google Scholar] [CrossRef]
- Peng, Y.J.; Zhang, H.; Feng, M.G.; Ying, S.H. SterylAcetyl hydrolase 1 (BbSay1) links lipid homeostasis to conidiogenesis and virulence in the entomopathogenic fungus Beauveria bassiana. J. Fungi 2022, 8, 292. [Google Scholar] [CrossRef]
- Gessler, N.N.; Filippovich, S.Y.; Bachurina, G.P.; Kharchenko, E.A.; Groza, N.V.; Belozerskaya, T.A. Oxylipins and oxylipin synthesis pathways in fungi. Appl. Biochem. Microbiol. 2017, 53, 628–639. [Google Scholar] [CrossRef]
- Peng, Y.; Yao, Y.; Pang, J.; Di, T.; Du, G.; Chen, B. Genetic diversity and virulence variation of Metarhizium rileyi from infected Spodoptera frugiperda in corn fields. Microorganisms 2024, 12, 264. [Google Scholar] [CrossRef]
- Wang, L.; Lai, Y.; Chen, J.; Cao, X.; Zheng, W.; Dong, L.; Zheng, Y.; Li, F.; Wei, G.; Wang, S. The ASH1-PEX16 regulatory pathway controls peroxisome biogenesis for appressorium-mediated insect infection by a fungal pathogen. Proc. Natl. Acad. Sci. USA 2023, 120, e2217145120. [Google Scholar] [CrossRef] [PubMed]
- Gebremariam, A.; Chekol, Y.; Assefa, F. Extracellular enzyme activity of entomopathogenic fungi, Beauveria bassiana and Metarhizium anisopliae and their pathogenicity potential as a bio-control agent against whitefly pests, Bemisia tabaci and Trialeurodes vaporariorum (Hemiptera: Aleyrodidae). BMC Res. Notes 2022, 15, 117. [Google Scholar] [CrossRef]
- Fan, L.; Li, B.; Wang, J.; Li, X.; Ma, F.; Du, F.; Li, H.; Lin, Y. Multifunctional regulation of NADPH oxidase in growth, microsclerotia formation and virulence in Metarhizium rileyi. Biotechnol. Lett. 2023, 45, 1441–1455. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Z.; Liu, X.; Song, Z.; Li, R.; Shao, C.; Yin, Y. Siderophore biosynthesis but not reductive iron assimilation is essential for the dimorphic fungus Nomuraea rileyi conidiation, dimorphism transition, resistance to oxidative stress, pigmented microsclerotium formation, and virulence. Front. Microbiol. 2016, 7, 931. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jiang, H.; Du, Y.; Keyhani, N.O.; Xia, Y.; Jin, K. Members of chitin synthase family in Metarhizium acridum differentially affect fungal growth, stress tolerances, cell wall integrity and virulence. PLoS Pathog. 2019, 15, e1007964. [Google Scholar] [CrossRef]
- Wang, D.; Lv, C.; Guan, Y.; Ni, X.; Wu, F. Dsk2 involves in conidiation, multi-stress tolerance and thermal adaptation in Beauveria bassiana. Microbiol. Rep. 2021, 13, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, L.; León, D.; Grijalba, E.; Moreno, N.; Cuartas-Otálora, P.; Quiroga-Cubides, G. Selection of inductors for thermotolerant conidia production of the entomopathogenic fungus Metarhizium rileyi Nm017. Biocontrol Sci. Technol. 2022, 32, 891–898. [Google Scholar] [CrossRef]
- Keyhani, N.O. Lipid biology in fungal stress and virulence: Entomopathogenic fungi. Fungal Biol. 2018, 122, 420–429. [Google Scholar] [CrossRef]
- Hou, J.; Ding, J.L.; Peng, Y.J.; Feng, M.G.; Ying, S.H. Genome-wide identification of BCS1 domain-containing proteins reveals the mitochondrial bcs1 essential for growth, stress response, and virulence of the filamentous entomopathogenic fungus Beauveria bassiana. Microbiol. Res. 2023, 267, 127262. [Google Scholar] [CrossRef] [PubMed]
