Multi-Functional Alginate Lyase AlgVR7 from Vibrio rumoiensis: Structural Insights and Catalytic Mechanisms
Abstract
:1. Introduction
2. Results
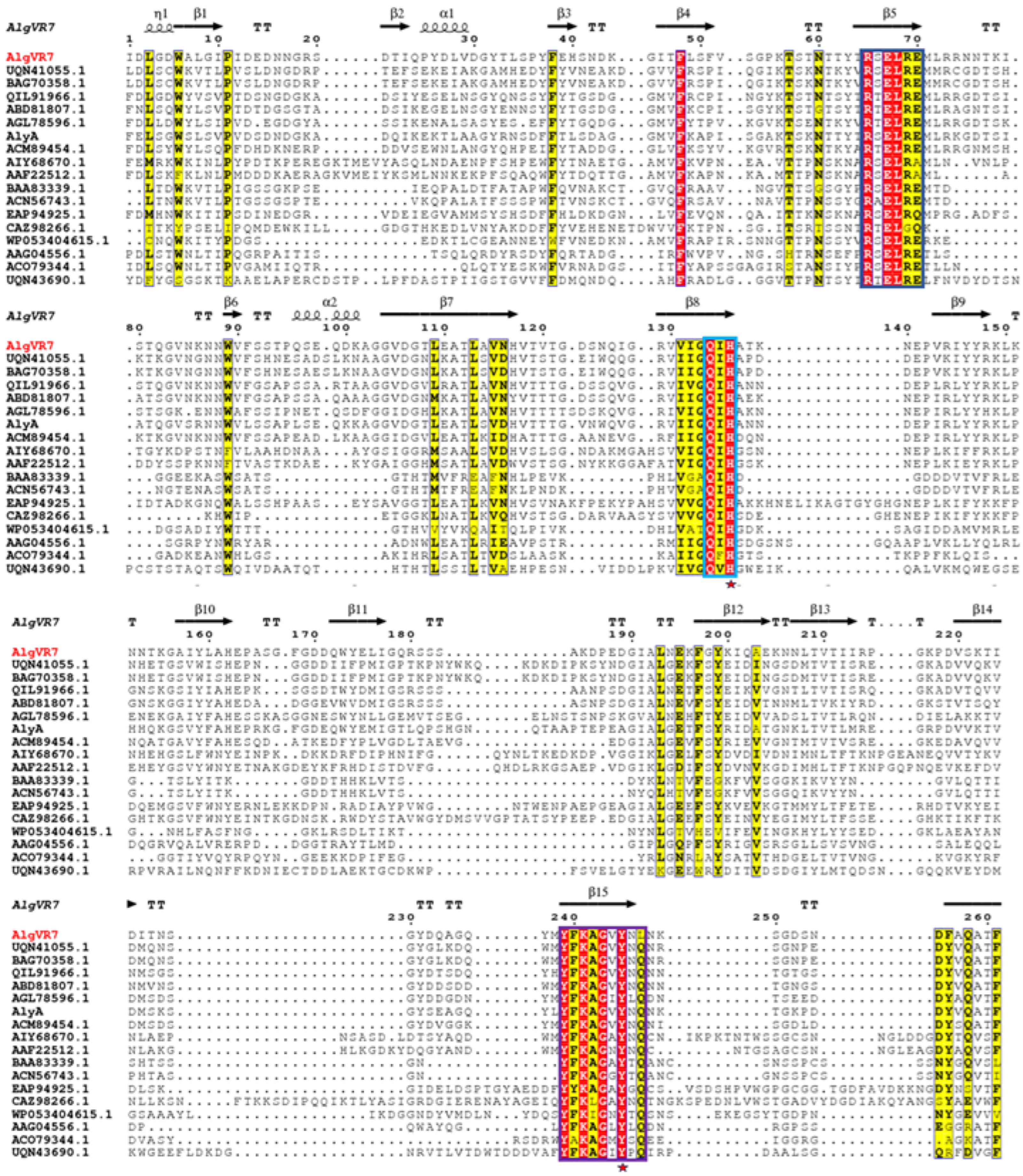
2.1. Phylogenetic Analysis and Sequence Comparison of AlgVR7
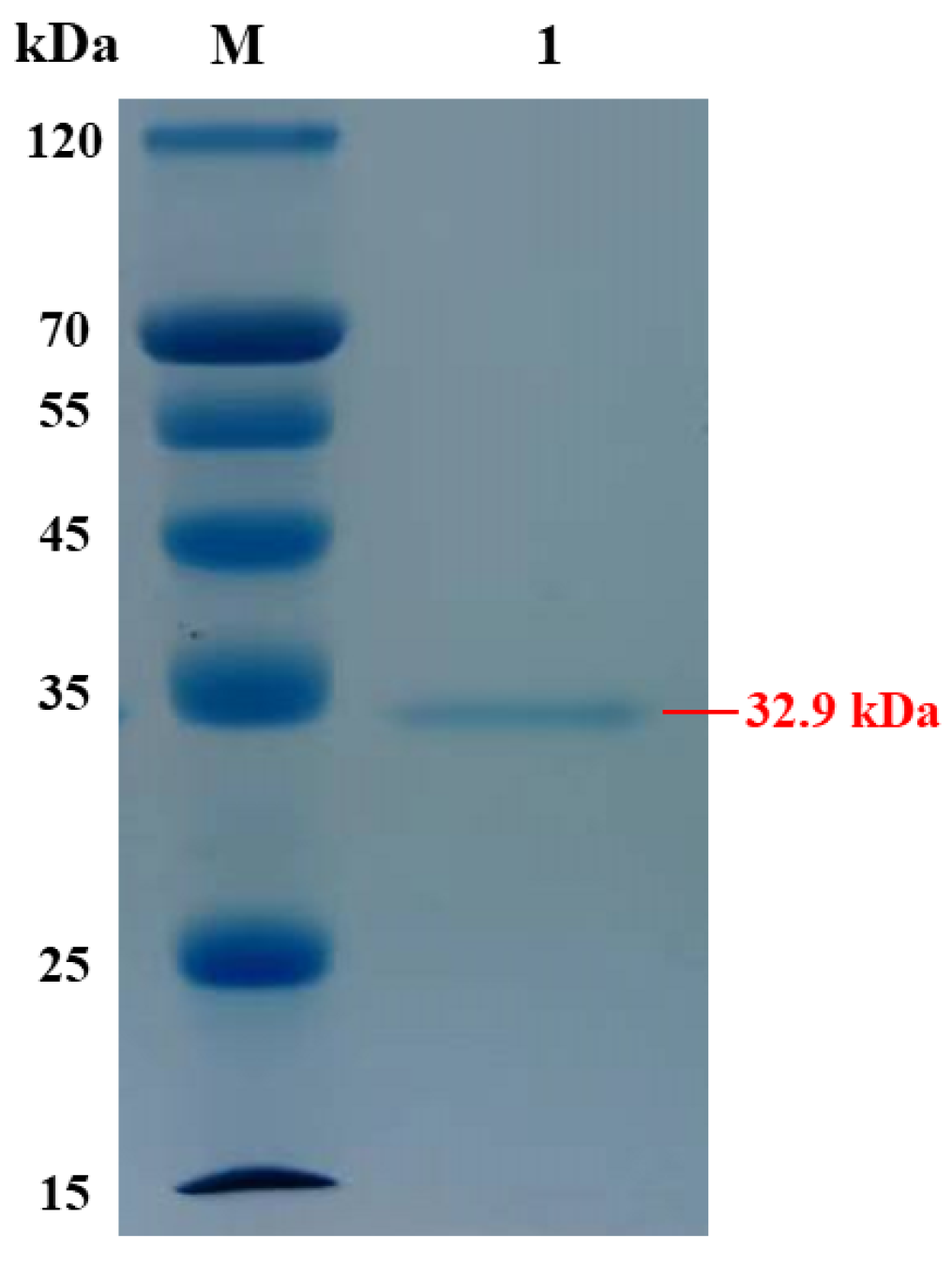
2.2. Expression and Purification of AlgVR7
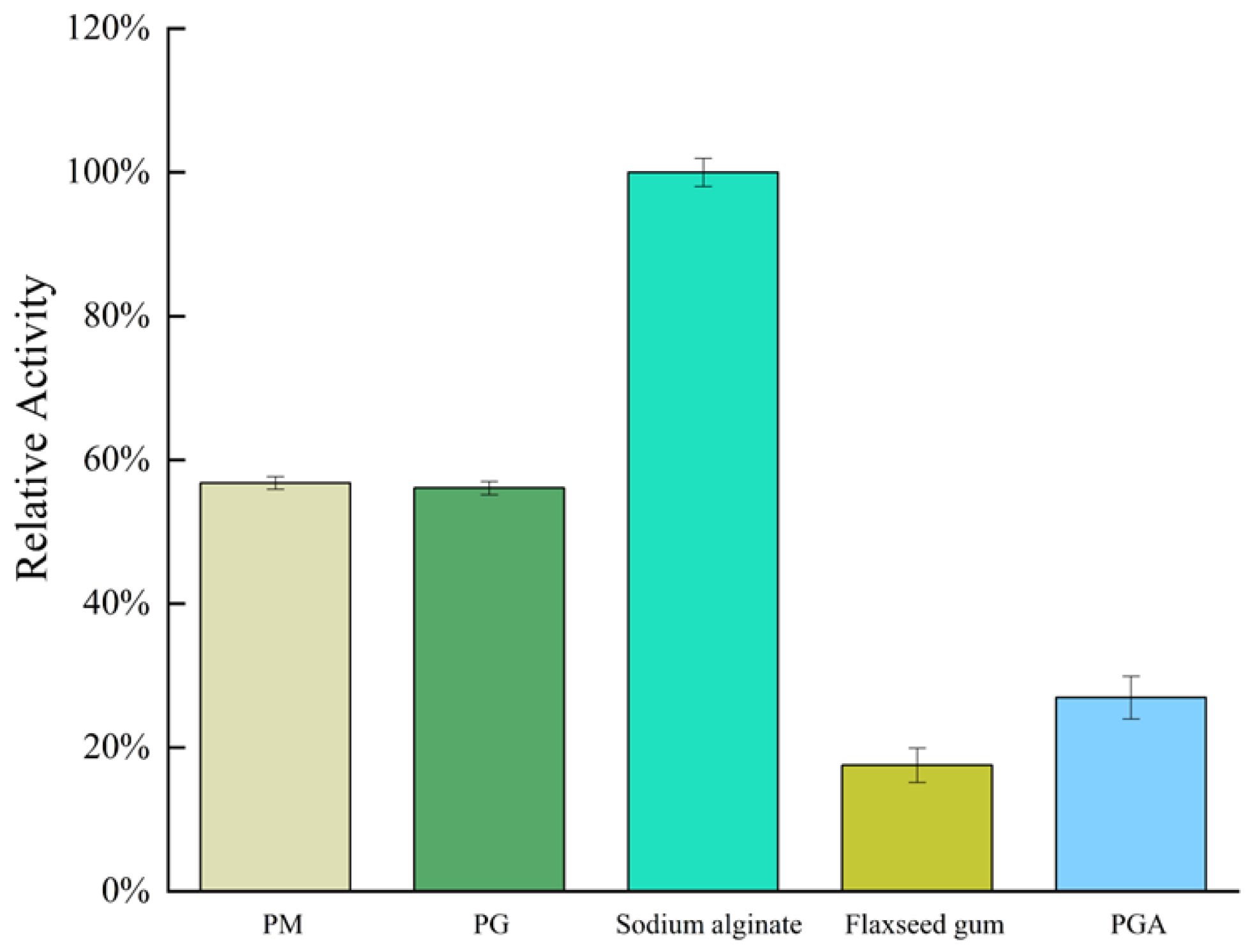
2.3. Substrate Specificity and Kinetic Parameters of AlgVR7
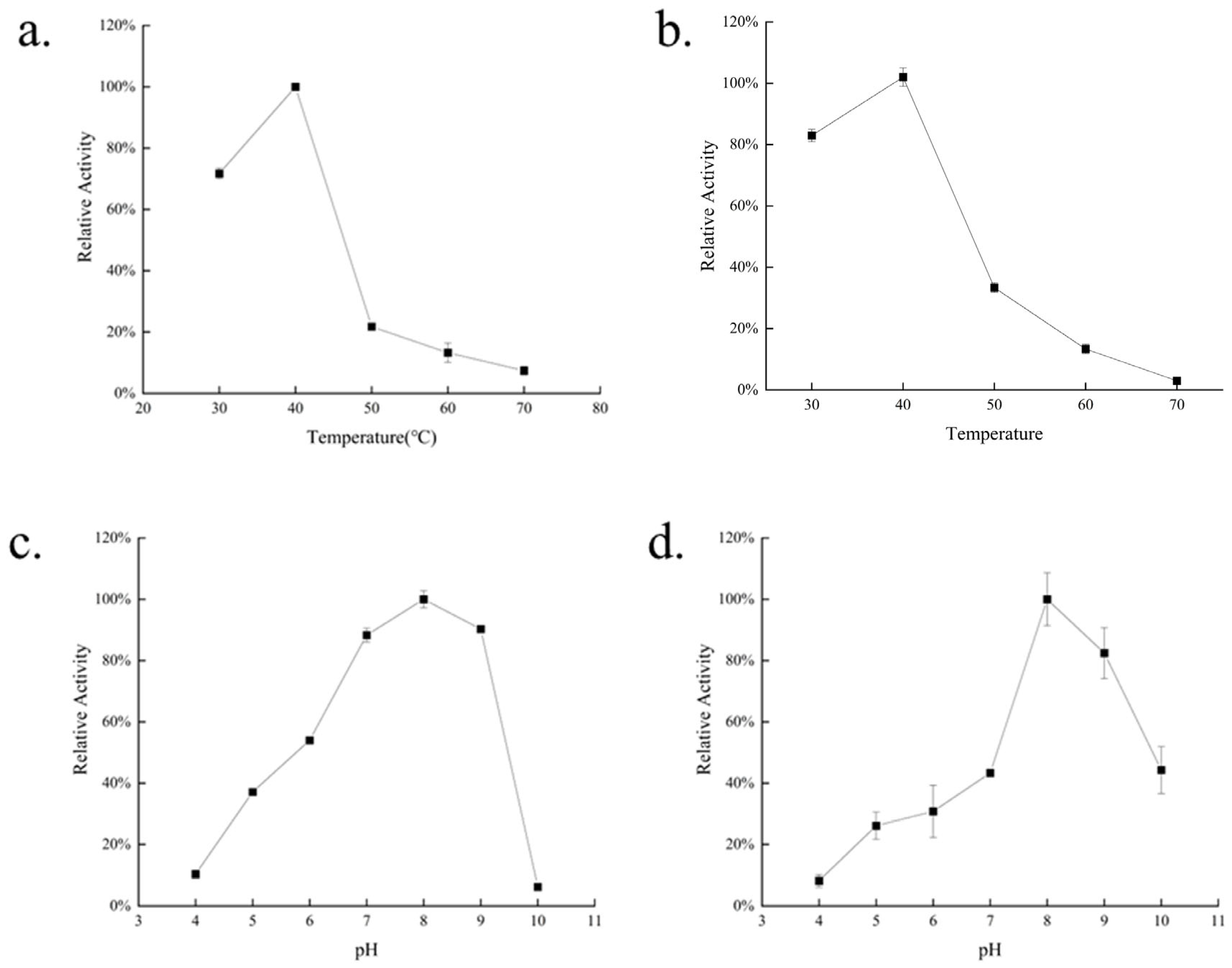
2.4. Biochemical Characterization of AlgVR7
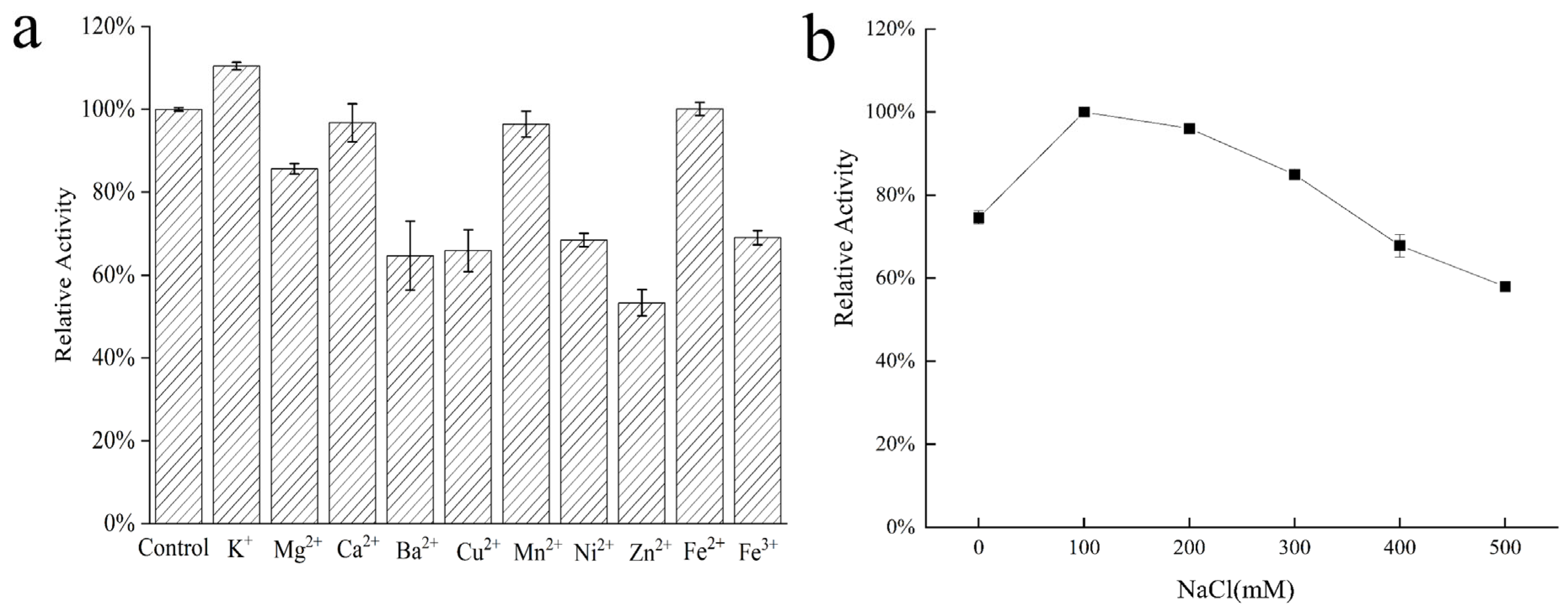
2.5. Effects of Metal Ions on AlgVR7 Stability
2.6. ESI-MS Analysis of the Degradation Products
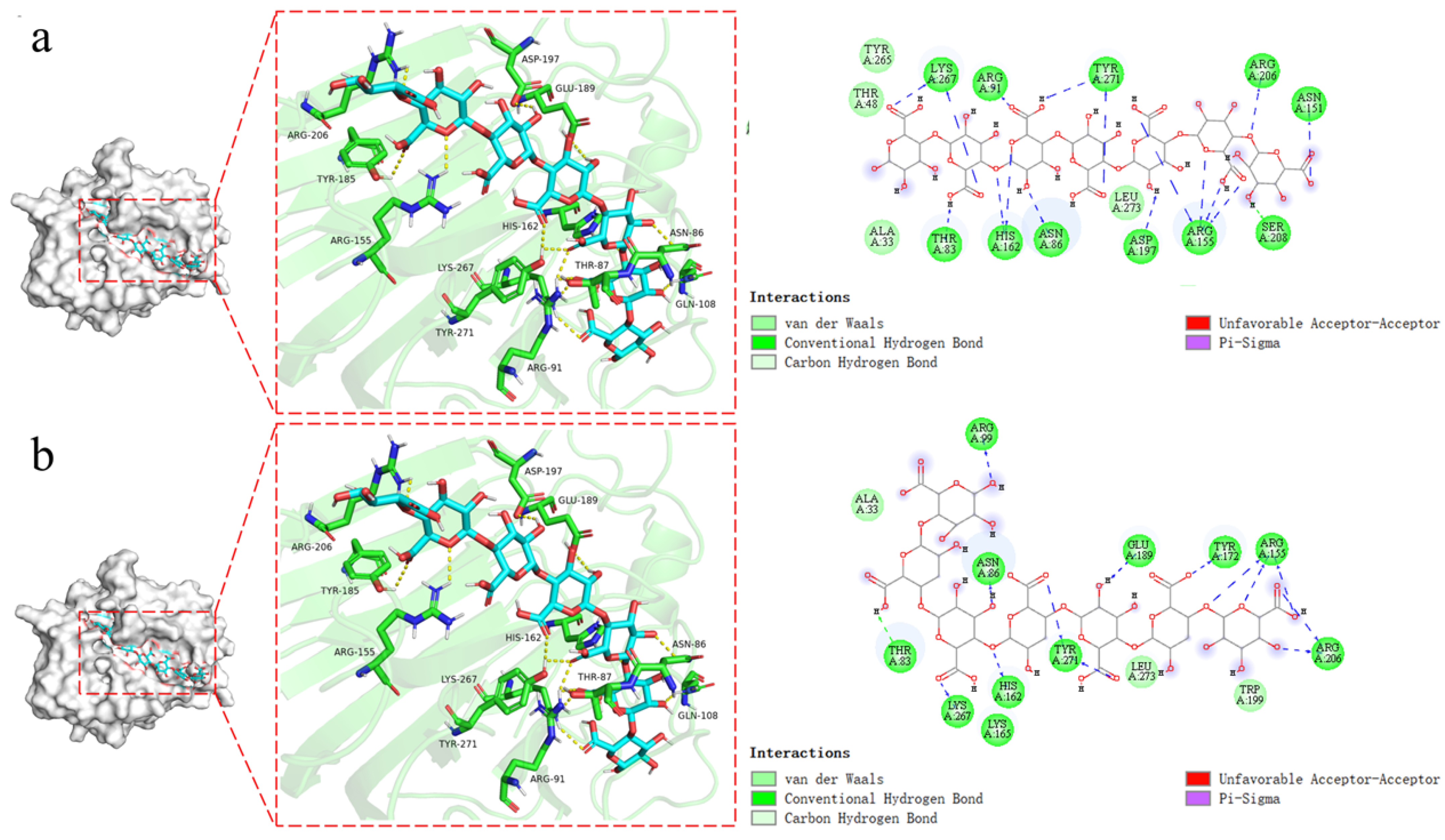
2.7. Substrate Recognizing and Molecular Docking
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Sequence Analysis of AlgVR7
4.3. Cloning of Alginate Lyase Gene AlgVR7
4.4. Enzymatic Activity Assay
4.5. Effects of Additives on Enzyme Stability
4.6. Substrate Specificity
4.7. ESI-MS Analysis of AlgVR7 Degradation Products
4.8. Molecular Docking
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Xiao, Z.; Li, K.; Li, T.; Zhang, F.; Xue, J.; Zhao, M.; Yin, H. Characterization and Mechanism Study of a Novel Pl7 Family Exolytic Alginate Lyase from Marine Bacteria Vibrio Sp. W13. Appl. Biochem. Biotechnol. 2023, 196, 68–84. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Zhang, X.; Li, Y.; Wang, L. Bacterial Alginate Metabolism: An Important Pathway for Bioconversion of Brown Algae. Biotechnol. Biofuels 2021, 14, 158. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ying, X.; Deng, S.; Li, W.; Peng, L.; Ma, L. Trehalose and Alginate Oligosaccharides Enhance the Stability of Myofibrillar Proteins in Shrimp (Litopenaeus vannamei) Muscle During Frozen Storage. J. Food Process. Preserv. 2022, 46, e16469. [Google Scholar] [CrossRef]
