Occurrence of Cryptosporidium spp. and Giardia spp. Infection in Humans in Latvia: Evidence of Underdiagnosed and Underreported Cases
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Fluorescent Microscopy Analyses
2.3. Data Analyses
3. Results
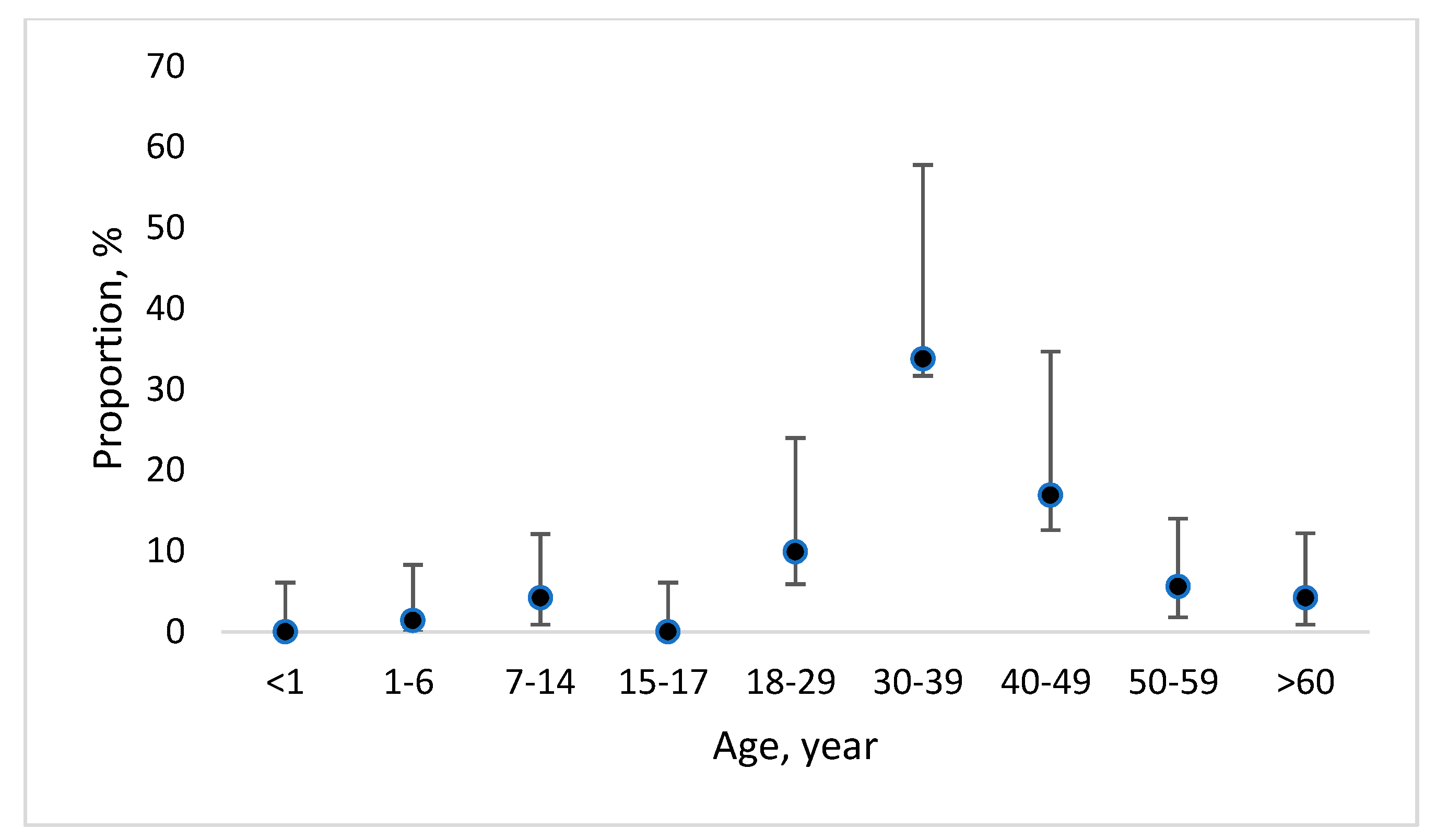
3.1. Long-Term Data on Officially Reported Cryptosporidiosis and Giardiasis Cases in Latvia
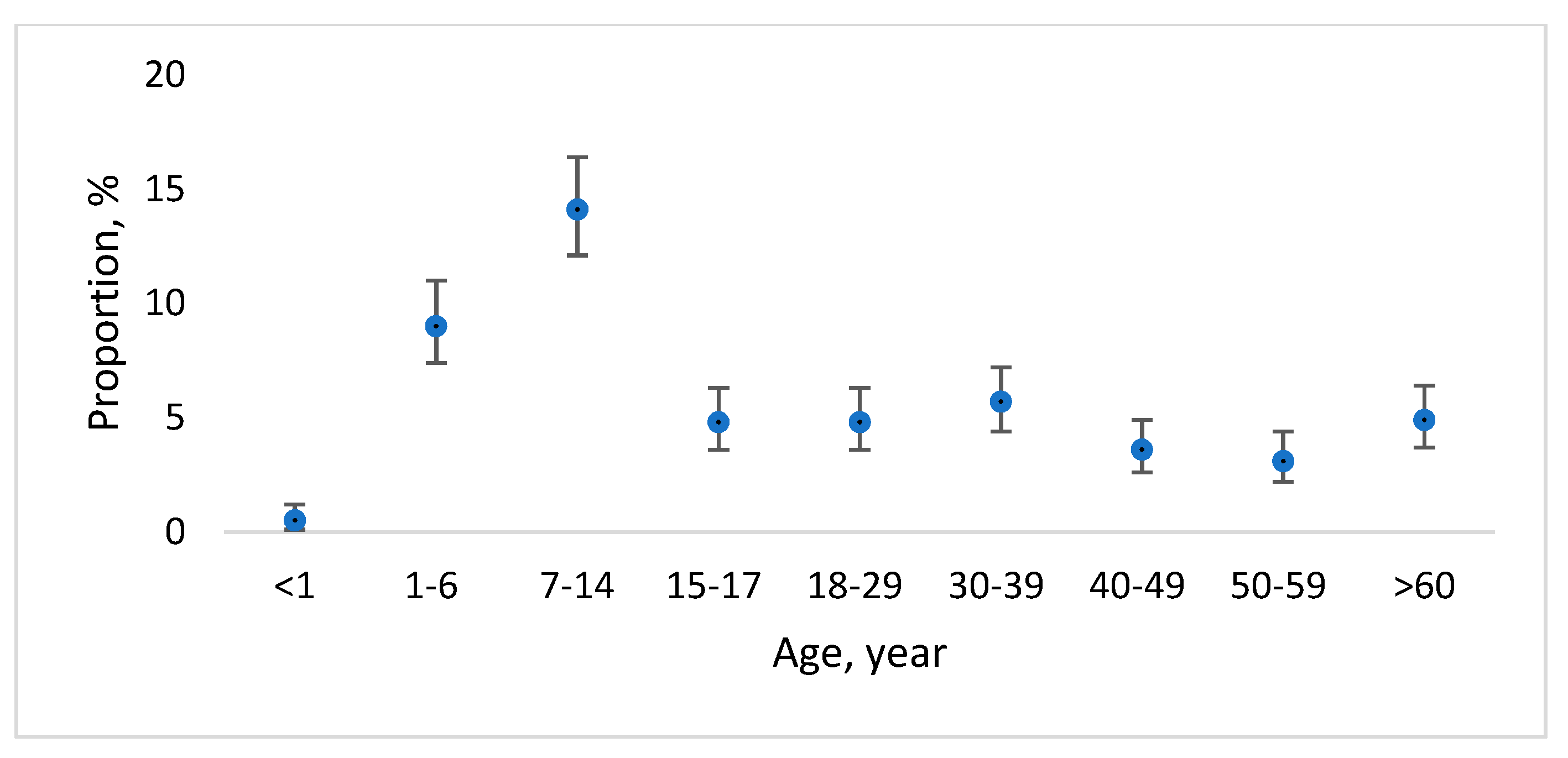
3.2. Cryptosporidium/Giardia Presence in Children within the Prospective Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Custodio, H. Protozoan parasites. Pediatr. Rev. 2016, 37, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Khalil, I.A.; Troeger, C.; Rao, P.C.; Blacker, B.F.; Brown, A.; Brewer, T.G.; Colombara, D.V.; De Hostos, E.L.; Engmann, C.; Guerrant, R.L.; et al. Morbidity, mortality, and long-term consequences associated with diarrhoea from Cryptosporidium infection in children younger than 5 years: A meta-analyses study. Lancet Glob. Health 2018, 6, e758–e768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotloff, K.L.; Nataro, J.P.; Blackwelder, W.C.; Nasrin, D.; Farag, T.H.; Panchalingam, S.; Wu, Y.; Sow, S.O.; Sur, D.; Breiman, R.F.; et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): A prospective, case-control study. Lancet 2013, 382, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Cacciò, S.M.; Thompson, R.A.; McLauchlin, J.; Smith, H.V. Unravelling Cryptosporidium and Giardia epidemiology. Trends Parasitol. 2005, 21, 430–437. [Google Scholar] [CrossRef]
- Savioli, L.; Smith, H.; Thompson, A. Giardia and Cryptosporidium join the ‘neglected diseases initiative’. Trends Parasitol. 2006, 22, 203–208. [Google Scholar] [CrossRef]