- Boguś, M.I.; Czygier, M.; Gołebiowski, M.; Kedra, E.; Kucińska, J.; Mazgajska, J.; Samborski, J.; Wieloch, W.; Włóka, E. Effects of insect cuticular fatty acids on in vitro growth and pathogenicity of the entomopathogenic fungus Conidiobolus coronatus. Exp. Parasitol. 2010, 125, 400–408. [Google Scholar] [CrossRef]
- Hou, J.; Zhang, H.; Ding, J.L.; Feng, M.G.; Ying, S.H. Transcriptomic investigation reveals a physiological mechanism for Beauveria bassiana to survive under linoleic acid stress. iScience 2023, 26, 106551. [Google Scholar] [CrossRef]
- Pang, J.; Peng, Y.; Di, T.; Du, G.; Chen, B. Virulence of Metarhizium rileyi is determined by its growth and antioxidant stress and the protective and detoxifying enzymes of Spodoptera frugiperda. Insects 2023, 14, 260. [Google Scholar] [CrossRef] [PubMed]
- Paixão, F.R.S.; Huarte-Bonnet, C.; Ribeiro-Silva, C.S.; Mascarin, G.M.; Fernandes, É.K.K.; Pedrini, N. Tolerance to abiotic factors of microsclerotia and Mycelial Pellets from Metarhizium robertsii, and molecular and ultrastructural changes during microsclerotial differentiation. Front. Fungal Biol. 2021, 2, 654737. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Chi, Z.; Liu, G.L.; Hu, Z.; Zhao, S.Z.; Chi, Z.M. Melanin biosynthesis in the desert-derived Aureobasidium melanogenum XJ5-1 is controlled mainly by the CWI signal pathway via a transcriptional activator Cmr1. Curr. Genet. 2020, 66, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Urquiza, A.; Keyhani, N.O. Action on the surface: Entomopathogenic fungi versus the insect cuticle. Insects 2013, 4, 357–374. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, S. Insect pathogenic fungi: Genomics, molecular interactions, and genetic improvements. Annu. Rev. Entomol. 2017, 62, 73–90. [Google Scholar] [CrossRef] [PubMed]
- Brzozowski, A.M.; Derewenda, U.; Derewenda, Z.S.; Dodson, G.G.; Lawson, D.M.; Turkenburg, J.P.; Bjorkling, F.; Huge-Jensen, B.; Patkar, S.A.; Thim, L. A model for interfacial activation in lipases from the structure of a fungal lipase-inhibitor complex. Nature 1991, 351, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.P.; Finlay, D.K. Glucose, glycolysis and lymphocyte responses. Mol. Immunol. 2015, 68, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Ortiz-Urquiza, A.; Garrett, T.; Pei, Y.; Keyhani, N.O. Involvement of a caleosin in lipid storage, spore dispersal, and virulence in the entomopathogenic filamentous fungus, Beauveria bassiana. Environ. Microbiol. 2015, 17, 4600–4614. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.; Sun, J.; Radhakrishnan, G.V.; Lee, T.; Bozsóki, Z.; Fort, S.; Gavrin, A.; Gysel, K.; Thygesen, M.B.; Andersen, K.R.; et al. A combination of chitooligosaccharide and lipochitooligosaccharide recognition promotes arbuscular mycorrhizal associations in Medicago truncatula. Nat. Commun. 2019, 10, 5047. [Google Scholar] [CrossRef]
- Kelly, S.; Radutoiu, S.; Stougaard, J. Legume LysM receptors mediate symbiotic and pathogenic signalling. Curr. Opin. Plant Biol. 2017, 39, 152–158. [Google Scholar] [CrossRef]
