Vitamin D Deficiency Is a Potential Risk for Blood Pressure Elevation and the Development of Hypertension
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measurements
2.3. Laboratory Analysis and Statistics
3. Results
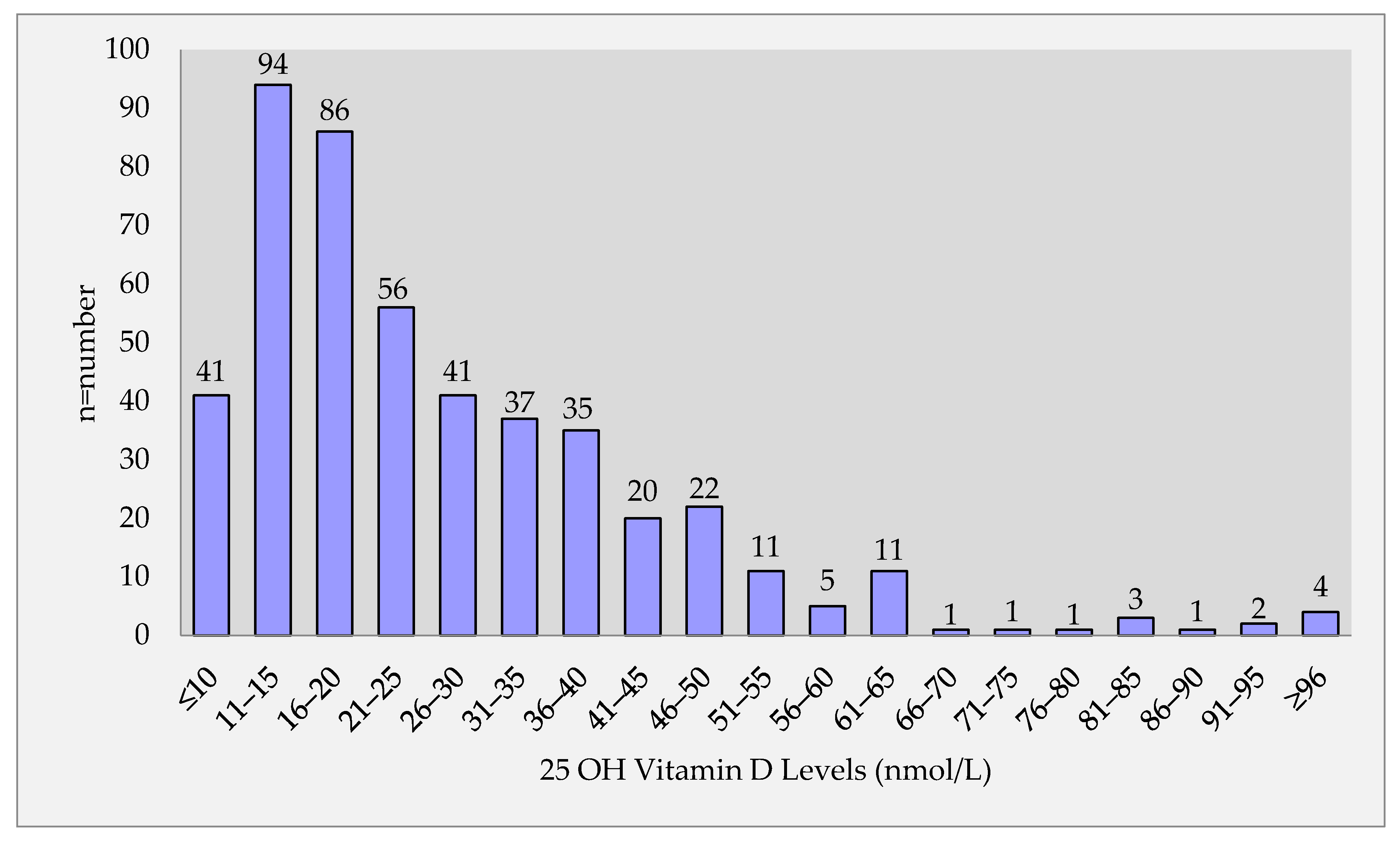
3.1. Associations of 25-OH Vitamin D and Normal-High Normal Blood Pressure
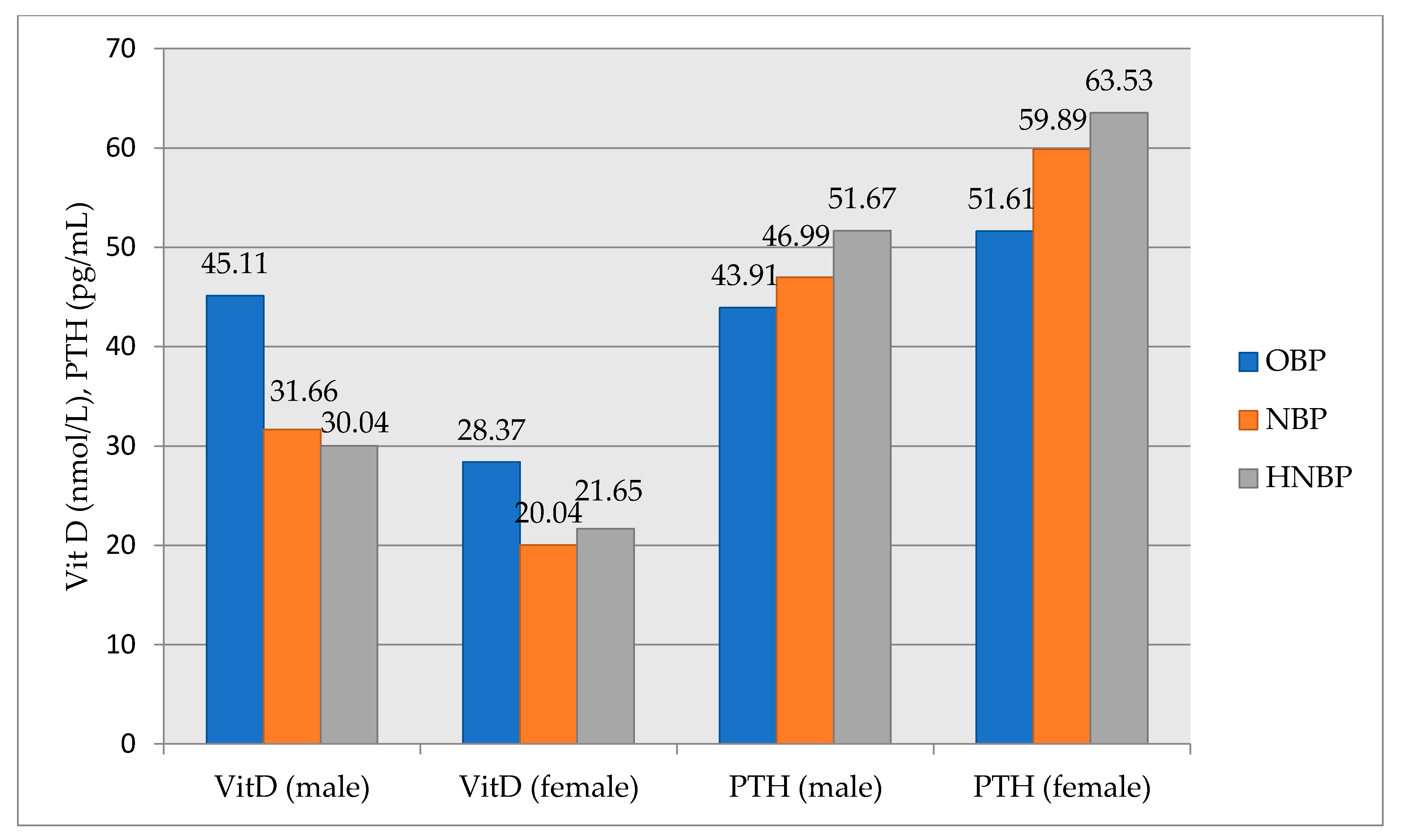
3.2. Associations of Other Metabolic Parameters and Non-Optimal Blood Pressure
3.3. Development of Incident Hypertension and Other Chronic Diseases during Follow-Up
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mithal, A.; Wahl, D.A.; Bonjour, J.P.; Burckhardt, P.; Dawson-Hughes, B.; Eisman, J.A.; El-Hajj Fuleihan, G.; Josse, R.G.; Lips, P.; Morales-Torres, J. IOF Committee of Scientific Advisors (CSA) Nutrition Working Group. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos Int. 2009, 20, 1807–1820. [Google Scholar] [CrossRef] [Green Version]
- Hekimsoy, Z.; Dinç, G.; Kafesçiler, S.; Onur, E.; Güvenç, Y.; Pala, T.; Güçlü, F.; Ozmen, B. Vitamin D status among adults in the Aegean region of Turkey. BMC Public Health 2010, 10, 782–789. [Google Scholar] [CrossRef] [Green Version]
- Cigerli, O.; Parildar, H.; Unal, A.D.; Tarcin, O.; Erdal, R.; GuvenerDemirag, N. Vitamin D deficiency is a problem for adult out-patients? A university hospital sample in Istanbul, Turkey. Public Health Nutr. 2013, 16, 1306–1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norman, P.E.; Powell, J.T. Vitamin D and cardiovascular disease. Circ. Res. 2014, 114, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Sassi, F.; Tamone, C.; D’Amelio, P. Vitamin D: Nutrient, Hormone, and Immunomodulator. Nutrients 2018, 10, 1656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costantini, E.; Sinjari, B.; Piscopo, F.; Porreca, A.; Reale, M.; Caputi, S.; Murmura, G. Evaluation of Salivary Cytokines and Vitamin D Levels in Periodontopathic Patients. Int. J. Mol. Sci. 2020, 21, 2669. [Google Scholar] [CrossRef] [Green Version]
- Mills, K.T.; Bundy, J.D.; Kelly, T.N.; Reed, J.E.; Kearney, P.M.; Reynolds, K.; Chen, J.; He, J. Global Disparities of Hypertension Prevalence and Control: A Systematic Analysis of Population-Based Studies From 90 Countries. Circulation 2016, 134, 441–450. [Google Scholar] [CrossRef]