- Bouwknegt, M.; Devleesschauwer, B.; Graham, H.; Robertson, L.J.; van der Giessen, J.W. Prioritisation of food-borne parasites in Europe, 2016. Eurosurveillance 2018, 23, 17–00161. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Ryan, U.M.; Xiao, L. Genetic diversity and population structure of Cryptosporidium. Trends Parasitol. 2018, 34, 997–1011. [Google Scholar] [CrossRef]
- Bolland, S.J.; Zahedi, A.; Oskam, C.; Murphy, B.; Ryan, U. Cryptosporidium bollandi n. sp. (Apicomplexa: Cryptosporidiiae) from angelfish (Pterophyllum scalare) and Oscar fish (Astronotus ocellatus). Exp. Parasitol. 2020, 217, 107956. [Google Scholar] [CrossRef]
- Holubová, N.; Tůmová, L.; Sak, B.; Hejzlarová, A.; Konečný, R.; McEvoy, J.; Kváč, M. Description of Cryptosporidium ornithophilus n. sp. (Apicomplexa: Cryptosporidiidae) in farmed ostriches. Parasites Vectors 2020, 13, 1–17. [Google Scholar] [CrossRef]
- Dillingham, R.A.; Lima, A.A.; Guerrant, R.L. Cryptosporidiosis: Epidemiology and impact. Microbes Infect. 2002, 4, 1059–1066. [Google Scholar] [CrossRef]
- Thompson, R.A.; Monis, P. Giardia—From genome to proteome. Adv. Parasitol. 2012, 78, 57–95. [Google Scholar] [CrossRef]
- Hillman, A.; Ash, A.; Elliot, A.; Lymbery, A.; Perez, C.; Thompson, R.A. Confirmation of a unique species of Giardia, parasitic in the quenda (Isoodon obesulus). Int. J. Parasitol. Parasites Wildl. 2016, 5, 110–115. [Google Scholar] [CrossRef] [Green Version]
- Cacciò, S.M.; Lalle, M.; Svärd, S.G. Host specificity in the Giardia duodenalis species complex. Infect. Genet. Evol. 2018, 66, 335–345. [Google Scholar] [CrossRef]