- Li, F.; Tang, Y.; Wei, L.; Yang, M.; Lu, Z.; Shi, F.; Zhan, F.; Li, Y.; Liao, W.; Lin, L. Alginate Oligosaccharide Modulates Immune Response, Fat Metabolism, and the Gut Bacterial Community in Grass Carp (Ctenopharyngodon idellus). Fish Shellfish. Immunol. 2022, 130, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Jiang, C.; Xu, J.; Liu, Z.; Mao, X. Characteristics and Applications of Alginate Lyases: A Review. Int. J. Biol. Macromol. 2020, 164, 1304–1320. [Google Scholar] [CrossRef]
- Chen, X.-L.; Dong, S.; Xu, F.; Dong, F.; Li, P.-Y.; Zhang, X.-Y.; Zhou, B.-C.; Zhang, Y.-Z.; Xie, B.-B. Characterization of a New Cold-Adapted and Salt-Activated Polysaccharide Lyase Family 7 Alginate Lyase from Pseudoalteromonas Sp. Sm0524. Front. Microbiol. 2016, 7, 1120. [Google Scholar] [CrossRef] [PubMed]
- Dharani, S.R.; Srinivasan, R.; Sarath, R.; Ramya, M. Recent Progress on Engineering Microbial Alginate Lyases Towards Their Versatile Role in Biotechnological Applications. Folia Microbiol. 2020, 65, 937–954. [Google Scholar] [CrossRef]
- Takagi, T.; Yokoi, T.; Shibata, T.; Morisaka, H.; Kuroda, K.; Ueda, M. Engineered Yeast Whole-Cell Biocatalyst for Direct Degradation of Alginate from Macroalgae and Production of Non-Commercialized Useful Monosaccharide from Alginate. Appl. Microbiol. Biotechnol. 2016, 100, 1723–1732. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Fu, L.; Wang, Z.; Cao, Z.; Zhao, L.; Seswita-Zilda, D.; Zhang, A.; Zhang, Q.; Li, J. A Novel Bifunctional Alginate Lyase and Antioxidant Activity of the Enzymatic Hydrolysates. J. Agric. Food Chem. 2024, 72, 4116–4126. [Google Scholar] [CrossRef]