- Barbarawi, M.; Kheiri, B.; Zayed, Y.; Barbarawi, O.; Dhillon, H.; Swaid, B.; Yelangi, A.; Sundus, S.; Bachuwa, G.; Alkotob, M.L.; et al. Vitamin D Supplementation and Cardiovascular Disease Risks in More Than 83,000 Individuals in 21 Randomized Clinical Trials: A Meta-analysis. JAMA Cardiol. 2019, 4, 765–776. [Google Scholar] [CrossRef]
- Vaidya, A.; Williams, J.S. The relationship between vitamin D and the renin-angiotensin system in the pathophysiology of hypertension, kidney disease, and diabetes. Metabolism 2012, 61, 450–458. [Google Scholar] [CrossRef] [Green Version]
- Egan, B.M.; Stevens-Fabry, S. Prehypertension--prevalence, health risks, and management strategies. Nat. Rev. Cardiol. 2015, 12, 289–300. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; AgabitiRosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J. Hypertens. 2018, 36, 1953–2041. [Google Scholar] [PubMed] [Green Version]
- Vimaleswaran, K.S.; Cavadino, A.; Berry, D.J.; LifeLines Cohort Study Investigators; Jorde, R.; Dieffenbach, A.K.; Lu, C.; Alves, A.C.; Heerspink, H.J.; Tikkanen, E.; et al. Association of vitamin D status with arterial blood pressure and hypertension risk: A mendelian randomisation study. Lancet Diabetes Endocrinol. 2014, 2, 719–729. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.K.; Il Kang, M.; Won Oh, K.; Kwon, H.S.; Lee, J.H.; Lee, W.C.; Yoon, K.H.; Son, H.Y. The association of serum vitamin D level with presence of metabolic syndrome and hypertension in middle-aged Korean subjects. Clin. Endocrinol. 2010, 73, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Ford, E.S.; Li, C.; Kris-Etherton, P.M.; Etherton, T.D.; Balluz, L.S. Independent associations of serum concentrations of 25-hydroxyvitamin D and parathyroid hormone with blood pressure among US adults. J. Hypertens. 2010, 28, 1821–1828. [Google Scholar] [CrossRef] [PubMed]
