Characteristics of Patients with Obstructive Sleep Apnea at High Risk for Cardiovascular Disease
Abstract
:1. Introduction
2. Materials and Methods
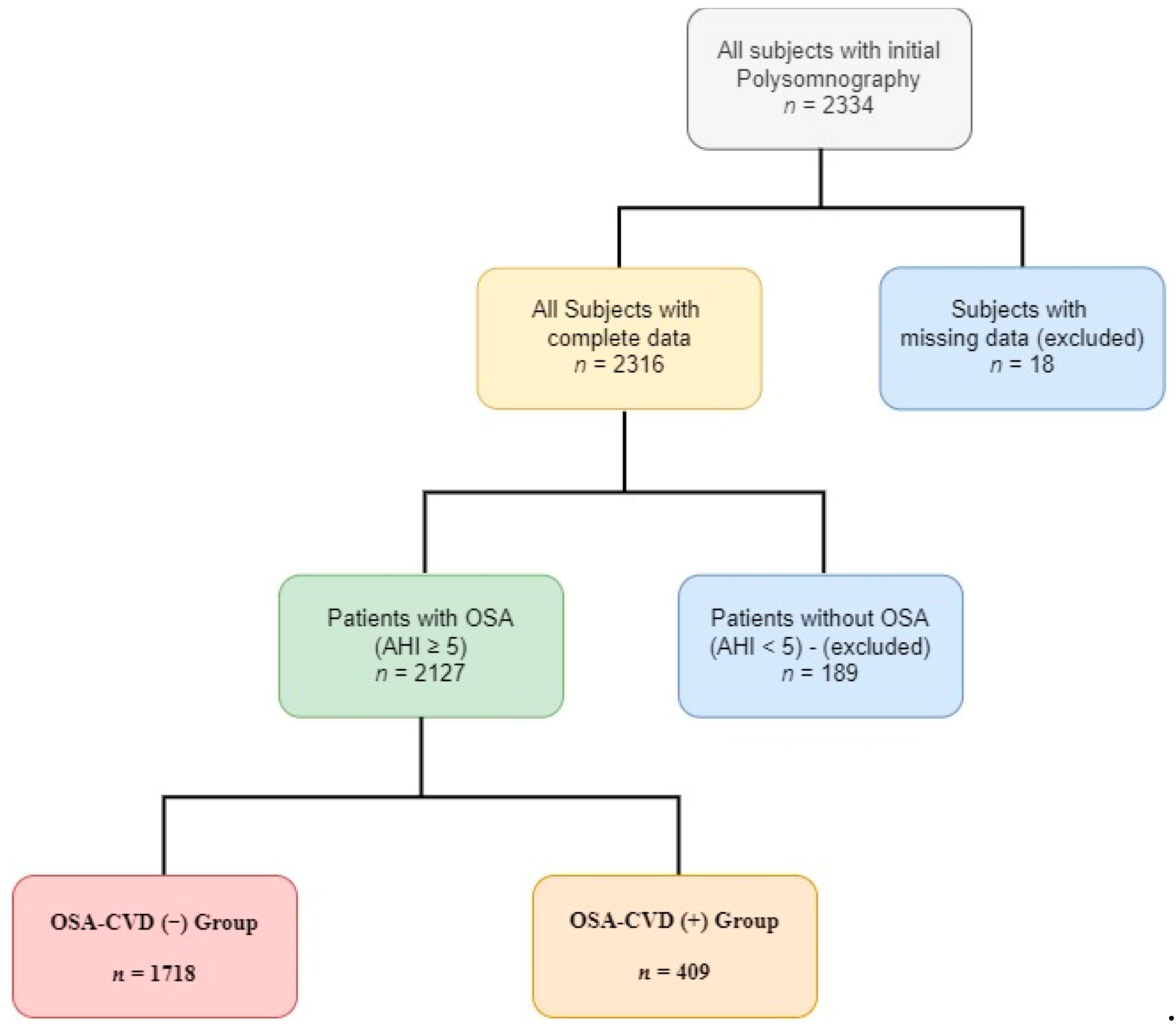
2.1. Population
2.2. Data Collection
2.3. Sleep Study
2.4. Statistical Analysis
3. Results
3.1. Patients
3.2. CVD Association with OSA Symptoms
3.3. Subgroup Analysis by Age, BMI, and OSA Severity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.M.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pépin, J.-L.; et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Respir. Med. 2019, 7, 687–698. [Google Scholar] [CrossRef] [Green Version]
- Xie, M.; Liu, X.; Cao, X.; Guo, M.; Li, X. Trends in prevalence and incidence of chronic respiratory diseases from 1990 to 2017. Respir. Res. 2020, 21, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Jehan, S.; Myers, A.K.; Zizi, F.; Pandi-Perumal, S.R.; Jean-Louis, G.; McFarlane, S.I. Obesity, obstructive sleep apnea and type 2 diabetes mellitus: Epidemiology and pathophysiologic insights. Sleep Med. Disord. Int. J. 2018, 2, 52–58. [Google Scholar]
- Umbro, I.; Fabiani, V.; Fabiani, M.; Angelico, F.; Del Ben, M. Association between non-alcoholic fatty liver disease and obstructive sleep apnea. World J. Gastroenterol. 2020, 26, 2669–2681. [Google Scholar] [CrossRef]
- Morsy, N.; Farrag, N.; Zaki, N.F.; Badawy, A.Y.; Abdelhafez, S.A.; El-Gilany, A.-H.; El Shafey, M.M.; Pandi-Perumal, S.R.; Spence, D.W.; BaHammam, A.S. Obstructive sleep apnea: Personal, societal, public health, and legal implications. Rev. Environ. Health 2019, 34, 153–169. [Google Scholar] [CrossRef]
- Garvey, J.F.; Pengo, M.F.; Drakatos, P.; Kent, B.D. Epidemiological aspects of obstructive sleep apnea. J. Thorac. Dis. 2015, 7, 920–929. [Google Scholar] [CrossRef]
- American Academy of Sleep Medicine. Hidden Health Crisis Costing America Billions: Underdiagnosing and Undertreating Obstructive Sleep Apnea Draining Health Care System; Frost & Sullivan: San Antonio, TX, USA, 2016. [Google Scholar]
- Knauert, M.; Naik, S.; Gillespie, M.B.; Kryger, M. Clinical consequences and economic costs of untreated obstructive sleep apnea syndrome. World J. Otorhinolaryngol. Head Neck Surg. 2015, 1, 17–27. [Google Scholar] [CrossRef] [Green Version]
- Saaresranta, T.; Hedner, J.; Bonsignore, M.R.; Riha, R.L.; McNicholas, W.T.; Penzel, T.; Anttalainen, U.; Kvamme, J.A.; Pretl, M.; Sliwinski, P.; et al. Clinical phenotypes and comorbidity in European sleep apnoea patients. PLoS ONE 2016, 11, e0163439. [Google Scholar] [CrossRef]
