Recent Advances in the Treatment of Insulin Resistance Targeting Molecular and Metabolic Pathways: Fighting a Losing Battle?
Abstract
:1. Introduction
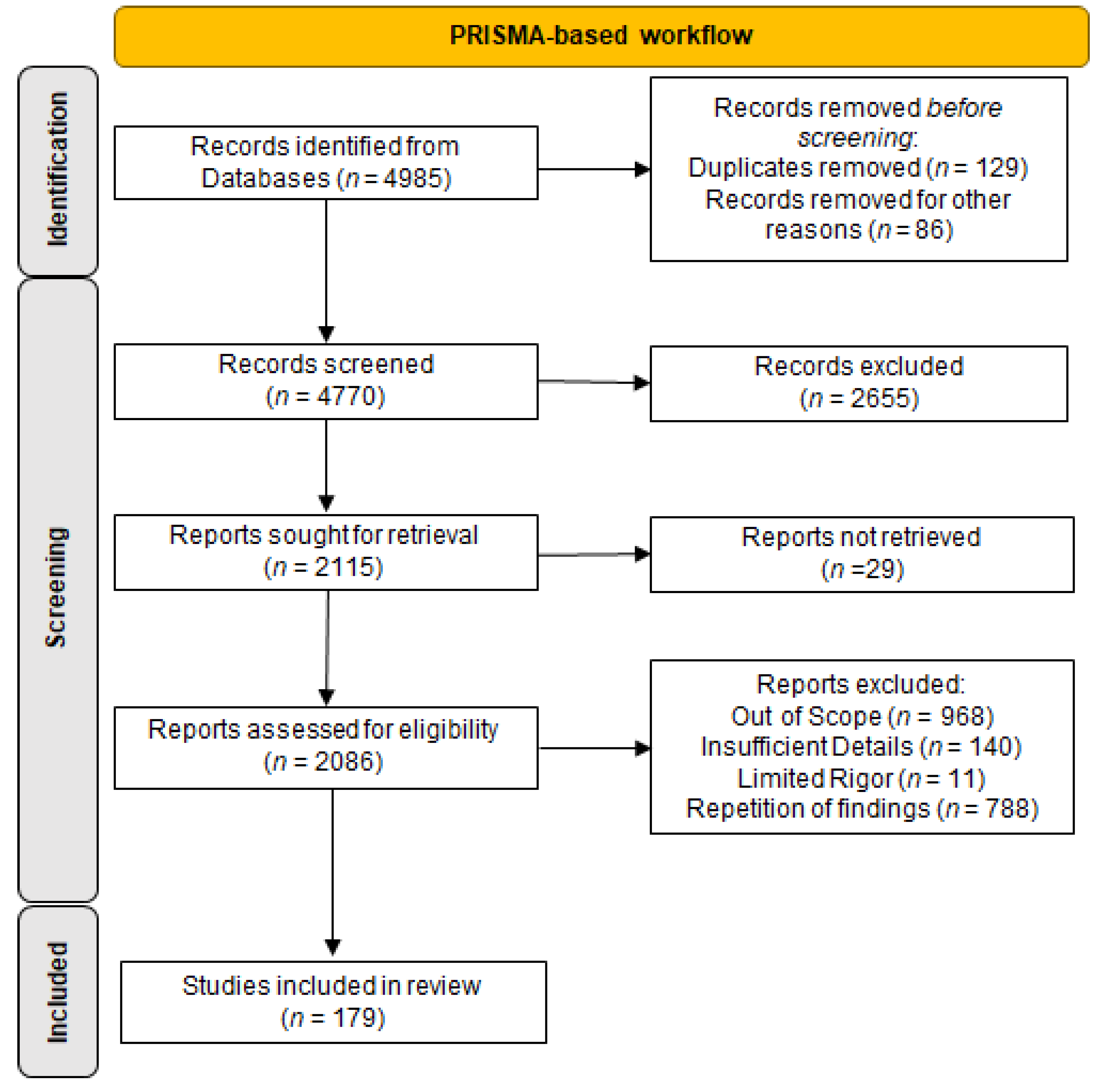
2. Materials and Methods
3. Discussion
3.1. Gut Microbiome Revitalization Promotes IR Alleviation
3.2. New Mechanistic Insights into IR Treatment and Prevention
3.3. Most Notable Innovations in IR Treatment Targeting Molecular Pathways
3.4. Well Known Molecules with Newly Discovered Anti-IR Features
3.5. Revision of New Strategies in IR Prevention Using Nature-Derived Compounds
3.6. Dietary Habits and Physical Activity Play a Crucial Role in IR Treatment
3.7. Cardiovascular Disease Treatment Alleviates IR Severity
3.8. Negative Results in Current Advances of IR Treatment and Prevention
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AhR | aryl hydrocarbon receptor |
| AMPK | AMP-activated protein kinase |
| ANGPLT3 | Angiopoietin-like 3 |
| apo A-I | apolipoprotein A-I |
| ATF4 | Activating Transcription Factor 4 |
| C9orf72 | pharmacological inhibition of chromosome 9 open reading frame 72 |
| CART | cocaine-and amphetamine-regulated transcript |
| CCL-2 | c-c motif chemokine ligand 2 |
| CHOP | C/EBP Homologous Protein |
| DM | Diabetes Mellitus |
| DPPIV | dipeptidyl peptidase IV |
| EcN-GM | Escherichia coli Nissle 1917 |
| EET | major epoxyeicosatrienoic acid |
| eGDR | estimated glucose disposal rate |
