Platelet-Rich Plasma-Releasate (PRPr) for the Treatment of Discogenic Low Back Pain Patients: Long-Term Follow-Up Survey
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Preparation of PRP Releasate (PRPr)
2.3. Follow-Up Survey
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Follow-Up Subjects
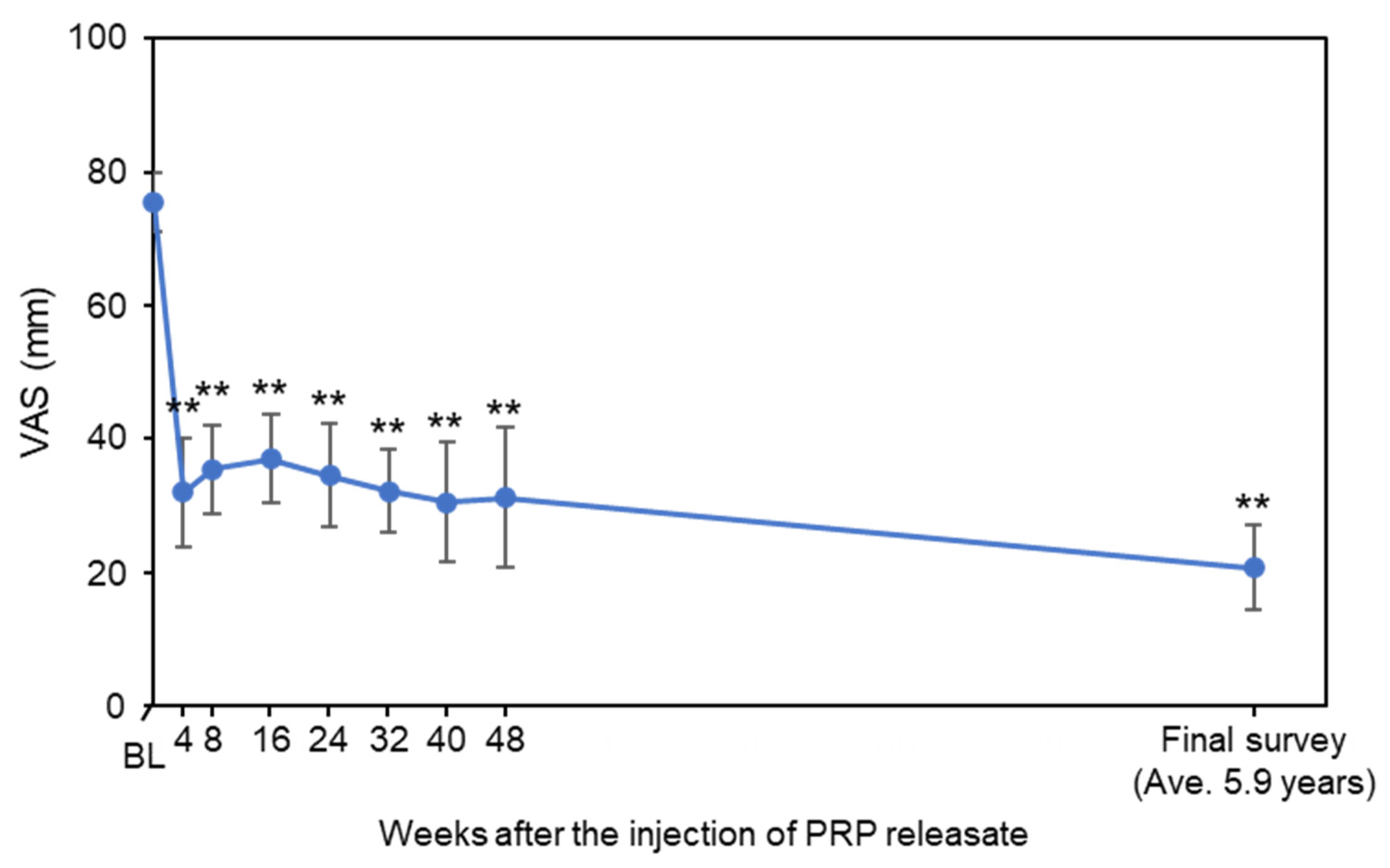
3.2. Assessment of Low Back Pain
3.3. Change in Disc Height
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Knezevic, N.N.; Candido, K.D.; Vlaeyen, J.W.S.; Van Zundert, J.; Cohen, S.P. Low back pain. Lancet 2021, 398, 78–92. [Google Scholar] [CrossRef]
- Chen, S.; Chen, M.; Wu, X.; Lin, S.; Tao, C.; Cao, H.; Shao, Z.; Xiao, G. Global, regional and national burden of low back pain 1990–2019: A systematic analysis of the Global Burden of Disease study 2019. J. Orthop. Transl. 2022, 32, 49–58. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Cheung, K.M.; Karppinen, J.; Chan, D.; Ho, D.W.; Song, Y.Q.; Sham, P.; Cheah, K.S.; Leong, J.C.; Luk, K.D. Prevalence and pattern of lumbar magnetic resonance imaging changes in a population study of one thousand forty-three individuals. Spine 2009, 34, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Samartzis, D.; Karppinen, J.; Mok, F.; Fong, D.Y.; Luk, K.D.; Cheung, K.M. A population-based study of juvenile disc degeneration and its association with overweight and obesity, low back pain, and diminished functional status. J. Bone Jt. Surg. 2011, 93, 662–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teraguchi, M.; Yoshimura, N.; Hashizume, H.; Muraki, S.; Yamada, H.; Minamide, A.; Oka, H.; Ishimoto, Y.; Nagata, K.; Kagotani, R.; et al. Prevalence and distribution of intervertebral disc degeneration over the entire spine in a population-based cohort: The Wakayama Spine Study. Osteoarthr. Cartil. 2014, 22, 104–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teraguchi, M.; Yoshimura, N.; Hashizume, H.; Muraki, S.; Yamada, H.; Oka, H.; Minamide, A.; Nakagawa, H.; Ishimoto, Y.; Nagata, K.; et al. The association of combination of disc degeneration, end plate signal change, and Schmorl node with low back pain in a large population study: The Wakayama Spine Study. Spine J. 2015, 15, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Hoyland, J.A.; Le Maitre, C.; Freemont, A.J. Investigation of the role of IL-1 and TNF in matrix degradation in the intervertebral disc. Rheumatology 2008, 47, 809–814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Maitre, C.L.; Freemont, A.J.; Hoyland, J.A. The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res. Ther. 2005, 7, R732–R745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abe, Y.; Akeda, K.; An, H.S.; Aoki, Y.; Pichika, R.; Muehleman, C.; Kimura, T.; Masuda, K. Proinflammatory cytokines stimulate the expression of nerve growth factor by human intervertebral disc cells. Spine 2007, 32, 635–642. [Google Scholar] [CrossRef] [Green Version]