- Mmbaga, B.T.; Houpt, E.R. Cryptosporidium and Giardia Infections in Children: A Review. Pediatr. Clin. N. Am. 2017, 64, 837–850. [Google Scholar] [CrossRef]
- Desai, N.T.; Sarkar, R.; Kang, G. Cryptosporidiosis: An under-recognized public health problem. Trop. Parasitol. 2012, 2, 91–98. [Google Scholar] [CrossRef] [Green Version]
- Rodney, D.A. Hunter’s Tropical Medicine and Emerging Infectious Diseases, 10th ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 707–711. [Google Scholar]
- Hörman, A.; Korpela, H.; Sutinen, J.; Wedel, H.; Hänninen, M.L. Meta-analysis in assessment of the prevalence and annual incidence of Giardia spp. and Cryptosporidium spp. infections in humans in the Nordic countries. Int. J. Parasitol. 2004, 34, 1337–1346. [Google Scholar] [CrossRef]
- Plutzer, J.; Lassen, B.; Jokelainen, P.; Djurković-Djaković, O.; Kucsera, I.; Dorbek-Kolin, E.; Šoba, B.; Sréter, T.; Imre, K.; Omeragić, J.; et al. Review of Cryptosporidium and Giardia in the eastern part of Europe, 2016. Eurosurveillance 2018, 23, 16–00825. [Google Scholar] [CrossRef]
- van der Giessen, J.; Deksne, G.; Gómez-Morales, M.A.; Troell, K.; Gomes, J.; Sotiraki, S.; Rozycki, M.; Kucsera, I.; Djurković-Djaković, O.; Robertson, L.J. Surveillance of foodborne parasitic diseases in Europe in a One Health approach. Parasite Epidemiol. Control 2021, 13, e00205. [Google Scholar] [CrossRef]
- O’Leary, J.K.; Sleator, R.D.; Lucey, B. Cryptosporidium spp. diagnosis and research in the 21st century. Food Waterborne Parasitol. 2021, 24, e00131. [Google Scholar] [CrossRef]
- Centre for Disease Prevention and Control of Latvia. Available online: www.spkc.gov.lv (accessed on 31 January 2022).