| FoxO1 | Forkhead box O1 |
| GABAA | γ-aminobutyric acid type a |
| GAL2 | saccharomyces cerevisiae |
| GLP-1 | glucagon-like peptide-1 |
| GLUT4 | glucose transporter type 4 |
| HbA1c | glycated hemoglobin |
| HFD | high-fat diet |
| HOMA-IR | homeostatic model assessment for insulin resistance |
| ICAM-1 | intercellular adhesion molecule-1 |
| IR | insulin resistance |
| IRβ | insulin receptor β |
| JNK | c-jun n-terminal kinase |
| KAT7 | Lysine Acetyltransferase 7 |
| mTORC1 | rapamycin complex 1 |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| NAFLD | non-alcoholic fatty liver disease |
| Nampt | nicotinamide phosphoribosyl transferase |
| NF-κβ | nuclear factor kappa B |
| Nogo | Reticulon-4 |
| p70S6K | ribosomal protein S6 kinase |
| P7C3 | pool 7, compound 3 |
| Pcyt2 | phosphate cytidylyltransferase 2 |
| p-eIF2α | Factor 2α |
| PIP5K1c | phosphatidylinositol-4-phosphate 5-kinase type 1 gamma |
| PKCε | protein kinase C |
| Pparα | fatty acid oxidation |
| RAGE | receptor for advanced glycation end products |
| SCFA | short-chain fatty acids |
| siRNA | small interfering RNA |
| SP1 | specificity protein 1 |
| STZ | streptozotocin |
| SVF | stromal vascular fraction |
| T1DM | type 1 diabetes mellitus |
| T2DM | Type 2 Diabetes Mellitus |
| TRB3 | Tribbles 3 |
| XBP1s | X Box Binding Protein 1 spliced |
References
- Diabetes. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 2 February 2022).