- García-Cosamalón, J.; Del Valle, M.E.; Calavia, M.G.; García-Suárez, O.; Muñiz, A.J.L.; Otero, J.; Vega, J.A. Intervertebral disc, sensory nerves and neurotrophins: Who is who in discogenic pain? J. Anat. 2010, 217, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Yamada, J.; Akeda, K.; Sano, T.; Iwasaki, T.; Takegami, N.; Sudo, A. Expression of Glial Cell Line-Derived Neurotrophic Factor in the Human Intervertebral Disc. Spine 2020, 45, E768–E775. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.X.; Hu, Y.G. Imaging Analysis of the High-Intensity Zone on Lumbar Spine Magnetic Resonance Images: Classification, Features and Correlation with Low Back Pain. J. Pain Res. 2021, 14, 2981–2989. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.X.; Hou, Z.T.; Hu, Y.G. Anterior High-Intensity Zone in Lumbar Discs: Prevalence and Association with Low Back Pain. Pain Med. 2020, 21, 2111–2116. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Hou, S.; Wu, W.; Zhang, C.; Yang, Y. The pathogenesis and clinical significance of a high-intensity zone (HIZ) of lumbar intervertebral disc on MR imaging in the patient with discogenic low back pain. Eur. Spine J. 2006, 15, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Videman, T.; Nurminen, M. The occurrence of anular tears and their relation to lifetime back pain history: A cadaveric study using barium sulfate discography. Spine 2004, 29, 2668–2676. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Akeda, K.; Miyazaki, S.; Yamada, J.; Muehleman, C.; Miyamoto, K.; Asanuma, Y.A.; Asanuma, K.; Fujiwara, T.; Lenz, M.E.; et al. NF-kB decoy oligodeoxynucleotide preserves disc height in a rabbit anular-puncture model and reduces pain induction in a rat xenograft-radiculopathy model. Eur. Cells Mater. 2021, 41, 90–109. [Google Scholar] [CrossRef] [PubMed]
- Masuda, K.; Imai, Y.; Okuma, M.; Muehleman, C.; Nakagawa, K.; Akeda, K.; Thonar, E.; Andersson, G.; An, H.S. Osteogenic protein-1 injection into a degenerated disc induces the restoration of disc height and structural changes in the rabbit anular puncture model. Spine 2006, 31, 742–754. [Google Scholar] [CrossRef] [PubMed]
- Chujo, T.; An, H.S.; Akeda, K.; Miyamoto, K.; Muehleman, C.; Attawia, M.; Andersson, G.; Masuda, K. Effects of growth differentiation factor-5 on the intervertebral disc—In vitro bovine study and in vivo rabbit disc degeneration model study. Spine 2006, 31, 2909–2917. [Google Scholar] [CrossRef] [PubMed]
- Sakai, D.; Schol, J.; Watanabe, M. Clinical Development of Regenerative Medicine Targeted for Intervertebral Disc Disease. Medicina 2022, 58, 267. [Google Scholar] [CrossRef]
