Immunohistochemistry Helps to Distinguish Noninvasive Follicular Thyroid Neoplasm with Papillary-like Nuclear Features/Noninvasive Encapsulated Follicular Variant of Papillary Thyroid Carcinoma with Other Follicular Thyroid Lesions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Case Selection
2.2. Immunohistochemical Staining
2.3. Immunohistochemical Evaluation
2.4. Statistical Analysis
3. Results
3.1. Clinicopathological Features
3.2. Immunohistochemical Features
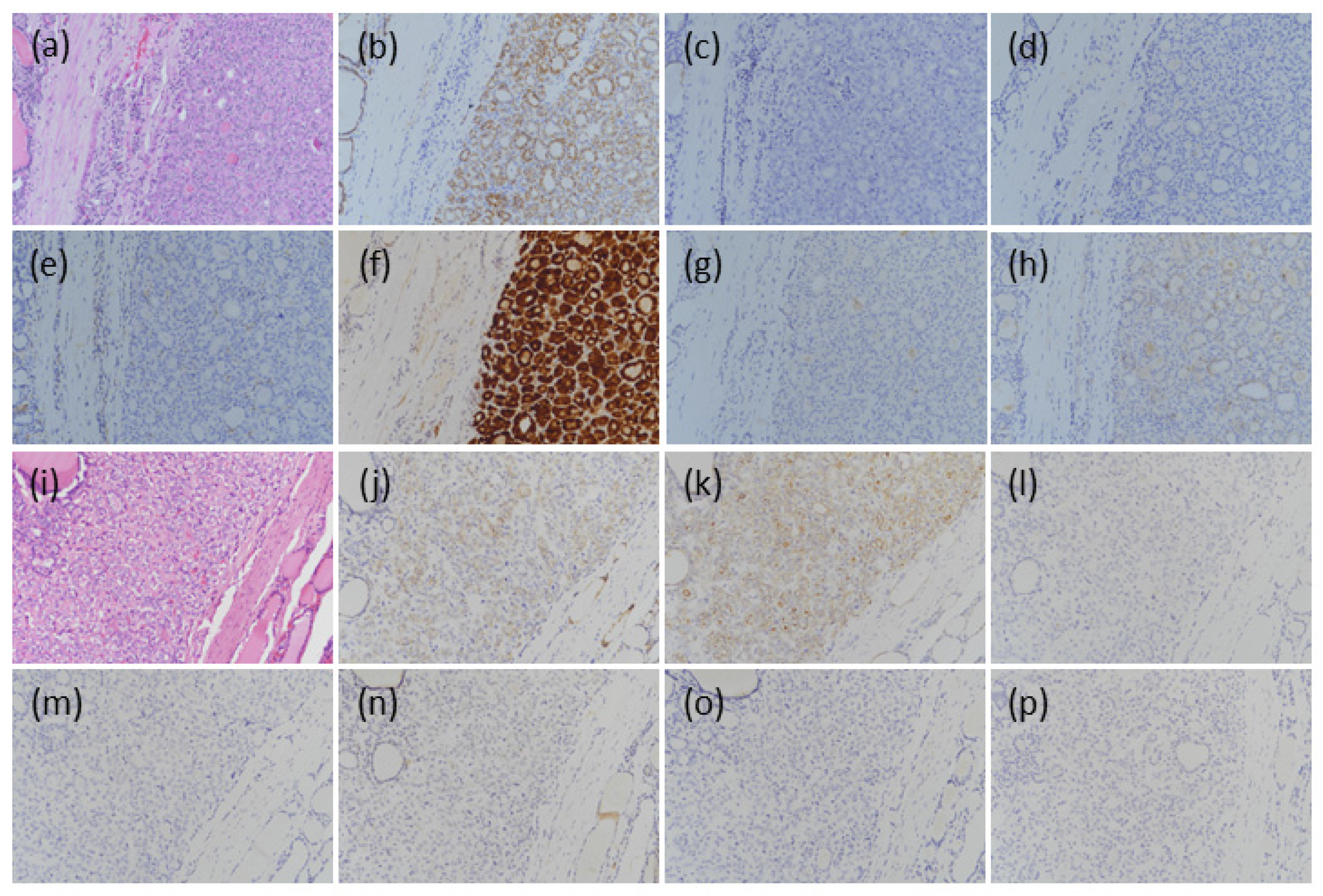
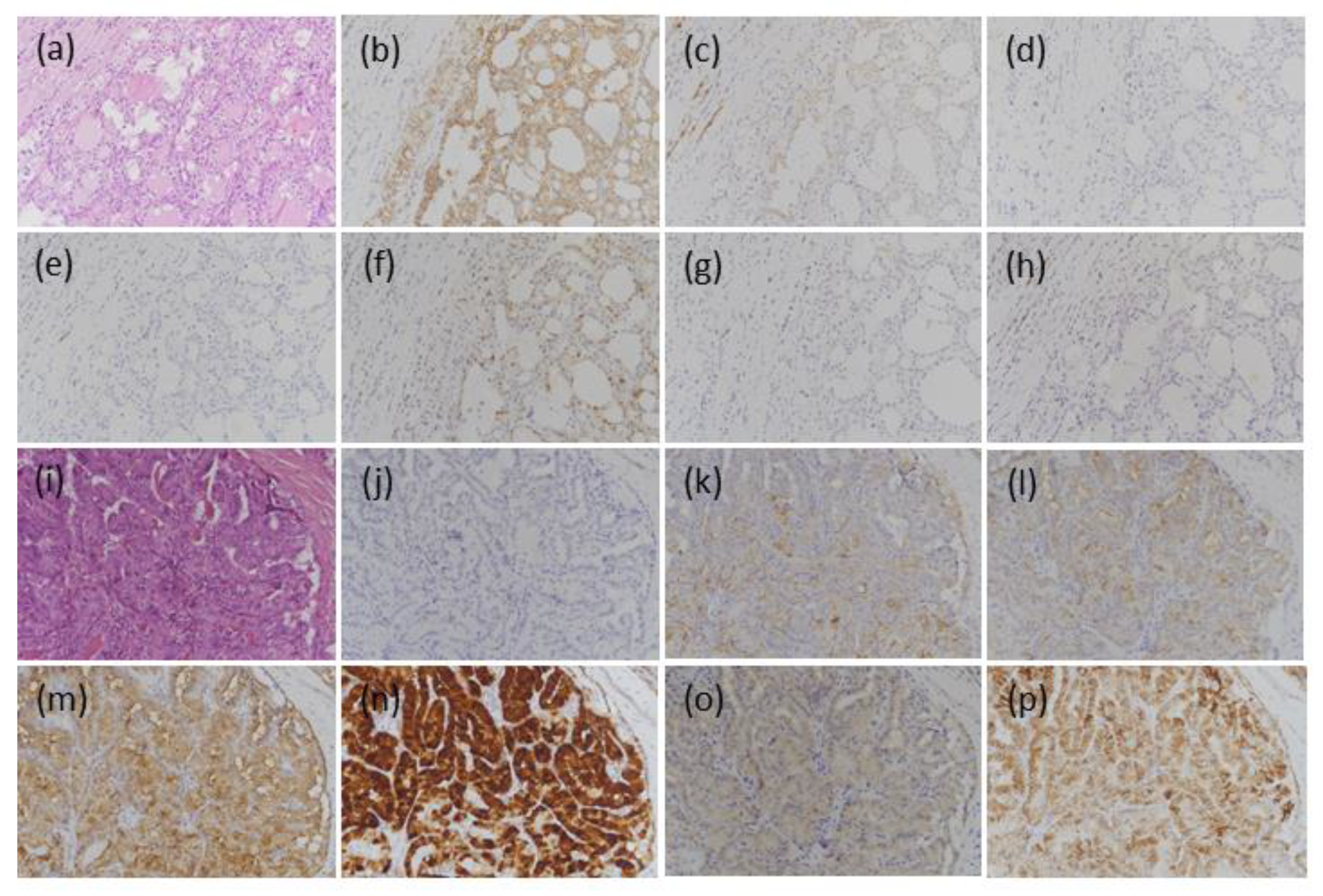
3.2.1. BFNs
3.2.2. NIFTP
3.2.3. NEFVPTC
3.2.4. IFVPTC
3.3. Comparisons between Various Lesions Using Single Markers
3.4. Diagnostic Application between BFNs and IFVPTC
3.5. Diagnostic Application between NIFTP/NEFVPTC and IFVPTC
3.6. Diagnostic Application between BFNs and NIFTP/NEFVPTC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nikiforov, Y.E.; Seethala, R.R.; Tallini, G.; Baloch, Z.W.; Basolo, F.; Thompson, L.D.; Barletta, J.A.; Wenig, B.M.; Al Ghuzlan, A.; Kakudo, K.; et al. Nomenclature Revision for Encapsulated Follicular Variant of Papillary Thyroid Carcinoma: A Paradigm Shift to Reduce Overtreatment of Indolent Tumors. JAMA Oncol. 2016, 2, 1023–1029. [Google Scholar] [CrossRef] [Green Version]
- Rosario, P.W.; Mourão, G.F. Noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP): A review for clinicians. Endocr. Relat. Cancer 2019, 26, R259–R266. [Google Scholar] [CrossRef]
- Pusztaszeri, M.; Bongiovanni, M. The impact of non-invasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) on the diagnosis of thyroid nodules. Gland Surg. 2019, 8, S86–S97. [Google Scholar] [CrossRef]
- Thompson, L.D. Ninety-four cases of encapsulated follicular variant of papillary thyroid carcinoma: A name change to Noninvasive Follicular Thyroid Neoplasm with Papillary-like Nuclear Features would help prevent overtreatment. Mod. Pathol. 2016, 29, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Rosario, P.W.; Mourao, G.F.; Nunes, M.B.; Nunes, M.S.; Calsolari, M.R. Noninvasive follicular thyroid neoplasm with papillary-like nuclear features. Endocr. Relat. Cancer 2016, 23, 893–897. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Bychkov, A.; Jung, C.K.; Hirokawa, M.; Sui, S.; Hong, S.; Lai, C.R.; Jain, D.; Canberk, S.; Kakudo, K. Interobserver and intraobserver variation in the morphological evaluation of noninvasive follicular thyroid neoplasm with papillary-like nuclear features in Asian practice. Pathol. Int. 2019, 69, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Lee, M.; Kwon, A.Y.; Choe, J.H.; Kim, J.H.; Kim, J.S.; Hahn, S.Y.; Shin, J.H.; Chung, M.K.; Son, Y.I.; et al. Molecular genotyping of noninvasive encapsulated follicular variant of papillary thyroid carcinoma. Histopathology 2018, 72, 648–661. [Google Scholar] [CrossRef]
- Cho, U.; Mete, O.; Kim, M.H.; Bae, J.S.; Jung, C.K. Molecular correlates and rate of lymph node metastasis of non-invasive follicular thyroid neoplasm with papillary-like nuclear features and invasive follicular variant papillary thyroid carcinoma: The impact of rigid criteria to distinguish non-invasive follicular thyroid neoplasm with papillary-like nuclear features. Mod. Pathol. 2017, 30, 810–825. [Google Scholar] [CrossRef] [PubMed]
- Parente, D.N.; Kluijfhout, W.P.; Bongers, P.J.; Verzijl, R.; Devon, K.M.; Rotstein, L.E.; Goldstein, D.P.; Asa, S.L.; Mete, O.; Pasternak, J.D. Clinical Safety of Renaming Encapsulated Follicular Variant of Papillary Thyroid Carcinoma: Is NIFTP Truly Benign? World J. Surg. 2018, 42, 321–326. [Google Scholar] [CrossRef]
- Alves, V.A.F.; Kakudo, K.; LiVolsi, V.; Lloyd, R.V.; Nikiforov, Y.E.; Nosé, V.; Papotti, M.; Thompson, L.D.R. Noninvasive Follicular Thyroid Neoplasm with Papillary-like Nuclear Features (NIFTP): Achieving Better Agreement by Refining Diagnostic Criteria. Clinics 2018, 73, e576. [Google Scholar] [CrossRef]
- Nikiforov, Y.; Baloch, Z.W.; Hodak, S.P.; Giordano, T.J.; Lloyd, R.V.; Seethala, R.R.; Wenig, B.M. Change in Diagnostic Criteria for Noninvasive Follicular Thyroid Neoplasm with Papillarylike Nuclear Features. JAMA Oncol. 2018, 4, 1125–1126. [Google Scholar] [CrossRef]
- Lloyd, R.V.; Asa, S.L.; LiVolsi, V.A.; Sadow, P.M.; Tischler, A.S.; Ghossein, R.A.; Tuttle, R.M.; Nikiforov, Y.E. The evolving diagnosis of noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP). Hum. Pathol. 2018, 74, 1–4. [Google Scholar] [CrossRef]
- Xu, B.; Serrette, R.; Tuttle, R.M.; Alzumaili, B.; Ganly, I.; Katabi, N.; Tallini, G.; Ghossein, R. How Many Papillae in Conventional Papillary Carcinoma? A Clinical Evidence-Based Pathology Study of 235 Unifocal Encapsulated Papillary Thyroid Carcinomas, with Emphasis on the Diagnosis of Noninvasive Follicular Thyroid Neoplasm with Papillary-like Nuclear Features. Thyroid 2019, 29, 1792–1803. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.N.; Sadow, P.M. Exploration of BRAFV600E as a diagnostic adjuvant in the non-invasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP). Hum. Pathol. 2018, 82, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, J.; Lu, J.; Gao, J.; Lu, T.; Ren, X.; Duan, H.; Liang, Z. Immunohistochemistry is highly sensitive and specific for detecting the BRAF V600E mutation in papillary thyroid carcinoma. Int. J. Clin. Exp. Pathol. 2015, 8, 15072–15078. [Google Scholar] [PubMed]
- Koperek, O.; Kornauth, C.; Capper, D.; Berghoff, A.S.; Asari, R.; Niederle, B.; von Deimling, A.; Birner, P.; Preusser, M. Immunohistochemical detection of the BRAF V600E-mutated protein in papillary thyroid carcinoma. Am. J. Surg. Pathol. 2012, 36, 844–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zargari, N.; Mokhtari, M. Evaluation of Diagnostic Utility of Immunohistochemistry Markers of TROP-2 and HBME-1 in the Diagnosis of Thyroid Carcinoma. Eur. Thyroid J. 2019, 8, 1–6. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Morreau, H.; Kievit, J.; Romijn, J.A.; Carrasco, N.; Smit, J.W. Combined immunostaining with galectin-3, fibronectin-1, CITED-1, Hector Battifora mesothelial-1, cytokeratin-19, peroxisome proliferator-activated receptor-{gamma}, and sodium/iodide symporter antibodies for the differential diagnosis of non-medullary thyroid carcinoma. Eur. J. Endocrinol. 2008, 158, 375–384. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.; Xu, S.; Yan, J.; Zhang, C.; Qin, S.; Wang, X.; Li, N. The value of tumor markers in the diagnosis of papillary thyroid carcinoma alone and in combination. Pol. J. Pathol. 2014, 3, 202–209. [Google Scholar] [CrossRef]
- Crescenzi, A.; Guidobaldi, L.; Nasrollah, N.; Taccogna, S.; Cicciarella Modica, D.D.; Turrini, L.; Nigri, G.; Romanelli, F.; Valabrega, S.; Giovanella, L.; et al. Immunohistochemistry for BRAF(V600E) antibody VE1 performed in core needle biopsy samples identifies mutated papillary thyroid cancers. Horm. Metab. Res. 2014, 46, 370–374. [Google Scholar] [CrossRef]
- Abdou, A.G.; Shabaan, M.; Abdallha, R.; Nabil, N. Diagnostic Value of TROP-2 and CK19 Expression in Papillary Thyroid Carcinoma in Both Surgical and Cytological Specimens. Clin. Pathol. 2019, 12, 2632010X19863047. [Google Scholar] [CrossRef] [Green Version]