- Zhu, B.; Sun, Y.; Ni, F.; Ning, L.; Yao, Z. Characterization of a New Endo-Type Alginate Lyase from Vibrio Sp. Nju-03. Int. J. Biol. Macromol. 2018, 108, 1140–1147. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Chen, P.; Zeng, Y.; Men, Y.; Mu, S.; Zhu, Y.; Chen, Y.; Sun, Y. The Characterization and Modification of a Novel Bifunctional and Robust Alginate Lyase Derived from Marinimicrobium Sp. H1. Mar. Drugs 2019, 17, 545. [Google Scholar] [CrossRef]
- Zhang, F.; Fu, Z.; Tang, L.; Zhang, Z.; Han, F.; Yu, W. Biochemical Characterization of a Novel Exo-Type Pl7 Alginate Lyase Vsaly7d from Marine Vibrio Sp. Qy108. Int. J. Mol. Sci. 2021, 22, 8402. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ji, D. Alginate Oligosaccharide Dp5 Exhibits Antitumor Effects in Osteosarcoma Patients Following Surgery. Front. Pharmacol. 2017, 8, 273086. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Kurachi, M.; Yamaguchi, K.; Oda, T. Induction of Multiple Cytokine Secretion from Raw264. 7 Cells by Alginate Oligosaccharides. Biosci. Biotechnol. Biochem. 2007, 71, 238–241. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, Y.; Dan, M.; Li, Y.; Zhao, G.; Wang, D. Characterization of Degradation Patterns and Enzymatic Properties of a Novel Alkali-Resistant Alginate Lyase Alyrm1 from Rubrivirga marina. Curr. Res. Food Sci. 2023, 6, 100414. [Google Scholar] [CrossRef]
- He, X.; Zhang, Y.; Wang, X.; Zhu, X.; Chen, L.; Liu, W.; Lyu, Q.; Ran, L.; Cheng, H.; Zhang, X. Characterization of Multiple Alginate Lyases in a Highly Efficient Alginate-Degrading Vibrio Strain and Its Degradation Strategy. Appl. Environ. Microbiol. 2022, 88, e01389-22. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Li, X.; Shi, H.; Zhou, J.; Tan, Z.; Yuan, M.; Yao, P.; Liu, X. Characterization of a Novel Alginate Lyase from Marine Bacterium Vibrio furnissii H1. Mar. Drugs 2018, 16, 30. [Google Scholar] [CrossRef]