- An, H.S.; Masuda, K.; Cs-Szabo, G.; Zhang, Y.; Chee, A.; Andersson, G.B.; Im, H.J.; Thonar, E.J.; Kwon, Y.M. Biologic repair and regeneration of the intervertebral disk. J. Am. Acad. Orthop. Surg. 2011, 19, 450–452. [Google Scholar] [CrossRef] [PubMed]
- Masuda, K.; An, H.S. Prevention of disc degeneration with growth factors. Eur. Spine J. 2006, 15 (Suppl. 3), S422–S432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masala, S.; Salimei, F.; Lacchè, A.; Marcia, S.; Massari, F. Overview on Percutaneous Therapies of Disc Diseases. Medicina 2019, 55, 471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Everts, P.; Onishi, K.; Jayaram, P.; Lana, J.F.; Mautner, K. Platelet-Rich Plasma: New Performance Understandings and Therapeutic Considerations in 2020. Int. J. Mol. Sci. 2020, 21, 7794. [Google Scholar] [CrossRef] [PubMed]
- Nurden, A.T. Platelets, inflammation and tissue regeneration. Thromb. Haemost. 2011, 105 (Suppl. 6), S13–S33. [Google Scholar] [CrossRef] [PubMed]
- Gilbertie, J.M.; Schaer, T.P.; Schubert, A.G.; Jacob, M.E.; Menegatti, S.; Lavoie, R.A.; Schnabel, L.V. Platelet-rich plasma lysate displays antibiofilm properties and restores antimicrobial activity against synovial fluid biofilms in vitro. J. Orthop. Res. 2020, 38, 1365–1374. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Guo, Y.; Kuss, M.; Shi, W.; Aldrich, A.L.; Untrauer, J.; Kielian, T.; Duan, B. Platelet-Rich Plasma for the Treatment of Tissue Infection: Preparation and Clinical Evaluation. Tissue Eng. Part B Rev. 2019, 25, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Rodeo, S.A. Platelet-Rich Plasma in Orthopaedic Surgery: A Critical Analysis Review. JBJS Rev. 2017, 5, e7. [Google Scholar] [CrossRef] [PubMed]
- Akeda, K.; Yamada, J.; Linn, E.T.; Sudo, A.; Masuda, K. Platelet-rich plasma in the management of chronic low back pain: A critical review. J. Pain Res. 2019, 12, 753–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muthu, S.; Jeyaraman, M.; Chellamuthu, G.; Jeyaraman, N.; Jain, R.; Khanna, M. Does the Intradiscal Injection of Platelet Rich Plasma Have Any Beneficial Role in the Management of Lumbar Disc Disease? Glob. Spine J. 2021, 2192568221998367. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Santiago, K.A.; Nguyen, J.T.; Solomon, J.L.; Lutz, G.E. Treatment of symptomatic degenerative intervertebral discs with autologous platelet-rich plasma: Follow-up at 5–9 years. Regen. Med. 2019, 14, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Tuakli-Wosornu, Y.A.; Terry, A.; Boachie-Adjei, K.; Harrison, J.R.; Gribbin, C.K.; LaSalle, E.E.; Nguyen, J.T.; Solomon, J.L.; Lutz, G.E. Lumbar Intradiskal Platelet-Rich Plasma (PRP) Injections: A Prospective, Double-Blind, Randomized Controlled Study. PM&R 2016, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Akeda, K.; Ohishi, K.; Masuda, K.; Bae, W.C.; Takegami, N.; Yamada, J.; Nakamura, T.; Sakakibara, T.; Kasai, Y.; Sudo, A. Intradiscal