- Cho, H.; Kim, J.Y.; Oh, Y.L. Diagnostic value of HBME-1, CK19, Galectin 3, and CD56 in the subtypes of follicular variant of papillary thyroid carcinoma. Pathol. Int. 2018, 68, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Tastekin, E.; Keskin, E.; Can, N.; Canberk, S.; Mut, A.; Erdogan, E.; Asa, N.; Güldiken, S.; Sezer, A.; Azatcam, M. CD56, CD57, HBME1, CK19, Galectin-3 and p63 immunohistochemical stains in differentiating diagnosis of thyroid benign/malign lesions and NIFTP. Pol. J. Pathol. 2019, 70, 286–294. [Google Scholar] [CrossRef] [Green Version]
- Sadiq, Q.; Sekhri, R.; Dibaba, D.T.; Zhao, Q.; Agarwal, S. HBME1 and CK19 expression in non-invasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) vs other follicular patterned thyroid lesions. World J. Surg. Oncol. 2021, 19, 143. [Google Scholar] [CrossRef]
- Fu, G.; Polyakova, O.; Chazen, R.S.; Freeman, J.L.; Witterick, I.J. Diagnostic Value of Galectin-3 in Distinguishing Invasive Encapsulated Carcinoma from Noninvasive Follicular Thyroid Neoplasms with Papillary-like Nuclear Features (NIFTP). Cancers 2021, 13, 2998. [Google Scholar] [CrossRef] [PubMed]
- Nikiforov, Y.E.; Baloch, Z.W.; Chan, J.K.C.; Derwahl, K.M.; Evans, H.L.; Ghossein, R.A.; Lloyd, R.V.; Oriola, J.; Osamura, R.Y.; Paschke, R.; et al. Follicular Adenoma. In WHO Classification of Tumours of Endocrine Organs, 4th ed.; Lloyd, R.V., Osamura, R.Y., Klöppel, G., Rosai, J., Eds.; IARC Press: Lyon, France, 2017; pp. 69–72. [Google Scholar]
- Lloyd, R.V.; Baloch, Z.W.; Sobrinho-Simões, M.; Tallini, G. Hürthle (oncocytic) Cell Tumours. In WHO Classification of Tumours of Endocrine Organs, 4th ed.; Lloyd, R.V., Osamura, R.Y., Klöppel, G., Rosai, J., Eds.; IARC Press: Lyon, France, 2017; pp. 96–99. [Google Scholar]
- Nikiforov, Y.E.; Ghossein, R.A.; Kakudo, K.; LiVolsi, V.; Papotti, M.; Randolph, G.W.; Tallini, G.; Thompson, L.D.R.; Tuttle, R.M. Noninvasive Follicular Thyroid Neoplasm with Papillary-like Nuclear Features. In WHO Classification of Tumours of Endocrine Organs, 4th ed.; Lloyd, R.V., Osamura, R.Y., Klöppel, G., Rosai, J., Eds.; IARC Press: Lyon, France, 2017; pp. 78–80. [Google Scholar]
- Xu, B.; Farhat, N.; Barletta, J.A.; Hung, Y.P.; Biase, D.; Casadei, G.P.; Onenerk, A.M.; Tuttle, R.M.; Roman, B.R.; Katabi, N. Should subcentimeter non-invasive encapsulated, follicular variant of papillary thyroid carcinoma be included in the noninvasive follicular thyroid neoplasm with papillary-like nuclear features category? Endocrine 2018, 59, 143–150. [Google Scholar] [CrossRef]