- Wolosowicz, M.; Lukaszuk, B.; Chabowski, A. The Causes of Insulin Resistance in Type 1 Diabetes Mellitus: Is There a Place for Quaternary Prevention? Int. J. Environ. Res. Public Health 2020, 17, 8651. [Google Scholar] [CrossRef] [PubMed]
- Bacha, F.; Bartz, S.K.; Jindal, I.; Puyau, M.R.; Adolph, A.; Sharma, S. 1262-P: Metabolic Flexibility across the Spectrum of Glycemic Regulation in Youth. Diabetes 2020, 69, e146000. [Google Scholar] [CrossRef]
- Courtney, C.H.; Olefsky, J.M. Insulin Resistance. In Mechanisms of Insulin Action: Medical Intelligence Unit; Springer: New York, NY, USA, 2021; pp. 185–209. [Google Scholar] [CrossRef]
- Schütten, M.T.; Kusters, Y.H.; Houben, A.J.; Niessen, H.E.; Roodt, J.O.; Scheijen, J.L.; van de Waardenburg, M.P.; Schalkwijk, C.G.; de Leeuw, P.W.; Stehouwer, C.D. Glucocorticoids affect metabolic but not muscle microvascular insulin sensitivity following high versus low salt intake. JCI Insight 2020, 5, e127530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartosiewicz, J.; Kaminski, T.; Pawlak, K.; Karbowska, M.; Tankiewicz-Kwedlo, A.; Pawlak, D. The activation of the kynurenine pathway in a rat model with renovascular hypertension. Exp. Biol. Med. 2017, 242, 750–761. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Shao, H.; Zheng, X. Amino acids at the intersection of nutrition and insulin sensitivity. Drug Discov. Today 2019, 24, 1038–1043. [Google Scholar] [CrossRef]
- Wolver, S.; Fadel, K.; Fieger, E.; Aburish, Z.; O’Rourke, B.; Chandler, T.-M.; Shimotani, D.; Clingempeel, N.; Jain, S.; Jain, A.; et al. Clinical Use of a Real-World Low Carbohydrate Diet Resulting in Reduction of Insulin Dose, Hemoglobin A1c, and Weight. Front. Nutr. 2021, 8, 485. [Google Scholar] [CrossRef]
- Gaesser, G.A.; Jones, J.M.; Angadi, S.S. Perspective: Does Glycemic Index Matter for Weight Loss and Obesity Prevention? Examination of the Evidence on “Fast” Compared with “Slow” Carbs. Adv. Nutr. Int. Rev. J. 2021, 12, 2076–2084. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Atkin, S.L.; Simental-Mendía, L.E.; Sahebkar, A. Molecular mechanisms by which aerobic exercise induces insulin sensitivity. J. Cell. Physiol. 2019, 234, 12385–12392. [Google Scholar] [CrossRef]
- Bailey, C.J. Treating insulin resistance: Future prospects. Diabetes Vasc. Dis. Res. 2007, 4, 20–31. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Wilmanski, T.; Diener, C.; Rappaport, N.; Patwardhan, S.; Wiedrick, J.; Lapidus, J.; Earls, J.C.; Zimmer, A.; Glusman, G.; Robinson, M.; et al. Gut microbiome pattern reflects healthy ageing and predicts survival in humans. Nat. Metab. 2021, 3, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Hills, R.D., Jr.; Pontefract, B.A.; Mishcon, H.R.; Black, C.A.; Sutton, S.C.; Theberge, C.R. Gut Microbiome: Profound Implications for Diet and Disease. Nutrients 2019, 11, 1613. [Google Scholar] [CrossRef] [Green Version]
- Liang, T.; Wu, L.; Xi, Y.; Li, Y.; Xie, X.; Fan, C.; Yang, L.; Yang, S.; Chen, X.; Zhang, J.; et al. Probiotics supplementation improves hyperglycemia, hypercholesterolemia, and hypertension in type 2 diabetes mellitus: An update of meta-analysis. Crit. Rev. Food Sci. Nutr. 2020, 61, 1670–1688. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Fogacci, F.; Bove, M.; Giovannini, M.; Borghi, C. Impact of a Short-Term Synbiotic Supplementation on Metabolic Syndrome and Systemic Inflammation in Elderly Patients: A Randomized Placebo-Controlled Clinical Trial. Eur. J. Nutr. 2021, 60, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Mejhert, N.; Rydén, M. Understanding the Complexity of Insulin Resistance. Nat. Rev. Endocrinol. 2022. online ahead of print. [Google Scholar] [CrossRef]