- Kunutsor, S.K.; Apekey, T.A.; Steur, M. Vitamin D and risk of future hypertension: Meta-analysis of 283,537 participants. Eur. J. Epidemiol. 2013, 28, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Jorde, R.; Figenschau, Y.; Emaus, N.; Hutchinson, M.; Grimnes, G. Serum 25-hydroxyvitamin D levels are strongly related to systolic blood pressure but do not predict future hypertension. Hypertension 2010, 55, 792–798. [Google Scholar] [CrossRef] [Green Version]
- Sabanayagam, C.; Shankar, A.; Somasundaram, S. Serum vitamin D level and prehypertension among subjects free of hypertension. Kidney Blood Press. Res. 2012, 35, 106–113. [Google Scholar] [CrossRef] [Green Version]
- Alpdemir, M.; Alpdemir, M.F. Meta Analysis Vitamin D deficiency status in Turkey: A meta-analysis. Int. J. Med. Biochem. 2019, 2, 118–131. [Google Scholar]
- Cashman, K.D.; Dowling, K.G.; Škrabáková, Z.; Gonzalez-Gross, M.; Valtueña, J.; De Henauw, S.; Moreno, L.; Damsgaard, C.T.; Michaelsen, K.F.; Mølgaard, C.; et al. Vitamin D deficiency in Europe: Pandemic? Am. J. Clin. Nutr. 2016, 103, 1033–1044. [Google Scholar] [CrossRef] [Green Version]
- Becher, U.M.; Endtmann, C.; Tiyerili, V.; Nickenig, G.; Werner, N. Endothelial damage and regeneration: The role of the renin-angiotensin-aldosterone system. Curr. Hypertens. Rep. 2011, 13, 86–92. [Google Scholar] [CrossRef]
- Briet, M.; Schiffrin, E.L. Vascular actions of aldosterone. J. Vasc. Res. 2013, 50, 89–99. [Google Scholar] [CrossRef]
- Li, Y.C.; Kong, J.; Wei, M.; Chen, Z.F.; Liu, S.Q.; Cao, L.P. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J. Clin. Investig. 2002, 110, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Forman, J.P.; Williams, J.S.; Fisher, N.D. Plasma 25-hydroxyvitamin D and regulation of the renin-angiotensin system in humans. Hypertension 2010, 55, 1283–1288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santoro, D.; Caccamo, D.; Lucisano, S.; Buemi, M.; Sebekova, K.; Teta, D.; De Nicola, L. Interplay of vitamin D, erythropoiesis, and the renin-angiotensin system. Biomed. Res. Int. 2015, 2015, 145828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ullah, M.I.; Uwaifo, G.I.; Nicholas, W.C.; Koch, C.A. Does vitamin d deficiency cause hypertension? Current evidence from clinical studies and potential mechanisms. Int. J. Endocrinol. 2010, 2010, 579640. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.C.; Qiao, G.; Uskokovic, M.; Xiang, W.; Zheng, W.; Kong, J. Vitamin D: A negative endocrine regulator of the renin-angiotensin system and blood pressure. J. Steroid Biochem. Mol. Biol. 2004, 89, 387–392. [Google Scholar] [CrossRef]