- Dean, A.G.; Sullivan, K.M.; Soe, M.M. OpenEpi: Open Source Epidemiologic Statistics for Public Health, Version. Available online: www.OpenEpi.com (accessed on 31 January 2022).
- European Centre for Disease Prevention and Control. Available online: http://atlas.ecdc.europa.eu/public/index.aspx (accessed on 31 January 2022).
- Mazjānis, I.; Tirāns, E. Infectious Diseases. Handbook, 2nd ed.; Autorkolektīvs: Riga, Latvia, 2006; p. 1008. (In Latvian) [Google Scholar]
- Åberg, R.; Sjöman, M.; Hemminki, K.; Pirnes, A.; Räsänen, S.; Kalanti, A.; Pohjanvirta, T.; Cacció, S.M.; Pihlajasaari, A.; Toikkanen, S.; et al. Cryptosporidium parvum caused a large outbreak linked to frisée salad in Finland, 2012. Zoonoses Public Health 2015, 62, 618–624. [Google Scholar] [CrossRef]
- Helsinki Commission: Climate Change in the Baltic Sea Area—HELCOM Thematic Assessment in 2007; Baltic Sea Environment Proceedings No 111. 2007. Available online: https://www.baltex-research.eu/BACC/material/Climate_change_report_07.pdf (accessed on 20 January 2022).
- Gertler, M.; Dürr, M.; Renner, P.; Poppert, S.; Askar, M.; Breidenbach, J.; Frank, C.; Preußel, K.; Schielke, A.; Werber, D.; et al. Outbreak of Cryptosporidium hominis following river flooding in the city of Halle (Saale), Germany, August 2013. BMC Infect. Dis. 2015, 15, 1–10. [Google Scholar]
- Messner, M.J.; Berger, P. Cryptosporidium infection risk: Results of new dose-response modeling. Risk Anal. 2016, 36, 1969–1982. [Google Scholar] [CrossRef]
- Coffey, C.M.; Collier, S.A.; Gleason, M.E.; Yoder, J.S.; Kirk, M.D.; Richardson, A.M.; Fullerton, K.; Benedict, K.M. Evolving Epidemiology of Reported Giardiasis Cases in the United States, 1995–2016. Clin. Infect. Dis. 2021, 72, 764–770. [Google Scholar] [CrossRef] [Green Version]
- Lassen, B.; Järvis, T. Eimeria and Cryptosporidium in Lithuanian cattle farms. Vet. Med. Zoot. 2009, 48, 24–28. [Google Scholar]
- Santoro, A.; Dorbek-Kolin, E.; Jeremejeva, J.; Tummeleht, L.; Orro, T.; Jokelainen, P.; Lassen, B. Molecular epidemiology of Cryptosporidium spp. in calves in Estonia: High prevalence of Cryptosporidium parvum shedding and 10 subtypes identified. Parasitology 2019, 146, 261–267. [Google Scholar] [CrossRef]
- Deksne, G.; Mateusa, M.; Cvetkova, S.; Derbakova, A.; Keidāne, D.; Troell, K.; Schares, G. Prevalence, risk factor and diversity of Cryptosporidium in cattle in Latvia. Vet. Parasitol. Reg. Stud. Rep. 2022, 28, 100677. [Google Scholar] [CrossRef]
- Robertson, L.J.; Campbell, A.T.; Smith, H.V. Survival of Cryptosporidium parvum oocysts under various environmental pressures. Appl. Environ. Microbiol. 1992, 58, 3494–3500. [Google Scholar] [CrossRef] [Green Version]