Injection of Autologous Platelet-Rich Plasma Releasate to Treat Discogenic Low Back Pain: A Preliminary Clinical Trial. Asian Spine J. 2017, 11, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Akeda, K.; Ohishi, K.; Takegami, N.; Sudo, T.; Yamada, J.; Fujiwara, T.; Niimi, R.; Matsumoto, T.; Nishimura, Y.; Ogura, T.; et al. Platelet-Rich Plasma Releasate versus Corticosteroid for the Treatment of Discogenic Low Back Pain: A Double-Blind Randomized Controlled Trial. J. Clin. Med. 2022, 11, 304. [Google Scholar] [CrossRef]
- Pfirrmann, C.W.; Metzdorf, A.; Zanetti, M.; Hodler, J.; Boos, N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine 2001, 26, 1873–1878. [Google Scholar] [CrossRef]
- Roland, M.; Morris, R. A study of the natural history of back pain. Part I: Development of a reliable and sensitive measure of disability in low-back pain. Spine 1983, 8, 141–144. [Google Scholar] [CrossRef]
- Ostelo, R.W.; Deyo, R.A.; Stratford, P.; Waddell, G.; Croft, P.; Von Korff, M.; Bouter, L.M.; de Vet, H.C. Interpreting change scores for pain and functional status in low back pain: Towards international consensus regarding minimal important change. Spine 2008, 33, 90–94. [Google Scholar] [CrossRef] [Green Version]
- Roach, K.E.; Brown, M.D.; Dunigan, K.M.; Kusek, C.L.; Walas, M. Test-retest reliability of patient reports of low back pain. J. Orthop. Sports Phys. Ther. 1997, 26, 253–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shafshak, T.S.; Elnemr, R. The Visual Analogue Scale Versus Numerical Rating Scale in Measuring Pain Severity and Predicting Disability in Low Back Pain. J. Clin. Rheumatol. 2021, 27, 282–285. [Google Scholar] [CrossRef]
- Roland, M.; Fairbank, J. The Roland—Morris Disability Questionnaire and the Oswestry Disability Questionnaire. Spine 2000, 25, 3115–3124. [Google Scholar] [CrossRef] [Green Version]
- Suzukamo, Y.; Fukuhara, S.; Kikuchi, S.; Konno, S.; Roland, M.; Iwamoto, Y.; Nakamura, T.; Committee on Science Project, Japanese Orthopaedic Association. Validation of the Japanese version of the Roland-Morris Disability Questionnaire. J. Orthop. Sci. 2003, 8, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Akeda, K.; Yamada, T.; Inoue, N.; Nishimura, A.; Sudo, A. Risk factors for lumbar intervertebral disc height narrowing: A population-based longitudinal study in the elderly. BMC Musculoskelet. Disord. 2015, 16, 344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obata, S.; Akeda, K.; Imanishi, T.; Masuda, K.; Bae, W.; Morimoto, R.; Asanuma, Y.; Kasai, Y.; Uchida, A.; Sudo, A. Effect of autologous platelet-rich plasma-releasate on intervertebral disc degeneration in the rabbit anular puncture model: A preclinical study. Arthritis Res. Ther. 2012, 14, R241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeLong, J.M.; Russell, R.P.; Mazzocca, A.D. Platelet-rich plasma: The PAW classification system. Arthrosc. J. Arthrosc. Relat. Surg. 2012, 28, 998–1009. [Google Scholar] [CrossRef] [PubMed]