- Anttalainen, U.; Grote, L.; Fietze, I.; Riha, R.L.; Ryan, S.; Staats, R.; Hedner, J.; Saaresranta, T. Insomnia symptoms combined with nocturnal hypoxia associate with cardiovascular comorbidity in the European sleep apnea cohort (ESADA). Sleep Breath. 2019, 23, 805–814. [Google Scholar] [CrossRef]
- Quan, W.; Zheng, D.; McEvoy, D.; Barbe, F.; Chen, R.; Liu, Z.; Loffler, K.; Lorenzi-Filho, G.; Luo, Y.; Mukherjee, S.; et al. High risk characteristics for recurrent cardiovascular events among patients with obstructive sleep apnoea in the SAVE study. EClinicalMedicine 2018, 2, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Johns, M.W. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef] [Green Version]
- Soldatos, C.R.; Dikeos, D.G.; Paparrigopoulos, T.J. Athens Insomnia Scale: Validation of an instrument based on ICD-10 criteria. J. Psychosom. Res. 2000, 48, 555–560. [Google Scholar] [CrossRef]
- Soldatos, C.R.; Dikeos, D.G.; Paparrigopoulos, T.J. The diagnostic validity of the Athens Insomnia Scale. J. Psychosom. Res. 2003, 55, 263–267. [Google Scholar] [CrossRef]
- Beck, A.T.; Beamesderfer, A. Assessment of depression: The Depression Inventory. Anxiety Disord. 1974, 7, 151–169. [Google Scholar] [CrossRef]
- Beck, A.T.; Steer, R.A.; Carbin, M.G. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin. Psychol. Rev. 1988, 8, 77–100. [Google Scholar] [CrossRef]
- Richter, P.; Werner, J.; Heerlein, A.; Kraus, A.; Sauer, H. On the validity of the Beck Depression Inventory. Psychopathology 1998, 31, 160–168. [Google Scholar] [CrossRef]
- Berry, R.B.; Brooks, R.; Gamaldo, C.E.; Harding, S.M.; Marcus, C.; Vaughn, B.V. The AASM manual for the scoring of sleep and associated events: Rules, terminology and technical speci-fications. Am. Acad. Sleep Med. 2016, 176. [Google Scholar]
- Mazzotti, D.R.; Keenan, B.T.; Lim, D.C.; Gottlieb, D.J.; Kim, J.; Pack, A.I. Symptom subtypes of obstructive sleep apnea predict incidence of cardiovascular outcomes. Am. J. Respir. Crit. Care Med. 2019, 200, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Pien, G.W.; Ratcliffe, S.; Björnsdottir, E.; Arnardottir, E.S.; Pack, A.; Benediktsdottir, B.; Gislason, T. The different clinical faces of obstructive sleep apnoea: A cluster analysis. Eur. Respir. J. 2014, 44, 1600–1607. [Google Scholar] [CrossRef] [Green Version]