- Liu, J.; Singh, B.; Tallini, G.; Carlson, D.L.; Katabi, N.; Shaha, A.; Tuttle, R.M.; Ghossein, R.A. Follicular variant of papillary thyroid carcinoma. Cancer 2006, 107, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Won, J.K.; Jung, K.C.; Kim, J.H.; Cho, S.W.; Park, D.J.; Park, Y.J. Clinical Characteristics of Subtypes of Follicular Variant Papillary Thyroid Carcinoma. Thyroid 2018, 28, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Pisanu, A.; Deplano, D.; Reccia, I.; Porceddu, G.; Uccheddu, A. Encapsulated papillary thyroid carcinoma is it a distinctive clinical entity with low-grade malignancy? J. Endocrinol. Investig. 2013, 36, 78–83. [Google Scholar] [CrossRef]
- Tallini, G.; Tuttle, R.M.; Ghossein, R.A. The History of the Follicular Variant of Papillary Thyroid Carcinoma. J. Clin. Endocrinol. Metab. 2017, 102, 15–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panicker, A.K.; Buhusi, M.; Thelen, K.; Maness, P.F. Cellular signalling mechanisms of neural cell adhesion molecules. Front. Biosci. 2003, 8, d900–d911. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Kim, Y.H.; Han, J.H.; Lee, K.B.; Sheen, S.S.; Lee, J.; Soh, E.Y.; Park, T.J. Silencing of homeobox B9 is associated with down-regulation of CD56 and extrathyroidal extension of tumor in papillary thyroid carcinoma. Hum. Pathol. 2012, 43, 1221–1228. [Google Scholar] [CrossRef]
- Palo, S.; Biligi, D.S. Differential diagnostic significance of HBME-1, CK19 and S100 in various thyroid lesions. Malays. J. Pathol. 2017, 1, 55–67. [Google Scholar]
- Wang, Y.; Huang, H.; Hu, F.; Li, J.; Zhang, L.; Pang, H. CITED1 contributes to the progression of papillary thyroid carcinoma via the Wnt/beta-catenin signaling pathway. Onco Targets Ther. 2019, 12, 6769–6777. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Shi, J.; Lin, F. The Potential Diagnostic Utility of TROP-2 in Thyroid Neoplasms. Appl. Immunohistochem. Mol. Morphol. 2017, 25, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Murtezaoglu, A.R.; Gucer, H. Diagnostic value of TROP-2 expression in papillary thyroid carcinoma and comparison with HBME-1, galectin-3 and cytokeratin 19. Pol. J. Pathol. 2017, 68, 1–10. [Google Scholar] [CrossRef]
- Giannini, R.; Ugolini, C.; Poma, A.; Urpì, M.; Niccoli, C.; Elisei, R.; Chiarugi, M.; Vitti, P.; Miccoli, P.; Basolo, F. Identification of two distinct molecular subtypes of non-invasive follicular neoplasm with papillary-like nuclear features by digital RNA counting. Thyroid 2017, 27, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