- He, L. Alterations of Gut Microbiota by Overnutrition Impact Gluconeogenic Gene Expression and Insulin Signaling. Int. J. Mol. Sci. 2021, 22, 2121. [Google Scholar] [CrossRef]
- Wu, R.; Zhou, L.; Chen, Y.; Ding, X.; Liu, Y.; Tong, B.; Lv, H.; Meng, X.; Li, J.; Jian, T.; et al. Sesquiterpene glycoside isolated from loquat leaf targets gut microbiota to prevent type 2 diabetes mellitus in db/db mice. Food Funct. 2022, 13, 1519–1534. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, J.; Xu, L.; Liu, Y.; Gu, J. The beneficial effects of genetically engineered Escherichia coli Nissle 1917 in obese C57BL/6J mice. Int. J. Obes. 2022. [Google Scholar] [CrossRef]
- Praveschotinunt, P.; Duraj-Thatte, A.M.; Gelfat, I.; Bahl, F.; Chou, D.B.; Joshi, N.S. Engineered E. coli Nissle 1917 for the delivery of matrix-tethered therapeutic domains to the gut. Nat. Commun. 2019, 10, 5580. [Google Scholar] [CrossRef] [Green Version]
- Mo, X.; Sun, Y.; Liang, X.; Li, L.; Hu, S.; Xu, Z.; Liu, S.; Zhang, Y.; Li, X.; Liu, L. Insoluble yeast β-glucan attenuates high-fat diet-induced obesity by regulating gut microbiota and its metabolites. Carbohydr. Polym. 2022, 281, 119046. [Google Scholar] [CrossRef]
- Muthuramalingam, K.; Singh, V.; Choi, C.; Choi, S.I.; Kim, Y.M.; Unno, T.; Cho, M. Dietary intervention using (1,3)/(1,6)-β-glucan, a fungus-derived soluble prebiotic ameliorates high-fat diet-induced metabolic distress and alters beneficially the gut microbiota in mice model. Eur. J. Nutr. 2019, 59, 2617–2629. [Google Scholar] [CrossRef] [PubMed]
- Effect of Metreleptin Therapy in the Treatment of Severe Insulin Resistance—Full Text View—ClinicalTrials.Gov. Available online: https://www.clinicaltrials.gov/ct2/show/NCT00085982 (accessed on 4 March 2022).
- Lin, J.; Wen, J.; Xiao, N.; Cai, Y.; Xiao, J.; Dai, W.; Chen, J.; Zeng, K.; Liu, F.; Du, B.; et al. Anti-diabetic and gut microbiota modulation effects of sacha inchi (Plukenetia volubilis L.) leaf extract in streptozotocin-induced type 1 diabetic mice. J. Sci. Food Agric. 2022. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Tanwar, B.; Sihag, M.K.; Sharma, V. Sacha inchi (Plukenetia volubilis L.): An emerging source of nutrients, omega-3 fatty acid and phytochemicals. Food Chem. 2022, 373, 131459. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Song, J.; Huang, Y.; Li, X.; Wang, H.; Zhang, Y.; Suo, H. Lactobacillus plantarum SHY130 isolated from yak yogurt attenuates hyperglycemia in C57BL/6J mice by regulating the enteroinsular axis. Food Funct. 2022, 13, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Salamone, D.; Rivellese, A.A.; Vetrani, C. The relationship between gut microbiota, short-chain fatty acids and type 2 diabetes mellitus: The possible role of dietary fibre. Acta Diabetol. 2021, 58, 1131–1138. [Google Scholar] [CrossRef]
- Hernandez, R.; Zhou, C. Recent Advances in Understanding the Role of IKKβ in Cardiometabolic Diseases. Front. Cardiovasc. Med. 2021, 8, 752337. [Google Scholar] [CrossRef]
