Acute Heart Failure in a Patient with Occult Barlow’s Disease Receiving Bevacizumab
Abstract
:1. Introduction
2. Case Presentation
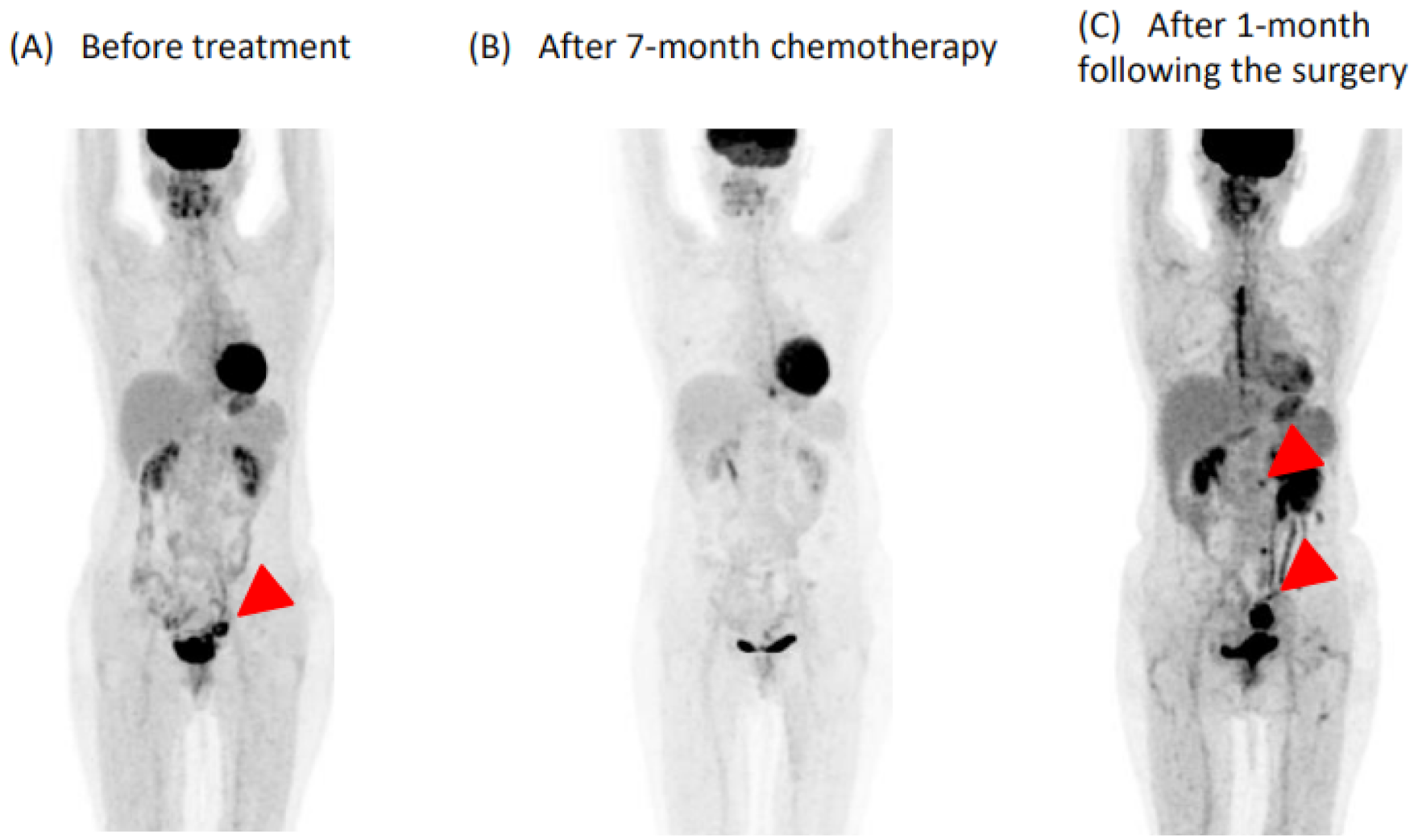
2.1. Before Admission
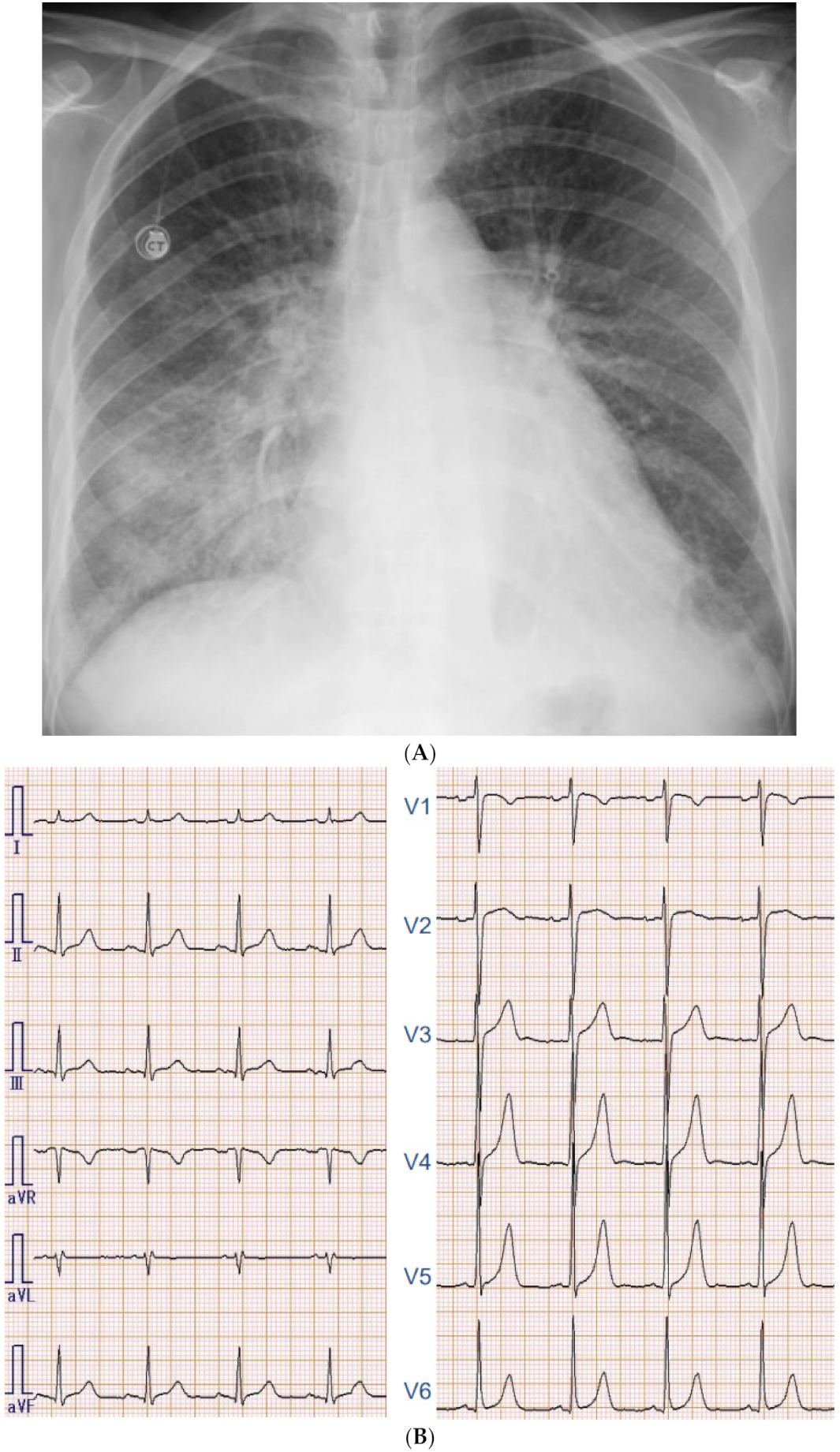
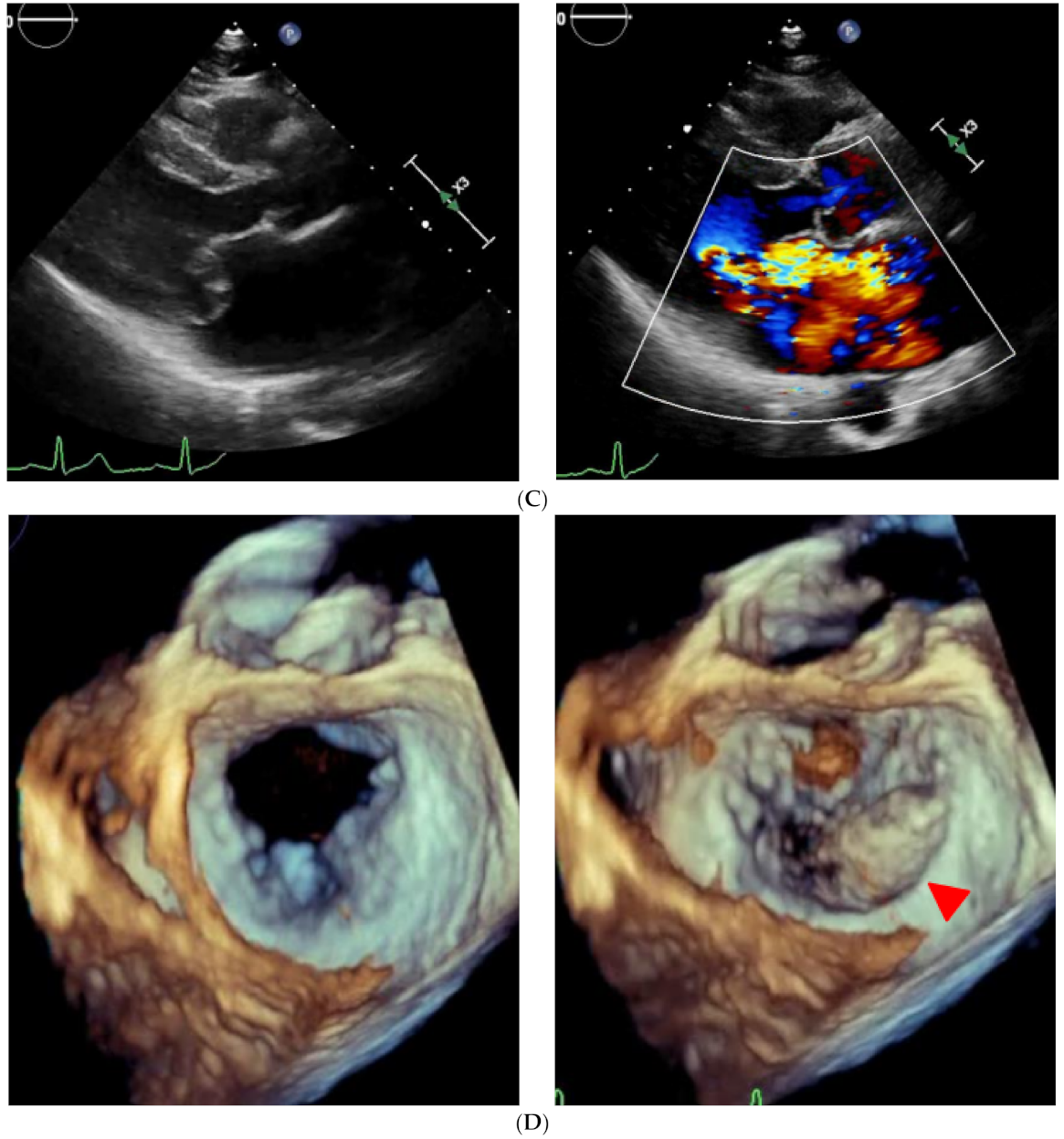
2.2. On Admission
2.3. In-Hospital Course
2.4. Post-Operative Course
3. Discussion
3.1. The Association between Bevacizumab and Heart Failure
3.2. Practical Implications of Cardio-Oncology Collaboration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ameri, P.; Canepa, M.; Anker, M.S.; Belenkov, Y.; Bergler-Klein, J.; Cohen-Solal, A.; Farmakis, D.; López-Fernández, T.; Lainscak, M.; Pudil, R.; et al. Cancer diagnosis in patients with heart failure: Epidemiology, clinical implications and gaps in knowledge. Eur. J. Heart Fail. 2018, 20, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Zamorano, J.L.; Lancellotti, P.; Rodriguez Muñoz, D.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.H.; Lyon, A.R.; et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 2768–2801. [Google Scholar] [CrossRef] [PubMed]
- Jarboe, J.; Gupta, A.; Saif, M.W. Therapeutic human monoclonal antibodies against cancer. Methods Mol. Biol. 2014, 1060, 61–77. [Google Scholar] [PubMed]