- Keenan, B.T.; Kim, J.; Singh, B.; Bittencourt, L.; Chen, N.-H.; Cistulli, P.A.; Magalang, U.J.; McArdle, N.; Mindel, J.W.; Benediktsdottir, B.; et al. Recognizable clinical subtypes of obstructive sleep apnea across international sleep centers: A cluster analysis. Sleep 2018, 41, 214. [Google Scholar] [CrossRef]
- Kim, J.; Keenan, B.T.; Lim, D.C.; Lee, S.K.; Pack, A.I.; Shin, C. Symptom-based subgroups of Koreans with obstructive sleep apnea. J. Clin. Sleep Med. 2018, 14, 437–443. [Google Scholar] [CrossRef] [Green Version]
- Frangopoulos, F.; Zannetos, S.; Nicolaou, I.; Economou, N.T.; Adamide, T.; Georgiou, A.; Nikolaidis, P.T.; Rosemann, T.; Knechtle, B.; Trakada, G. The complex interaction between the major sleep symptoms, the severity of obstructive sleep apnea, and sleep quality. Front. Psychiatry 2021, 12, 630162. [Google Scholar] [CrossRef]
- Ogilvie, R.P.; Lakshminarayan, K.; Iber, C.; Patel, S.; Lutsey, P.L. Joint effects of OSA and self-reported sleepiness on incident CHD and stroke. Sleep Med. 2018, 44, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Rajan, S.; McKee, M.; Rangarajan, S.; Bangdiwala, S.; Rosengren, A.; Gupta, R.; Kutty, V.R.; Wielgosz, A.; Lear, S.; AlHabib, K.F.; et al. Association of symptoms of depression with cardiovascular disease and mortality in low-, middle-, and high-income countries. JAMA Psychiatry 2020, 77, 1052–1063. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.-L.; Tsao, H.-M.; Hsu, C.-C.; Wu, K.-M.; Hsu, T.-S.; Wu, Y.-T.; Hu, G.-C. Bidirectional association between obstructive sleep apnea and depression. Medicine 2016, 95, e4833. [Google Scholar] [CrossRef]
- Ferenchick, E.K.; Ramanuj, P.; Pincus, H.A. Depression in primary care: Part 1—Screening and diagnosis. BMJ 2019, 365, l794. [Google Scholar] [CrossRef] [Green Version]
- Edwards, B.A.; Redline, S.; Sands, S.A.; Owens, R.L. More than the sum of the respiratory events: Personalized medicine approaches for obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2019, 200, 691–703. [Google Scholar] [CrossRef] [PubMed]
| Total Population According to CVD | ||||
|---|---|---|---|---|
| All OSA Patients (n = 2127) | OSA-CVD (−) Group (n = 1718) | OSA-CVD (+) Group (n = 409) | p Value | |
| Demographics | ||||
| Age(Years) | 55 ± 14 | 54 ± 14 | 64 ± 12 | <0.001 |
| Age ≥ 60 years | 851 (40%) | 567 (33%) | 274 (67%) | <0.001 |
| BMI (kg/m2) | 33 ± 7 | 34 ± 7 | 35 ± 6 | 0.002 |
| BMI ≥ 30 | 1531 (72%) | 1202 (70%) | 339 (83%) | <0.001 |
| Neck circumference (cm) | 43 ± 5 | 42 ± 5 | 43 ± 4 | <0.001 |
| Waist circumference (cm) | 115 ± 15 | 114 ± 16 | 119± 13 | <0.001 |
| Hip circumference (cm) | 115 ± 15 | 115 ± 16 | 118 ± 14 | <0.001 |