- Tsitsigiannis, D.I.; Keller, N.P. Oxylipins as developmental and host-fungal communication signals. Trends Microbiol. 2007, 15, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.R.; Peng, Y.L.; Dickman, M.B.; Sharon, A. The dawn of fungal pathogen genomics. Annu. Rev. Phytopathol. 2006, 44, 337–366. [Google Scholar] [CrossRef] [PubMed]
- Niu, M.; Steffan, B.N.; Fischer, G.J.; Venkatesh, N.; Raffa, N.L.; Wettstein, M.A.; Bok, J.W.; Greco, C.; Zhao, C.; Berthier, E.; et al. Fungal oxylipins direct programmed developmental switches in filamentous fungi. Nat. Commun. 2020, 11, 5158. [Google Scholar] [CrossRef] [PubMed]
- Lopez, S.; Bermudez, B.; Montserrat-de la Paz, S.; Jaramillo, S.; Varela, L.M.; Ortega-Gomez, A.; Abia, R.; Muriana, F.J. Membrane composition and dynamics: A target of bioactive virgin olive oil constituents. Biochim. Biophys. Acta 2014, 1838, 1638–1656. [Google Scholar] [CrossRef] [PubMed]
- Du, A.; Wang, Z.; Huang, T.; Xue, S.; Jiang, C.; Qiu, G.; Yuan, K. Fatty acids in cancer: Metabolic functions and potential treatment. MedComm-Oncology 2023, 2, e25. [Google Scholar] [CrossRef]
- Eccles, J.A.; Baldwin, W.S. Detoxification Cytochrome P450s (CYPs) in families 1-3 produce functional oxylipins from polyunsaturated fatty acids. Cells 2022, 12, 82. [Google Scholar] [CrossRef] [PubMed]
- Rangel, D.E.; Braga, G.U.; Fernandes, É.K.K.; Keyser, C.A.; Hallsworth, J.E.; Roberts, D.W. Stress tolerance and virulence of insect-pathogenic fungi are determined by environmental conditions during conidial formation. Curr. Genet. 2015, 61, 383–404. [Google Scholar] [CrossRef]
- Barbieri, A.; Rico, I.B.; Silveira, C.; Feltrin, C.; Dall Agnol, B.; Schrank, A.; Lozina, L.; Klafke, G.M.; Reck, J. Field efficacy of Metarhizium anisopliae oil formulations against Rhipicephalus microplus ticks using a cattle spray race. Ticks Tick Borne Dis. 2023, 14, 102147. [Google Scholar] [CrossRef]
- Brito, E.S.; de Paula, A.R.; Vieira, L.P.; Dolinski, C.; Samuels, R.I. Combining vegetable oil and sub-lethal concentrations of Imidacloprid with Beauveria bassiana and Metarhizium anisopliae against adult guava weevil Conotrachelus psidii (Coleoptera: Curculionidae). Biocontrol Sci. Technol. 2008, 18, 665–673. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, G.; Zhang, X.; Du, G.; Wang, W.; Yao, Y.; Jin, S.; Cai, H.; Peng, Y.; Chen, B. Oleic Acid and Linoleic Acid Enhances the Biocontrol Potential of Metarhizium rileyi. J. Fungi 2024, 10, 521. https://doi.org/10.3390/jof10080521
Wang G, Zhang X, Du G, Wang W, Yao Y, Jin S, Cai H, Peng Y, Chen B. Oleic Acid and Linoleic Acid Enhances the Biocontrol Potential of Metarhizium rileyi. Journal of Fungi. 2024; 10(8):521. https://doi.org/10.3390/jof10080521
Chicago/Turabian StyleWang, Guang, Xu Zhang, Guangzu Du, Wenqian Wang, Yunhao Yao, Sitong Jin, Haosheng Cai, Yuejin Peng, and Bin Chen. 2024. "Oleic Acid and Linoleic Acid Enhances the Biocontrol Potential of Metarhizium rileyi" Journal of Fungi 10, no. 8: 521. https://doi.org/10.3390/jof10080521
APA StyleWang, G., Zhang, X., Du, G., Wang, W., Yao, Y., Jin, S., Cai, H., Peng, Y., & Chen, B. (2024). Oleic Acid and Linoleic Acid Enhances the Biocontrol Potential of Metarhizium rileyi. Journal of Fungi, 10(8), 521. https://doi.org/10.3390/jof10080521