- Yu, Y.-Y.; Cui, S.-C.; Zheng, T.-N.; Ma, H.-J.; Xie, Z.-F.; Jiang, H.-W.; Li, Y.-F.; Zhu, K.-X.; Huang, C.-G.; Li, J.; et al. Sarsasapogenin improves adipose tissue inflammation and ameliorates insulin resistance in high-fat diet-fed C57BL/6J mice. Acta Pharmacol. Sin. 2021, 42, 272–281. [Google Scholar] [CrossRef]
- Hua, X.; Sun, D.; Zhang, W.; Fu, J.; Tong, J.; Sun, S.; Zeng, F.; Ouyang, S.; Zhang, G.; Wang, S.; et al. P7C3-A20 alleviates fatty liver by shaping gut microbiota and inducing FGF21/FGF1, via the AMP-activated protein kinase/CREB regulated transcription coactivator 2 pathway. J. Cereb. Blood Flow Metab. 2021, 178, 2111–2130. [Google Scholar] [CrossRef]
- Shabkhiz, F.; Khalafi, M.; Rosenkranz, S.; Karimi, P.; Moghadami, K. Resistance training attenuates circulating FGF-21 and myostatin and improves insulin resistance in elderly men with and without type 2 diabetes mellitus: A randomised controlled clinical trial. Eur. J. Sport Sci. 2021, 21, 636–645. [Google Scholar] [CrossRef]
- Kiluk, P.; Baran, A.; Kaminski, T.W.; Maciaszek, M.; Flisiak, I. The Level of FGF 21 as a New Risk Factor for the Occurrence of Cardiometabolic Disorders amongst the Psoriatic Patients. J. Clin. Med. 2019, 8, 2206. [Google Scholar] [CrossRef] [Green Version]
- Qiang, W.; Shen, T.; Noman, M.; Guo, J.; Jin, Z.; Lin, D.; Pan, J.; Lu, H.; Li, X.; Gong, F. Fibroblast Growth Factor 21 Augments Autophagy and Reduces Apoptosis in Damaged Liver to Improve Tissue Regeneration in Zebrafish. Front. Cell Dev. Biol. 2021, 9, 756743. [Google Scholar] [CrossRef] [PubMed]
- Fangmann, D.; Geisler, C.; Schlicht, K.; Hartmann, K.; Köpke, J.; Tiede, A.; Settgast, U.; Türk, K.; Schulte, D.M.; Altmann, K.; et al. Differential effects of protein intake versus intake of a defined oligopeptide on FGF-21 in obese human subjects in vivo. Clin. Nutr. 2021, 40, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Xing, Y.; He, L.; Xiu, Z.; Yang, L.; Han, A.; Jia, Q.; Dong, Y. Effect of 1-Deoxynojirimycin on insulin resistance in prediabetic mice based on next-generation sequencing and intestinal microbiota study. J. Ethnopharmacol. 2022, 289, 115029. [Google Scholar] [CrossRef] [PubMed]
- Alipourfard, I.; Bakhtiyari, S.; Gheysarzadeh, A.; Di Renzo, L.; De Lorenzo, A.; Mikeladze, D.; Khamoushi, A. The Key Role of Akt Protein Kinase in Metabolic-Inflammatory Pathways Cross-Talk: TNF-α Down-Regulation and Improving of Insulin Resistance in HepG2 Cell Line. Curr. Mol. Med. 2021, 21, 257–264. [Google Scholar] [CrossRef] [PubMed]
- De Araújo Henriques Ferreira, G.; Magnani, M.; Cabral, L.; Brandão, L.R.; Noronha, M.F.; de Campos Cruz, J.; de Souza, E.L.; de Brito Alves, J.L. Potentially Probiotic Limosilactobacillus Fermentum Fruit-Derived Strains Alleviate Cardiometabolic Disorders and Gut Microbiota Impairment in Male Rats Fed a High-Fat Diet. Probiotics Antimicrob. Proteins 2022. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, Y.; Schwenger, K.J.P.; Allard, J.P. Manipulation of intestinal microbiome as potential treatment for insulin resistance and type 2 diabetes. Eur. J. Nutr. 2021, 60, 2361–2379. [Google Scholar] [CrossRef]
- Dewi, L.; Rosidi, A.; Noer, E.R.; Ayuningtyas, A. The Prospect for Type 2 Diabetes Mellitus Combined with Exercise and Synbiotics: A Perspective. Curr. Diabetes Rev. 2021, 17, e012821190875. [Google Scholar] [CrossRef]
- Effects of Oral Glutamine Supplementation on Insulin Resistance and Functional Intestinal Disorders in Obese Patients. Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04883515 (accessed on 4 March 2022).