| Waist/Hipcircumference ratio | 1.0 ± 0.16 | 1.0 ± 0.18 | 1.0 ± 0.08 | 0.57 |
| Smoking status | ||||
| Never, n (%) | 681 (32%) | 567 (33%) | 106 (26%) | |
| Current Smoking, n (%) | 617 (29%) | 533 (31%) | 82 (20%) | |
| Former, n (%) | 820 (39%) | 618 (36%) | 221 (54%) | <0.001 |
| Pack-years | 23 (0, 50) | 20 (0, 45) | 35 (0, 45) | <0.001 |
| Co-morbidities | ||||
| Hypertension | 1021 (48%) | 722 (42%) | 303 (74%) | <0.001 |
| Diabetes type II | 425 (20%) | 275 (16%) | 139 (34%) | <0.001 |
| COPD | 362 (17%) | 241 (14%) | 127 (31%) | <0.001 |
| Bronchial asthma | 149 (7%) | 104 (6%) | 29 (7%) | 0.63 |
| Hypothyroidism | 319 (15%) | 258 (15%) | 61 (15%) | 0.80 |
| Dyslipidemia | 872 (41%) | 653 (38%) | 213 (52%) | <0.001 |
| Depression | 255 (12%) | 206 (12%) | 62 (15%) | 0.08 |
| Total Population According to CVD | ||||
|---|---|---|---|---|
| All OSA Patients (n = 2127) | OSA-CVD (−) Group (n = 1718) | OSA-CVD (+) Group (n = 409) | p Value | |
| TRT (min) | 421 ± 108 | 420 ± 117 | 424 ± 54 | 0.45 |
| TST (min) | 258 ± 61 | 261 ± 61 | 245 ± 61 | <0.001 |
| Sleepefficiency, % | 62 ± 12 | 63 ± 12 | 58 ± 13 | <0.001 |
| WASO (min) | 110 (91, 142) | 107 (88, 136) | 125 (88, 136) | <0.001 |
| Sleep Latency | 41 (26, 65) | 39 (26, 63) | 47 (26, 63) | <0.001 |
| REM Latency | 255 ± 83 | 253 ± 82 | 264 ± 86 | 0.015 |
| NREM (%) | 90 ± 6 | 90 ± 7 | 91 ± 3 | 0.001 |
| SWS (%) | 7 (5, 9) | 7 (6, 9) | 6 (6, 9) | <0.001 |
| REM(%) | 9 (7, 11) | 9 (7, 12) | 8 (7, 12) | <0.001 |
| AHI | 36 (21, 63) | 34 (20, 62) | 43 (20, 62) | <0.001 |
| REM AHI | 48 ± 28 | 47 ± 28 | 54 ± 26 | <0.001 |
| AI | 46 ± 14 | 46 ± 14 | 49 ± 13 | <0.001 |
| ODI | 40 (24, 66) | 38 (22, 65) | 48 (22, 65) | <0.001 |
| Mean SaO2 | 92 (90, 93) | 92 (90, 94) | 91 (90, 94) | <0.001 |
| Lowest SaO2 | 80 (74, 84) | 80 (75, 84) | 78 (75, 84) | <0.001 |
| TST90 (min) | 59 (19, 131) | 51 (16, 122) | 86 (16, 122) | <0.001 |
| Severity of OSA (%) | ||||
| 5 ≤ AHI < 15 | 278 (13%) | 265 (15%) | 13 (3%) | |
| 15 ≤ AHI < 30 | 543 (26%) | 445(26%) | 98 (24%) | |
| AHI ≥ 30 | 1306 (61%) | 1008 (59%) | 298 (73%) | <0.001 |
| Total Population According to CVD | ||||
|---|---|---|---|---|
| All OSA Patients (n = 2127) | OSA-CVD (−) Group (n = 1718) | OSA-CVD (+) Group (n = 409) | p Value | |
| Nocturnal Symptoms | ||||
| Snoring | 2084 (98%) | 1683 (98%) | 404 (99%) | 0.413 |
| Witnessed apneas | 2084 (98%) | 1667 (97%) | 405 (99%) | 0.042 |
| Athens Insomnia Scale Score | 8 ± 5 | 8 ± 5 | 8 ± 5 | 0.79 |