- Zhu, B.; Ni, F.; Ning, L.; Sun, Y.; Yao, Z. Cloning and Characterization of a New Ph-Stable Alginate Lyase with High Salt Tolerance from Marine Vibrio. Sp. Nj-04. Int. J. Biol. Macromol. 2018, 115, 1063–1070. [Google Scholar] [CrossRef]
- Chao, Y.; Wang, S.; Wu, S.; Wei, J.; Chen, H. Cloning and Characterization of an Alginate Lyase from Marine Vibrio. Sp. Qd-5. Biochem. Mol. Biol. 2017. [Google Scholar] [CrossRef]
- Han, F.; Gong, Q.-H.; Song, K.; Li, J.-B.; Yu, W.-G. Cloning, Sequence Analysis and Expression of Gene Alyvi Encoding Alginate Lyase from Marine Bacterium Vibrio Sp. Qy101. DNA Seq. J. DNA Seq. Mapp. 2004, 15, 344–350. [Google Scholar] [CrossRef]
- Long, L.; Hu, Q.; Wang, X.; Li, H.; Li, Z.; Jiang, Z.; Ni, H.; Li, Q.; Zhu, Y. A Bifunctional Exolytic Alginate Lyase from Microbulbifer Sp. Alw1 with Salt Activation and Calcium-Dependent Catalysis. Enzym. Microb. Technol. 2022, 161, 110109. [Google Scholar] [CrossRef]
- Meng, Q.; Zhou, L.; Hassanin, H.A.M.; Jiang, B.; Liu, Y.; Chen, J.; Zhang, T. A New Role of Family 32 Carbohydrate Binding Module in Alginate Lyase from Vibrio natriegens Sk42. 001 in Altering Its Catalytic Activity, Thermostability and Product Distribution. Food Biosci. 2021, 42, 101112. [Google Scholar] [CrossRef]
- Yang, J.; Cui, D.; Chen, D.; Chen, W.; Ma, S.; Shen, H. Purification and Characterization of a Novel Endolytic Alginate Lyase from Microbulbifer Sp. Sh-1 and Its Agricultural Application. Mar. Drugs 2020, 18, 184. [Google Scholar] [CrossRef]