- Lyu, K.; Zhang, D.; Song, J.D.; Li, X.; Perry, R.J.; Samuel, V.T.; Shulman, G.I. Short-term overnutrition induces white adipose tissue insulin resistance through sn-1,2-diacylglycerol/PKCε/insulin receptor Thr1160 phosphorylation. JCI Insight 2021, 6, e139946. [Google Scholar] [CrossRef]
- Lule, K.O.; Akarsu, E.; Sayiner, Z.A.; Lule, N.O.; Balci, S.O.; Demirel, C.; Bozdag, Z.; Korkmaz, M.; Yilmaz, I. The effects of metformin, pioglitazone, exenatide and exercise on fatty liver in obese diabetic rats: The role of IRS-1 and SOCS-3 molecules. Inflammopharmacology 2022, 30, 243–250. [Google Scholar] [CrossRef]
- Jeon, S.-M. Regulation and function of AMPK in physiology and diseases. Exp. Mol. Med. 2016, 48, e245. [Google Scholar] [CrossRef]
- Khodadadi, M.; Jafari-Gharabaghlou, D.; Zarghami, N. An update on mode of action of metformin in modulation of meta-inflammation and inflammaging. Pharmacol. Rep. 2022. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Dludla, P.V.; Nyambuya, T.M.; Johnson, R.; Silvestri, S.; Orlando, P.; Mazibuko-Mbeje, S.E.; Gabuza, K.B.; Mxinwa, V.; Mokgalaboni, K.; Tiano, L.; et al. Metformin and heart failure–related outcomes in patients with or without diabetes: A systematic review of randomized controlled trials. Heart Fail. Rev. 2021, 26, 1437–1445. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Recarte, D.; Palomer, X.; Wahli, W.; Vázquez-Carrera, M. The PPARβ/δ-AMPK Connection in the Treatment of Insulin Resistance. Int. J. Mol. Sci. 2021, 22, 8555. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Jiang, F.; Huang, B.; Zheng, W.; Jiang, Y.; Cai, G.; Liu, D.; Hu, C.Y.; Wang, C. Dihydromyricetin Ameliorates Inflammation-Induced Insulin Resistance via Phospholipase C-CaMKK-AMPK Signal Pathway. Oxidative Med. Cell. Longev. 2021, 2021, 8542809. [Google Scholar] [CrossRef]