| BFN | NIFTP | NEFVPTC | IFVPTC | |
|---|---|---|---|---|
| Female/male | 16/5 | 12/4 | 15/1 | 8/2 |
| Mean age, years | 44.0 | 50.9 | 37.1 | 43.6 |
| Mean size, cm (range) | 2.42 (0.7–4.8) | 2.40 (0.2–6) | 2.26(0.5–3.2) | 1.68 (1.1–2.6) |
| Multifocality | 8/21 | 0 | 2/16 | 4/10 |
| Extrathyroidal extension | 0 | 0 | 0 | 4/10 |
| Lymph node metastases | 0 | 0 | 0 | 4/10 |
| Distant metastasis | 0 | 0 | 0 | 0 |
| FOP, months, median, (range) | 41.5 (1.0–102) | 45.0 (1.0–91.0) | 47.5(1.0–98.0) | 70.0 (1.0–87.0) |
| CD56 * | 21/21 (100%) | 13/16 (82.4%) | 12/16 (75.0%) | 1/10 (10.0%) |
| CK19 | 4/21 (19.0%) | 12/16 (75%) | 14/16 (87.5%) | 10/10 (100%) |
| HBME-1 | 2/21 (9.5%) | 9/16 (56.2%) | 9/16 (56.2%) | 10/10 (100%) |
| Gal-3 | 1/21 (4.8%) | 1/16 (6.3%) | 2/16 (12.5%) | 10/10 (100%) |
| CITED1 | 12/21 (57.1%) | 11/16 (68.8%) | 11/16 (68.8%) | 10/10 (100%) |
| VE1 | 0/21 (0%) | 0/16 (0%) | 1/16 (6.3%) | 4/10 (40.0%) |
| TROP-2 | 4/21 (19.0%) | 1/16 (6.3%) | 1/16 (6.3%) | 10/10 (100%) |
| IHC Markers | BFN vs. NIFTP | BFN vs. NEFVPTC | BFN vs. IFVPTC | NIFTP vs. NEFVPTC | NIFTP vs. IFVPTC | NEFVPTC vs. IFVPTC |
|---|---|---|---|---|---|---|
| CD56 | p = 0.0412 * | p = 0.0167 * | p < 0.0001 * | p = 0.6738 | p = 0.0005 * | p = 0.0016 * |
| CK19 | p = 0.0008 * † | p < 0.0001 * ‡ | p < 0.0001 * | p = 0.3726 | p = 0.0919 | p = 0.2538 |
| HBME-1 | p = 0.0024 * | p = 0.0024 * | p < 0.0001 * | p = 1.0000 | p = 0.0164 * | p = 0.0164 * |
| Gal-3 | p = 0.8449 | p = 0.3994 | p < 0.0001 * | p = 0.5506 | p < 0.0001 * | p < 0.0001 * |
| CITED1 | p = 0.4768 | p = 0.4768 | p = 0.0156 * | p = 0.7141 | p = 0.0538 # | p = 0.0538 # |
| VE1 | p = 0.4111 | p = 0.2519 | p = 0.0023 * | p = 0.3173 | p = 0.0070 * | p = 0.0372 * |
| TROP-2 | p = 0.2658 | p = 0.2658 | p < 0.0001 * | p = 1.0000 | p < 0.0001 * | p < 0.0001 * |
| IHC Markers | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) |
|---|---|---|---|---|---|
| CD56 | 90.0 | 100 | 100 | 95.5 | 96.8 |
| CK19 | 100 | 81.0 | 71.4 | 100 | 87.1 |
| HBME-1 | 100 | 90.5 | 83.3 | 100 | 93.5 |
| Gal-3 | 100 | 95.2 | 90.9 | 100 | 96.8 |
| CITED1 | 100 | 42.9 | 45.5 | 100 | 61.3 |
| VE1 | 40.0 | 100 | 100 | 77.8 | 80.6 |
| TROP-2 * | 100 | 81.0 | 71.4 | 100 | 87.1 |
| Gal-3 and/or HBME-1 and/or TROP-2 | 100 | 100 | 100 | 100 | 100 |
| IHC Markers | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) |