- Wang, X.-H.; Sun, X.-H.; Chen, X.-L.; Li, P.-Y.; Qin, Q.-L.; Zhang, Y.-Q.; Xu, F. Synergy of the Two Alginate Lyase Domains of a Novel Alginate Lyase from Vibrio Sp. Nc2 in Alginate Degradation. Appl. Environ. Microbiol. 2022, 88, e01559-22. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Nibu, Y.; Murata, K.; Yoshida, S.; Kusakabe, I. Characterization of a Novel Alginate Lyase from Flavobacterium multivolum K-11. Food Sci. Technol. Int. 1997, 4, 388–392. [Google Scholar] [CrossRef]
- Tang, L.; Guo, E.; Zhang, L.; Wang, Y.; Gao, S.; Bao, M.; Han, F.; Yu, W. The Function of Cbm32 in Alginate Lyase Vxaly7b on the Activity on Both Soluble Sodium Alginate and Alginate Gel. Front. Microbiol. 2022, 12, 798819. [Google Scholar] [CrossRef]
- Xu, F.; Chen, X.-L.; Sun, X.-H.; Dong, F.; Li, C.-Y.; Li, P.-Y.; Ding, H.; Chen, Y.; Zhang, Y.-Z.; Wang, P. Structural and Molecular Basis for the Substrate Positioning Mechanism of a New Pl7 Subfamily Alginate Lyase from the Arctic. J. Biol. Chem. 2020, 295, 16380–16392. [Google Scholar] [CrossRef]
- Tang, L.; Bao, M.; Wang, Y.; Fu, Z.; Han, F.; Yu, W. Effects of Module Truncation of a New Alginate Lyase Vxaly7c from Marine Vibrio Xiamenensis Qy104 on Biochemical Characteristics and Product Distribution. Int. J. Mol. Sci. 2022, 23, 4795. [Google Scholar] [CrossRef]
- Das, R.; Kayastha, A.M. β-Amylase: General Properties, Mechanism and Panorama of Applications by Immobilization on Nano-Structures. Biocatal. Enzym. Basics Appl. 2019, 17–38. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, L.; Chen, Y.; Ni, H.; Xiao, A.; Cai, H. Characterization of an Extracellular Biofunctional Alginate Lyase from Marine Microbulbifer Sp. Alw1 and Antioxidant Activity of Enzymatic Hydrolysates. Microbiol. Res. 2016, 182, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Wang, P.; Zhang, Y.