| Athens Insomnia Scale Score ≥ 6 (%) | 1426 (67%) | 1168 (68%) | 249 (61%) | 0.12 |
| Frequentawakenings | 1723 (81%) | 1374 (80%) | 356 (87%) | 0.001 |
| Nocturia | 1617 (76%) | 1271 (74%) | 344 (84%) | <0.001 |
| Diurnalsymptoms | ||||
| ESS score | 11 ± 5 | 10 ± 5 | 11 ± 5 | 0.39 |
| ESS ≥ 10 | 1170 (55%) | 928 (54%) | 233 (57%) | 0.26 |
| ESS ≥ 16 | 383 (18%) | 309 (18%) | 74 (18%) | 0.94 |
| Morningheadache | 404 (19%) | 326 (19%) | 82 (20%) | 0.45 |
| Driving Problems | 447 (21%) | 361 (21%) | 82 (20%) | 0.69 |
| BDI score | 8 (4, 14) | 8 (4, 13) | 10 (4, 13) | <0.001 |
| BDI ≥ 10 | 915 (43%) | 721 (42%) | 213 (52%) | 0.005 |
| B | S.E. | p-Value | OR (95%CI) | |
|---|---|---|---|---|
| Males versus Females | 0.506 | 0.207 | 0.014 | 1.659 (1.106–2.487) |
| Age > 60 years | 0.905 | 0.156 | <0.001 | 2.471 (1.819–3.357) |
| Body mass index ≥ 30 | 0.367 | 0.197 | 0.062 | 1.443 (0.982–2.122) |
| Smoking (Current/Former) | 0.295 | 0.181 | 0.103 | 1.342 (0.942–1.913) |
| Hypertension | 0.898 | 0.166 | <0.001 | 2.453 (1.771–3.399) |
| Type 2 diabetes | 0.418 | 0.176 | <0.018 | 1.519 (1.075–2.146) |
| Dyslipidaemia | 0.215 | 0.151 | 0.154 | 1.240 (0.922–1.668) |
| Moderate/Severe OSA | 0.658 | 0.349 | 0.03 | 1.931 (0.975–3.826) |
| BDI ≥ 10 | 0.409 | 0.154 | 0.008 | 1.505 (1.113–2.036) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouloukaki, I.; Fanaridis, M.; Stathakis, G.; Ermidou, C.; Kallergis, E.; Moniaki, V.; Mauroudi, E.; Schiza, S.E. Characteristics of Patients with Obstructive Sleep Apnea at High Risk for Cardiovascular Disease. Medicina 2021, 57, 1265. https://doi.org/10.3390/medicina57111265
Bouloukaki I, Fanaridis M, Stathakis G, Ermidou C, Kallergis E, Moniaki V, Mauroudi E, Schiza SE. Characteristics of Patients with Obstructive Sleep Apnea at High Risk for Cardiovascular Disease. Medicina. 2021; 57(11):1265. https://doi.org/10.3390/medicina57111265
Chicago/Turabian StyleBouloukaki, Izolde, Michail Fanaridis, Georgios Stathakis, Christina Ermidou, Eleftherios Kallergis, Violeta Moniaki, Eleni Mauroudi, and Sophia E. Schiza. 2021. "Characteristics of Patients with Obstructive Sleep Apnea at High Risk for Cardiovascular Disease" Medicina 57, no. 11: 1265. https://doi.org/10.3390/medicina57111265
APA StyleBouloukaki, I., Fanaridis, M., Stathakis, G., Ermidou, C., Kallergis, E., Moniaki, V., Mauroudi, E., & Schiza, S. E. (2021). Characteristics of Patients with Obstructive Sleep Apnea at High Risk for Cardiovascular Disease. Medicina, 57(11), 1265. https://doi.org/10.3390/medicina57111265