- Vaidya, A.; Sun, B.; Forman, J.P.; Hopkins, P.N.; Brown, N.J.; Kolatkar, N.S.; Williams, G.H.; Williams, J.S. The Fok1 vitamin D receptor gene polymorphism is associated with plasma renin activity in Caucasians. Clin. Endocrinol. 2011, 74, 783–790. [Google Scholar] [CrossRef]
- Szymczak-Pajor, I.; Śliwińska, A. Analysis of Association between Vitamin D Deficiency and Insulin Resistance. Nutrients 2019, 11, 794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carthy, E.P.; Yamashita, W.; Hsu, A.; Ooi, B.S. 1,25-Dihydroxyvitamin D3 and rat vascular smooth muscle cell growth. Hypertension 1989, 13, 954–959. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Sun, Y.; Agrawal, D.K. Vitamin D deficiency and essential hypertension. J. Am. Soc. Hypertens. 2015, 9, 885–901. [Google Scholar] [CrossRef] [Green Version]
- Vaidya, A.; Sun, B.; Larson, C.; Forman, J.P.; Williams, J.S. Vitamin D3 therapy corrects the tissue sensitivity to angiotensin ii akin to the action of a converting enzyme inhibitor in obese hypertensives: An interventional study. J. Clin. Endocrinol. Metab. 2012, 97, 2456–2465. [Google Scholar] [CrossRef] [PubMed]
- Arora, P.; Song, Y.; Dusek, J.; Plotnikoff, G.; Sabatine, M.S.; Cheng, S.; Valcour, A.; Swales, H.; Taylor, B.; Carney, E.; et al. Vitamin D therapy in individuals with prehypertension or hypertension: The DAYLIGHT trial. Circulation 2015, 131, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.L.; Vanwoerkom, R.C.; Horne, B.D.; Bair, T.L.; May, H.T.; Lappé, D.L.; Muhlestein, J.B. Parathyroid hormone, vitamin D, renal dysfunction, and cardiovascular disease: Dependent or independent risk factors? Am. Heart J. 2011, 162, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Bosworth, C.; Sachs, M.C.; Duprez, D.; Hoofnagle, A.N.; Ix, J.H.; Jacobs, D.R.; Peralta, C.A.; Siscovick, D.S.; Kestenbaum, B.; de Boer, I.H. Parathyroid hormone and arterial dysfunction in the multi-ethnic study of atherosclerosis. Clin. Endocrinol. 2013, 79, 429–436. [Google Scholar] [CrossRef]