-Z.; Chen, X.-L. Diversity of Three-Dimensional Structures and Catalytic Mechanisms of Alginate Lyases. Appl. Environ. Microbiol. 2018, 84, e02040-17. [Google Scholar] [CrossRef]
- Janeček, Š.; Mareček, F.; MacGregor, E.A.; Svensson, B. Starch-Binding Domains as CBM Families-History, Occurrence, Structure, Function and Evolution. Biotechnol. Adv. 2019, 37, 107451. [Google Scholar] [CrossRef]
- Tanaka, M.; Kumakura, D.; Mino, S.; Doi, H.; Ogura, Y.; Hayashi, T.; Yumoto, I.; Cai, M.; Zhou, Y.G.; Gomez-Gil, B.; et al. Genomic Characterization of Closely Related Species in the Rumoiensis Clade Infers Ecogenomic Signatures to Non-Marine Environments. Environ. Microbiol. 2020, 22, 3205–3217. [Google Scholar] [CrossRef] [PubMed]
- Badur, A.H.; Jagtap, S.S.; Yalamanchili, G.; Lee, J.-K.; Zhao, H.; Rao, C.V. Alginate Lyases from Alginate-Degrading Vibrio Splendidus 12b01 Are Endolytic. Appl. Environ. Microbiol. 2015, 81, 1865–1873. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.-W.; Huang, L.-S.-X.; Tan, H.-D.; Qin, Y.-Q.; Du, Y.-G.; Yin, H. Characterization of a New Endo-Type Polym-Specific Alginate Lyase from Pseudomonas Sp. Biotechnol. Lett. 2015, 37, 409–415. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Shao, Y.; Jiao, C.; Yang, Q.-M.; Weng, H.-F.; Xiao, A.-F. Characterization and Application of an Alginate Lyase, Aly1281 from Marine Bacterium Pseudoalteromonas carrageenovora Asy5. Mar. Drugs 2020, 18, 95. [Google Scholar] [CrossRef]