- Li, M.; Kroetz, D.L. Bevacizumab-induced hypertension: Clinical presentation and molecular understanding. Pharmacol Ther. 2018, 182, 152–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Wijngaarden, A.L.; Kruithof, B.P.T.; Vinella, T.; Barge-Schaapveld, D.Q.C.M.; Ajmone Marsan, N. Characterization of Degenerative Mitral Valve Disease: Differences between Fibroelastic Deficiency and Barlow’s Disease. J. Cardiovasc. Dev. Dis. 2021, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Totzeck, M.; Mincu, R.I.; Rassaf, T. Cardiovascular Adverse Events in Patients with Cancer Treated with Bevacizumab: A Meta-Analysis of More Than 20,000 Patients. J. Am. Heart Assoc. 2017, 6, e006278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel-Qadir, H.; Ethier, J.L.; Lee, D.S.; Thavendiranathan, P.; Amir, E. Cardiovascular toxicity of angiogenesis inhibitors in treatment of malignancy: A systematic review and meta-analysis. Cancer Treat Rev. 2017, 53, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Mayer, E.L.; Je, Y.; Rosenberg, J.E.; Nguyen, P.L.; Azzi, G.R.; Bellmunt, J.; Burstein, H.J.; Schutz, F.A. Congestive heart failure risk in patients with breast cancer treated with bevacizumab. J. Clin. Oncol. 2011, 29, 632–638. [Google Scholar] [CrossRef] [Green Version]