- King, B.J.; Monis, P.T. Critical processes affecting Cryptosporidium oocyst survival in the environment. Parasitology 2007, 134, 309. [Google Scholar] [CrossRef]
- Olson, M.E.; Goh, J.; Phillips, M.; Guselle, N.; McAllister, T.A. Giardia cyst and Cryptosporidium oocyst survival in water, soil, and cattle feces. J. Environ. Qual. 1999, 28, 1991–1996. [Google Scholar] [CrossRef]
- Enserink, R.; van den Wijngaard, C.; Bruijning-Verhagen, P.; van Asten, L.; Mughini-Gras, L.; Duizer, E.; Kortbeek, T.; Scholts, R.; Nagelkerke, N.; Smit, H.A.; et al. Gastroenteritis attributable to 16 enteropathogens in children attending day care: Significant effects of rotavirus, norovirus, astrovirus, Cryptosporidium and Giardia. Pediatr. Infect. Dis. J. 2015, 34, 5–10. [Google Scholar] [CrossRef]
| Factor | Not Analyzed | Cryptosporidium spp. | Giardia spp. | |||
|---|---|---|---|---|---|---|
| No. of Infected | Proportion, % | No. of Infected | Proportion, % | |||
| (95% CI) | (95% CI) | |||||
| Age group | <1 | 123 | 4 | 3.3 (1.0–8.3) | 3 | 2.4 (0.5–7.2) |
| 1–6 | 313 | 22 | 7.0 (4.6–10.5) | 23 | 7.3 (4.9–10.8) | |
| 6–14 | 108 | 6 | 5.6 (2.3–11.8) | 8 | 7.4 (3.6–14.1) | |
| 15–17 | 40 | 3 | 7.5 (1.9–20.6) | 8 | 20 (10.2–35.0) | |
| Gender | Female | 268 | 11 | 4.1 (2.2–7.3) | 23 | 8.6 (5.7–12.6) |
| Male | 316 | 24 | 7.6 (5.1–11.1) | 19 | 6.0 (3.8–9.3) | |
| Total | 584 | 35 | 6.0 (4.3–8.1) | 42 | 7.2 (5.3–9.6) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deksne, G.; Krūmiņš, A.; Mateusa, M.; Morozovs, V.; Šveisberga, D.P.; Korotinska, R.; Bormane, A.; Vīksna, L.; Krūmiņa, A. Occurrence of Cryptosporidium spp. and Giardia spp. Infection in Humans in Latvia: Evidence of Underdiagnosed and Underreported Cases. Medicina 2022, 58, 471. https://doi.org/10.3390/medicina58040471
Deksne G, Krūmiņš A, Mateusa M, Morozovs V, Šveisberga DP, Korotinska R, Bormane A, Vīksna L, Krūmiņa A. Occurrence of Cryptosporidium spp. and Giardia spp. Infection in Humans in Latvia: Evidence of Underdiagnosed and Underreported Cases. Medicina. 2022; 58(4):471. https://doi.org/10.3390/medicina58040471
Chicago/Turabian StyleDeksne, Gunita, Agris Krūmiņš, Maira Mateusa, Vladimirs Morozovs, Dārta Paula Šveisberga, Rita Korotinska, Antra Bormane, Ludmila Vīksna, and Angelika Krūmiņa. 2022. "Occurrence of Cryptosporidium spp. and Giardia spp. Infection in Humans in Latvia: Evidence of Underdiagnosed and Underreported Cases" Medicina 58, no. 4: 471. https://doi.org/10.3390/medicina58040471
APA StyleDeksne, G., Krūmiņš, A., Mateusa, M., Morozovs, V., Šveisberga, D. P., Korotinska, R., Bormane, A., Vīksna, L., & Krūmiņa, A. (2022). Occurrence of Cryptosporidium spp. and Giardia spp. Infection in Humans in Latvia: Evidence of Underdiagnosed and Underreported Cases. Medicina, 58(4), 471. https://doi.org/10.3390/medicina58040471