- Luigi, P.; Chiara, F.M.; Laura, Z.; Cristiano, M.; Giuseppina, C.; Luciano, C.; Giuseppe, P.; Sabrina, C.; Susanna, S.; Antonio, C.; et al. Arterial Hypertension, Metabolic Syndrome and Subclinical Cardiovascular Organ Damage in Patients with Asymptomatic Primary Hyperparathyroidism before and after Parathyroidectomy: Preliminary Results. Int. J. Endocrinol. 2012, 2012, 408295. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhang, D.Z. Circulating parathyroid hormone and risk of hypertension: A meta-analysis. Clin. Chim. Acta 2018, 482, 40–45. [Google Scholar] [CrossRef]
- Van Ballegooijen, A.J.; Kestenbaum, B.; Sachs, M.C.; de Boer, I.H.; Siscovick, D.S.; Hoofnagle, A.N.; Ix, J.H.; Visser, M.; Brouwer, I.A. Association of 25-hydroxyvitamin D and parathyroid hormone with incident hypertension: MESA (Multi-Ethnic Study of Atherosclerosis). J. Am. Coll. Cardiol. 2014, 63, 1214–1222. [Google Scholar] [CrossRef] [Green Version]
- Tomaschitz, A.; Pilz, S.; Ritz, E.; Grammer, T.; Drechsler, C.; Boehm, B.O.; März, W. Independent association between 1,25-dihydroxyvitamin D, 25-hydroxyvitamin D and the renin-angiotensin system: The Ludwigshafen Risk and Cardiovascular Health (LURIC) study. Clin. Chim. Acta 2010, 411, 1354–1360. [Google Scholar] [CrossRef]
- Pirro, M.; Manfredelli, M.R.; Helou, R.S.; Scarponi, A.M.; Schillaci, G.; Bagaglia, F.; Melis, F.; Mannarino, E. Association of parathyroid hormone and 25-OH-vitamin D levels with arterial stiffness in postmenopausal women with vitamin D insufficiency. J. Atheroscler. Thromb. 2012, 19, 924–931. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Kim, J. Association of Serum 25-Hydroxyvitamin D and Parathyroid Hormone with Hypertension in Middle-Aged and Older Korean Adults. Am. J. Hypertens. 2016, 29, 96–103. [Google Scholar] [CrossRef] [Green Version]
- Melguizo-Rodríguez, L.; Costela-Ruiz, V.J.; García-Recio, E.; De Luna-Bertos, E.; Ruiz, C.; Illescas-Montes, R. Role of Vitamin D in the Metabolic Syndrome. Nutrients 2021, 13, 830. [Google Scholar] [CrossRef]