- Kawada, A.; Hiura, N.; Shiraiwa, M.; Tajima, S.; Hiruma, M.; Hara, K.; Ishibashi, A.; Takahara, H. Stimulation of Human Keratinocyte Growth by Alginate Oligosaccharides, a Possible Co-Factor for Epidermal Growth Factor in Cell Culture. Febs. Lett. 1997, 408, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, L.; Che, J.; Li, X.; Li, J.; Wang, J.; Xu, Y. In Vitro Non-Specific Immunostimulatory Effect of Alginate Oligosaccharides with Different Molecular Weights and Compositions on Sea Cucumber (Apostichopus japonicus) Coelomocytes. Aquaculture 2014, 434, 434–441. [Google Scholar] [CrossRef]
- Vuoristo, K.S.; Fredriksen, L.; Oftebro, M.; Arntzen, M.Ø.; Aarstad, O.A.; Stokke, R.; Steen, I.H.; Hansen, L.D.; Schüller, R.B.; Aachmann, F.L.; et al. Production, Characterization and Application of an Alginate Lyase, Amorpl7a, from Hot Vents in the Arctic Mid-Ocean Ridge. J. Agric. Food Chem. 2019, 67, 2936–2945. [Google Scholar] [CrossRef]
- Du, M.; Li, X.; Qi, W.; Li, Y.; Wang, L. Identification and Characterization of a Critical Loop for the High Activity of Alginate Lyase Vaaly2 from the Pl7_5 Subfamily. Front. Microbiol. 2024, 14, 1333597. [Google Scholar] [CrossRef] [PubMed]


| Substrate | Sodium Alginate | polyM | polyG |
|---|---|---|---|
| Specific activity (U/mg) | 6854.6 | 3893.6 | 3845.0 |
| Km (μmol) | 16.6 | 13.1 | 22.8 |
| Vmax (μmol/s) | 186.8 | 150.9 | 176.8 |
| Kcat (s−1) | 16.9 | 13.7 | 11.4 |
| Kcat/Km (s−1/μmol) | 1.0 | 1.1 | 0.5 |
| Organism | Enzyme | Optimal pH/Temperature (°C) | Substrate Specificity (U/mg) | Km/Vmax/Kcat (Alginate, polyM, and polyG) | Reference |
|---|---|---|---|---|---|
| Vibrio rumoiensis 402 | AlgVR7 | 8.0/40 | 6854.6 | Km = 16.6, 13.1, 22.8 μmol Vmax = 186.8, 150.9, 176.8 μmol/s Kcat = 16.9, 13.7, 11.4 s−1 | This study |
| Vibrio pelagius WXL662 | VpAly-I | 6.0/40 | 194.0 | N.D | [16] |