- Tsai, H.T.; Marshall, J.L.; Weiss, S.R.; Huang, C.Y.; Warren, J.L.; Freedman, A.N.; Fu, A.Z.; Sansbury, L.B.; Potosky, A.L. Bevacizumab use and risk of cardiovascular adverse events among elderly patients with colorectal cancer receiving chemotherapy: A population-based study. Ann. Oncol. 2013, 24, 1574–1579. [Google Scholar] [CrossRef]
- Stewart, M.H.; Jahangir, E.; Polin, N.M. Valvular Heart Disease in Cancer Patients: Etiology, Diagnosis, and Management. Curr. Treat. Options Cardiovasc. Med. 2017, 19, 53. [Google Scholar] [CrossRef] [PubMed]
- Imbalzano, E.; Vatrano, M.; Ghiadoni, L.; Mandraffino, G.; Dalbeni, A.; Khandheria, B.K.; Costantino, R.; Trapani, G.; Manganaro, R.; Cusmà Piccione, M.; et al. Arterial stiffness and mitral regurgitation in arterial hypertension: An intriguing pathophysiological link. Vascul. Pharmacol. 2018, 111, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Sonaglioni, A.; Albini, A.; Fossile, E.; Pessi, M.A.; Nicolosi, G.L.; Lombardo, M.; Anzà, C.; Ambrosio, G. Speckle-Tracking Echocardiography for Cardioncological Evaluation in Bevacizumab-Treated Colorectal Cancer Patients. Cardiovasc. Toxicol. 2020, 20, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Nhola, L.F.; Abdelmoneim, S.S.; Villarraga, H.R.; Kohli, M.; Grothey, A.; Bordun, K.A.; Cheung, M.; Best, R.; Cheung, D.; Huang, R.; et al. Echocardiographic Assessment for the Detection of Cardiotoxicity Due to Vascular Endothelial Growth Factor Inhibitor Therapy in Metastatic Renal Cell and Colorectal Cancers. J. Am. Soc. Echocardiogr. 