- Slominski, A.T.; Kim, T.K.; Li, W.; Postlethwaite, A.; Tieu, E.W.; Tang, E.K.Y.; Tuckey, R.C. Detection of novel CYP11A1-derived secosteroids in the human epidermis and serum and pig adrenal gland. Sci. Rep. 2015, 8, 14875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkinson, C.; Desai, R.; Slominski, A.T.; Tuckey, R.C.; Hewison, M.; Handelsman, D.J. Simultaneous measurement of 13 circulating vitamin D3 and D2 mono and dihydroxy metabolites using liquid chromatography mass spectrometry. Clin. Chem. Lab. Med. 2021, 59, 1642–1652. [Google Scholar] [CrossRef] [PubMed]
- Slominski, R.M.; Raman, C.; Elmets, C.; Jetten, A.M.; Slominski, A.T.; Tuckey, R.C. The significance of CYP11A1 expression in skin physiology and pathology. Mol. Cell. Endocrinol. 2021, 530, 111238. [Google Scholar] [CrossRef]
- Alvarez, J.A.; Ashraf, A. Role of vitamin d in insulin secretion and insulin sensitivity for glucose homeostasis. Int. J. Endocrinol. 2010, 2010, 351385. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| OBP (n = 202) | NOBP (n = 289) | ||||
|---|---|---|---|---|---|
| Mean | SS | Mean | SS | p | |
| Age, years | 34.32 | 8.50 | 37.81 | 9.87 | <0.001 |
| SBP, mm Hg | 107.65 | 6.61 | 130.53 | 6.53 | <0.001 |
| DBP, mmHg | 67.53 | 6.19 | 81.73 | 5.90 | <0.001 |
| MABP, mmHg | 80.90 | 5.74 | 98.00 | 5.53 | <0.001 |
| Height, cm | 164.90 | 8.54 | 164.90 | 9.74 | = 0.998 |
| Weight, kg | 68.32 | 12.41 | 80.69 | 14.43 | <0.001 |
| Body mass index, kg/m2 | 25.10 | 4.50 | 29.73 | 5.14 | <0.001 |
| Waist circumference, cm | 83.82 | 10.26 | 95.57 | 11.06 | <0.001 |
| Fasted glucose, mg/dL | 91.06 | 11.04 | 95.23 | 11.84 | <0.001 |
| Creatinine, mg/dL | 0.67 | 0.15 | 0.66 | 0.14 | 0.871 |
| LDL, mg/dL | 115.23 | 31.24 | 122.85 | 36.39 | = 0.017 |
| Triglyceride, mg/dL | 99.04 | 55.84 | 151.23 | 133.01 | <0.001 |
| Total cholesterol, mg/dL | 184.73 | 37.15 | 197.59 | 44.39 | <0.001 |
| Uric acid, mg/dL | 4.06 | 0.99 | 4.70 | 1.30 | <0.001 |
| AST, U/L | 20.24 | 6.05 | 23.50 | 11.25 | <0.001 |
| ALT, U/L | 18.77 | 10.39 | 26.45 | 22.83 | <0.001 |
| GGT, U/L | 19.45 | 14.70 | 29.26 | 26.31 | <0.001 |
| ALP, U/L | 72.92 | 24.28 | 81.48 | 25.24 | <0.001 |
| Albumin, g/L | 4.36 | 0.31 | 4.32 | 0.29 | = 0.216 |
| Calcium, mg/dL | 9.57 | 0.50 | 9.60 | 0.48 | = 0.518 |