- Gato-Calvo, L.; Hermida-Gomez, T.; Romero, C.R.; Burguera, E.F.; Blanco, F.J. Anti-Inflammatory Effects of Novel Standardized Platelet Rich Plasma Releasates on Knee Osteoarthritic Chondrocytes and Cartilage in vitro. Curr. Pharm. Biotechnol. 2019, 20, 920–933. [Google Scholar] [CrossRef] [PubMed]
- Fewtrell, M.S.; Kennedy, K.; Singhal, A.; Martin, R.M.; Ness, A.; Hadders-Algra, M.; Koletzko, B.; Lucas, A. How much loss to follow-up is acceptable in long-term randomised trials and prospective studies? Arch. Dis. Child. 2008, 93, 458–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Patient ID | Age | Gender | VAS | RDQ | Disc Level | MRI Phirmann’s Grade | X-ray at Final Follow-Up |
|---|---|---|---|---|---|---|---|
| #01 | 4X | M | 70 | 11 | L4/L5 | III | − |
| #02 | 2X | F | 80 | 12 | L4/L5 | III | + |
| #04 | 4X | M | 80 | 8 | L4/L5 | III | + |
| #05 | 2X | F | 80 | 17 | L4/L5 | IV | − |
| #06 | 3X | F | 50 | 8 | L4/L5 | III | + |
| #07 | 2X | M | 90 | 15 | L5/S1 | III | + |
| #08 | 4X | M | 70 | 13 | L5/S1 | III | + |
| #09 | 2X | F | 90 | 16 | L4/L5 | IV | + |
| #12 | 4X | M | 90 | 22 | L4/L5 | III | + |
| #13 | 2X | F | 80 | 10 | L4/L5 | III | − |
| #14 | 2X | F | 50 | 6 | L4/L5 | III | − |
| ≥30% Reduction | ≥50% Reduction | ≥75% Reduction | |
|---|---|---|---|
| VAS | 91% (10/11) | 64% (7/11) | 64% (7/11) |
| RDQ | 91% (10/11) | 91% (10/11) | 82% (9/11) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akeda, K.; Takegami, N.; Yamada, J.; Fujiwara, T.; Ohishi, K.; Tamaru, S.; Sudo, A. Platelet-Rich Plasma-Releasate (PRPr) for the Treatment of Discogenic Low Back Pain Patients: Long-Term Follow-Up Survey. Medicina 2022, 58, 428. https://doi.org/10.3390/medicina58030428
Akeda K, Takegami N, Yamada J, Fujiwara T, Ohishi K, Tamaru S, Sudo A. Platelet-Rich Plasma-Releasate (PRPr) for the Treatment of Discogenic Low Back Pain Patients: Long-Term Follow-Up Survey. Medicina. 2022; 58(3):428. https://doi.org/10.3390/medicina58030428
Chicago/Turabian StyleAkeda, Koji, Norihiko Takegami, Junichi Yamada, Tatsuhiko Fujiwara, Kohshi Ohishi, Satoshi Tamaru, and Akihiro Sudo. 2022. "Platelet-Rich Plasma-Releasate (PRPr) for the Treatment of Discogenic Low Back Pain Patients: Long-Term Follow-Up Survey" Medicina 58, no. 3: 428. https://doi.org/10.3390/medicina58030428
APA StyleAkeda, K., Takegami, N., Yamada, J., Fujiwara, T., Ohishi, K., Tamaru, S., & Sudo, A. (2022). Platelet-Rich Plasma-Releasate (PRPr) for the Treatment of Discogenic Low Back Pain Patients: Long-Term Follow-Up Survey. Medicina, 58(3), 428. https://doi.org/10.3390/medicina58030428