| V. furnissii H1 | AlyH1 | 7.5/40 | 2.40 * | Km = 2.28 mg/mL Vmax = 2.81 U/mg toward alginate | [17] |
| Vibrio sp. QY101 | AlyVI | 7.5/40 | N.D | Km = 0.2223, N.D, 0.3274 mg/mL Vmax = 3.6, N.D, 2.8321 U/mg | [18] |
| Vibrio sp. NJ-04 | AlgNJ-04 | 7.0/30 | 2416 | Km = 0.49, 0.86, 0.24 mM Vmax = 72, 95, 35 pmol/s; Kcat = 59, 77, 29 s−1 | [10] |
| Vibrio sp. NJU-03 | AlgNJU-03 | 7.0/30 | 6468.9 | Km = 8.50, 10.94, 4.00 mM Vmax = 1.67, 0.30, 2.50 nmol/s | [19] |
| Vibrio sp. SY08 | AlySY08 | 7.5/40 | 1070 | N.D | [20] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Z.; Liang, S.; Jiang, W.; Wang, L.; Wang, Y.; Wang, H.; Wang, L.; Cong, Y.; Lu, Y.; Yang, G. Multi-Functional Alginate Lyase AlgVR7 from Vibrio rumoiensis: Structural Insights and Catalytic Mechanisms. Mar. Drugs 2025, 23, 124. https://doi.org/10.3390/md23030124
Huang Z, Liang S, Jiang W, Wang L, Wang Y, Wang H, Wang L, Cong Y, Lu Y, Yang G. Multi-Functional Alginate Lyase AlgVR7 from Vibrio rumoiensis: Structural Insights and Catalytic Mechanisms. Marine Drugs. 2025; 23(3):124. https://doi.org/10.3390/md23030124
Chicago/Turabian StyleHuang, Zhe, Shuai Liang, Wulong Jiang, Li Wang, Yuan Wang, Hua Wang, Lianshun Wang, Yuting Cong, Yanan Lu, and Guojun Yang. 2025. "Multi-Functional Alginate Lyase AlgVR7 from Vibrio rumoiensis: Structural Insights and Catalytic Mechanisms" Marine Drugs 23, no. 3: 124. https://doi.org/10.3390/md23030124
APA StyleHuang, Z., Liang, S., Jiang, W., Wang, L., Wang, Y., Wang, H., Wang, L., Cong, Y., Lu, Y., & Yang, G. (2025). Multi-Functional Alginate Lyase AlgVR7 from Vibrio rumoiensis: Structural Insights and Catalytic Mechanisms. Marine Drugs, 23(3), 124. https://doi.org/10.3390/md23030124