| Phosphor, mg/dL | 3.42 | 0.56 | 3.35 | 0.59 | = 0.258 |
| PTH, pg/mL | 49.60 | 23.98 | 58.08 | 26.33 | <0.001 |
| Vitamin D (25-hydroxyvitamin D), nmol/L | 32.53 | 31.50 | 24.41 | 14.40 | <0.001 |
| HbA1c, % | 5.33 | 0.46 | 5.52 | 0.39 | <0.001 |
| Fasted insulin, mU/L | 6.96 | 7.13 | 9.58 | 7.61 | <0.001 |
| HOMA-IR | 1.60 | 1.74 | 2.32 | 2.08 | <0.001 |
| C peptide, ng/mL | 2.32 | 0.92 | 3.16 | 1.68 | <0.001 |
| FT3, ng/dL | 3.23 | 0.38 | 3.22 | 0.40 | = 0.246 |
| FT4, ng/dL | 0.84 | 0.13 | 0.82 | 0.15 | = 0.330 |
| TSH, mIU/L | 1.81 | 1.31 | 1.95 | 1.32 | = 0.238 |
| CRP, mg/L | 0.96 | 2.17 | 2.08 | 4.05 | <0.001 |
| p | OR | %95 Confidence Interval | ||
|---|---|---|---|---|
| Low | High | |||
| Age | 0.047 | 1.024 | 1.000 | 1.048 |
| 25 (OH)vitamin D | 0.021 | 0.985 | 0.972 | 0.998 |
| HOMA-IR | 0.047 | 1.229 | 1.002 | 1.507 |
| BMI | 0.000 | 1.199 | 1.133 | 1.270 |
| Constant | 0.000 | 0.005 | ||
| OBP (n = 202) | NOBP (n = 289) | p-Value | |
|---|---|---|---|
| Development of HT | 18 | 61 | <0.001 |
| Development of DM | 15 | 37 | =0.07 |
| Development of CAD | 11 | 21 | =0.46 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karadeniz, Y.; Özpamuk-Karadeniz, F.; Ahbab, S.; Ataoğlu, E.; Can, G. Vitamin D Deficiency Is a Potential Risk for Blood Pressure Elevation and the Development of Hypertension. Medicina 2021, 57, 1297. https://doi.org/10.3390/medicina57121297
Karadeniz Y, Özpamuk-Karadeniz F, Ahbab S, Ataoğlu E, Can G. Vitamin D Deficiency Is a Potential Risk for Blood Pressure Elevation and the Development of Hypertension. Medicina. 2021; 57(12):1297. https://doi.org/10.3390/medicina57121297
Chicago/Turabian StyleKaradeniz, Yusuf, Fatma Özpamuk-Karadeniz, Süleyman Ahbab, Esra Ataoğlu, and Günay Can. 2021. "Vitamin D Deficiency Is a Potential Risk for Blood Pressure Elevation and the Development of Hypertension" Medicina 57, no. 12: 1297. https://doi.org/10.3390/medicina57121297
APA StyleKaradeniz, Y., Özpamuk-Karadeniz, F., Ahbab, S., Ataoğlu, E., & Can, G. (2021). Vitamin D Deficiency Is a Potential Risk for Blood Pressure Elevation and the Development of Hypertension. Medicina, 57(12), 1297. https://doi.org/10.3390/medicina57121297