- Li, C.; Sun, L. Mitochondria-Associated Membranes (MAMs): A Novel Therapeutic Target for Treating Metabolic Syndrome. Curr. Med. Chem. 2021, 28, 1347–1362. [Google Scholar] [CrossRef]
- Hüttl, M.; Markova, I.; Miklankova, D.; Zapletalova, I.; Poruba, M.; Racova, Z.; Vecera, R.; Malinska, H. The Beneficial Additive Effect of Silymarin in Metformin Therapy of Liver Steatosis in a Pre-Diabetic Model. Pharmaceutics 2021, 14, 45. [Google Scholar] [CrossRef]
- Ni, X.; Wang, H. Silymarin attenuated hepatic steatosis through regulation of lipid metabolism and oxidative stress in a mouse model of nonalcoholic fatty liver disease (NAFLD). Am. J. Transl. Res. 2016, 8, 1073–1081. [Google Scholar]
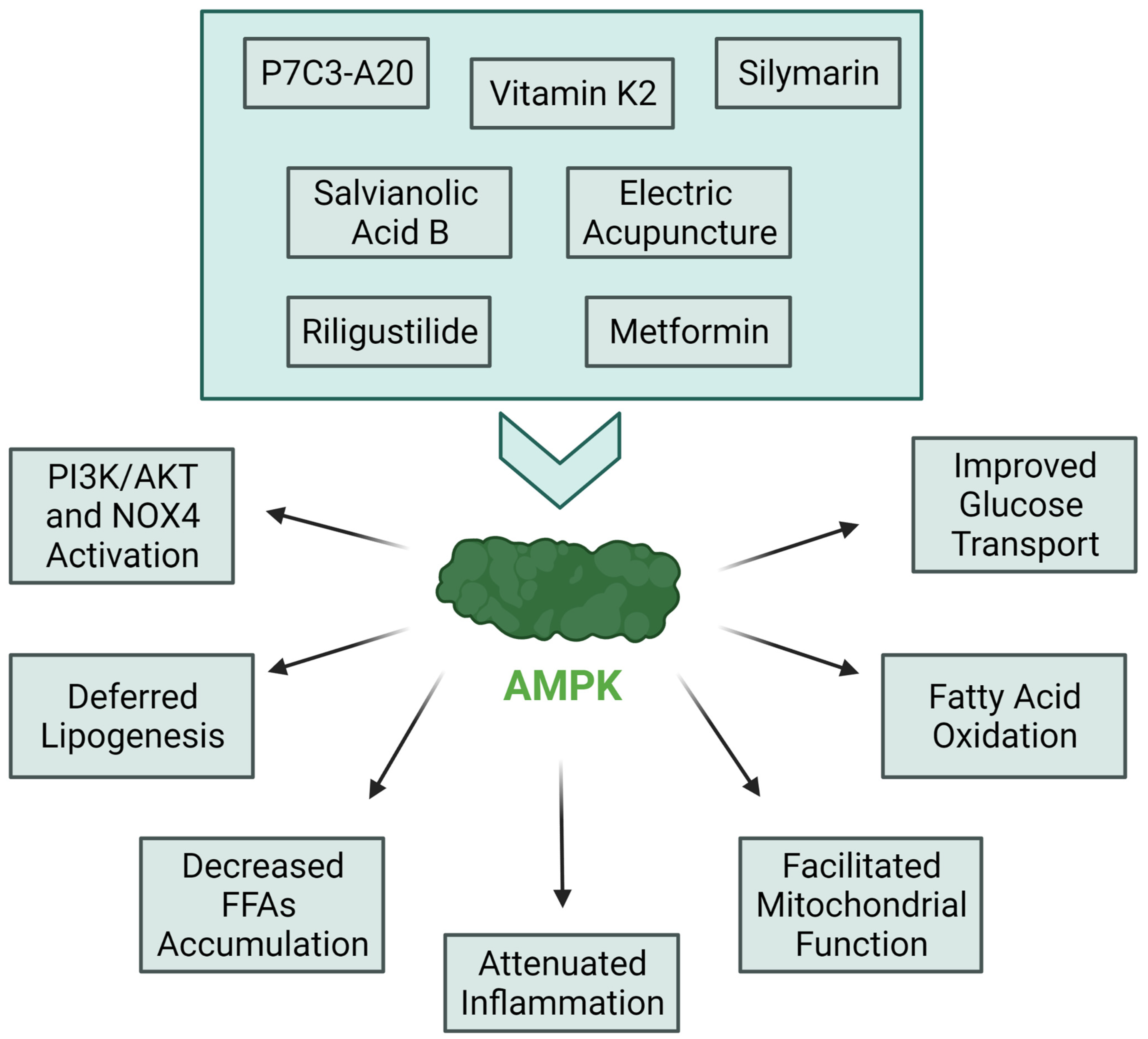
- Entezari, M.; Hashemi, D.; Taheriazam, A.; Zabolian, A.; Mohammadi, S.; Fakhri, F.; Hashemi, M.; Hushmandi, K.; Ashrafizadeh, M.; Zarrabi, A.; et al. AMPK signaling in diabetes mellitus, insulin resistance and diabetic complications: A pre-clinical and clinical investigation. Biomed. Pharmacother. 2022, 146, 112563. [Google Scholar] [CrossRef]
- Meng, Q.; Qi, X.; Fu, Y.; Chen, Q.; Cheng, P.; Yu, X.; Sun, X.; Wu, J.; Li, W.; Zhang, Q.; et al. Flavonoids extracted from mulberry (Morus alba L.) leaf improve skeletal muscle mitochondrial function by activating AMPK in type 2 diabetes. J. Ethnopharmacol. 2020, 248, 112326. [Google Scholar] [CrossRef]
- Ruderman, N.B.; Carling, D.; Prentki, M.; Cacicedo, J.M. AMPK, insulin resistance, and the metabolic syndrome. J. Clin. Investig. 2013, 123, 2764–2772. [Google Scholar] [CrossRef]