2019, 32, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Karlstaedt, A.; Barrett, M.; Hu, R.; Gammons, S.T.; Ky, B. Cardio-Oncology: Understanding the Intersections Between Cardiac Metabolism and Cancer Biology. JACC Basic Transl. Sci. 2021, 6, 705–718. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izumida, T.; Imamura, T.; Ueno, Y.; Fukahara, K.; Kinugawa, K. Acute Heart Failure in a Patient with Occult Barlow’s Disease Receiving Bevacizumab. Medicina 2021, 57, 998. https://doi.org/10.3390/medicina57100998
Izumida T, Imamura T, Ueno Y, Fukahara K, Kinugawa K. Acute Heart Failure in a Patient with Occult Barlow’s Disease Receiving Bevacizumab. Medicina. 2021; 57(10):998. https://doi.org/10.3390/medicina57100998
Chicago/Turabian StyleIzumida, Toshihide, Teruhiko Imamura, Yohei Ueno, Kazuaki Fukahara, and Koichiro Kinugawa. 2021. "Acute Heart Failure in a Patient with Occult Barlow’s Disease Receiving Bevacizumab" Medicina 57, no. 10: 998. https://doi.org/10.3390/medicina57100998
APA StyleIzumida, T., Imamura, T., Ueno, Y., Fukahara, K., & Kinugawa, K. (2021). Acute Heart Failure in a Patient with Occult Barlow’s Disease Receiving Bevacizumab. Medicina, 57(10), 998. https://doi.org/10.3390/medicina57100998