|---|---|---|---|---|---|
| CD56 | 90.0 | 78.1 | 56.3 | 96.2 | 81.0 |
| HBME-1 | 100 | 43.8 | 35.7 | 100 | 57.1 |
| Gal-3 | 100 | 90.6 | 76.9 | 100 | 92.9 |
| CITED1 | 100 | 31.3 | 31.3 | 100 | 47.6 |
| VE1 | 40.0 | 96.9 | 80.0 | 83.8 | 83.3 |
| TROP-2 | 100 | 93.8 | 83.3 | 100 | 95.2 |
| Gal-3 and TROP-2 | 100 | 100 | 100 | 100 | 100 |
| IHC Markers | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) |
|---|---|---|---|---|---|
| Single marker: | |||||
| CD56 | 21.9 | 100 | 100 | 45.7 | 52.8 |
| CK19 | 81.3 | 81.0 | 86.7 | 73.9 | 81.1 |
| HBME-1 | 56.3 | 90.5 | 90.0 | 57.6 | 69.8 |
| Double markers: | |||||
| CK19 and/or HBME-1 | 96.9 | 77.3 | 86.1 | 94.4 | 88.9 |
| CD56 and/or CK19 | 84.4 | 81.0 | 87.1 | 77.3 | 83.0 |
| CD56 and/or HBME-1 | 71.9 | 90.5 | 92.0 | 67.9 | 79.2 |
| Triple markers: | |||||
| CD56 and/or CK19 and/or HBME-1 | 96.9 | 76.2 | 86.1 | 94.1 | 88.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuang, H.-W.; Wang, J.-S.; Tsai, J.-W.; Hsu, C.-T.; Lin, K.-J. Immunohistochemistry Helps to Distinguish Noninvasive Follicular Thyroid Neoplasm with Papillary-like Nuclear Features/Noninvasive Encapsulated Follicular Variant of Papillary Thyroid Carcinoma with Other Follicular Thyroid Lesions. Medicina 2021, 57, 1246. https://doi.org/10.3390/medicina57111246
Chuang H-W, Wang J-S, Tsai J-W, Hsu C-T, Lin K-J. Immunohistochemistry Helps to Distinguish Noninvasive Follicular Thyroid Neoplasm with Papillary-like Nuclear Features/Noninvasive Encapsulated Follicular Variant of Papillary Thyroid Carcinoma with Other Follicular Thyroid Lesions. Medicina. 2021; 57(11):1246. https://doi.org/10.3390/medicina57111246
Chicago/Turabian StyleChuang, Hao-Wen, Jyh-Seng Wang, Jen-Wei Tsai, Chao-Tien Hsu, and Kai-Jen Lin. 2021. "Immunohistochemistry Helps to Distinguish Noninvasive Follicular Thyroid Neoplasm with Papillary-like Nuclear Features/Noninvasive Encapsulated Follicular Variant of Papillary Thyroid Carcinoma with Other Follicular Thyroid Lesions" Medicina 57, no. 11: 1246. https://doi.org/10.3390/medicina57111246
APA StyleChuang, H. -W., Wang, J. -S., Tsai, J. -W., Hsu, C. -T., & Lin, K. -J. (2021). Immunohistochemistry Helps to Distinguish Noninvasive Follicular Thyroid Neoplasm with Papillary-like Nuclear Features/Noninvasive Encapsulated Follicular Variant of Papillary Thyroid Carcinoma with Other Follicular Thyroid Lesions. Medicina, 57(11), 1246. https://doi.org/10.3390/medicina57111246