- Maqbool, A.; Watt, N.T.; Haywood, N.; Viswambharan, H.; Skromna, A.; Makava, N.; Visnagri, A.; Shawer, H.M.; Bridge, K.; Muminov, S.K.; et al. Divergent Effects of Genetic and Pharmacological Inhibition of Nox2 NADPH Oxidase on Insulin Resistance-Related Vascular Damage. Am. J. Physiol. Cell Physiol. 2020, 318, C64–C74. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-X.; Zhang, L.-Z.; Zhang, H.-H.; Lai, L.-F.; Wang, Y.-Q.; Sun, J.; Xu, N.-G.; Li, Z.-X. Low-frequency electroacupuncture improves disordered hepatic energy metabolism in insulin-resistant Zucker diabetic fatty rats via the AMPK/mTORC1/p70S6K signaling pathway. Acupunct. Med. 2022. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Cang, X.; Wang, Y.; Zeng, J.; Gao, J.; Yu, Q.; Lu, C.; Xu, F.; Lin, J.; Zhu, J.; Wang, X. C9orf72 knockdown alleviates hepatic insulin resistance by promoting lipophagy. Biochem. Biophys. Res. Commun. 2022, 588, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Guan, P.; Xu, L.; Liu, B.; Li, M.; Xu, Z.; Huang, X.; Han, L. Riligustilide alleviates hepatic insulin resistance and gluconeogenesis in T2DM mice through multitarget actions. Phytother. Res. 2022, 36, 462–474. [Google Scholar] [CrossRef]
- Habibi, J.; Chen, D.; Hulse, J.L.; Whaley-Connell, A.T.; Sowers, J.R.; Jia, G. Targeting mineralocorticoid receptors in diet-induced hepatic steatosis and insulin resistance. Am. J. Physiol. Integr. Comp. Physiol. 2022, 322, R253–R262. [Google Scholar] [CrossRef]
- Xiang, J.; Zhang, C.; Di, T.; Chen, L.; Zhao, W.; Wei, L.; Zhou, S.; Wu, X.; Wang, G.; Zhang, Y. Salvianolic acid B alleviates diabetic endothelial and mitochondrial dysfunction by down-regulating apoptosis and mitophagy of endothelial cells. Bioengineered 2022, 13, 3486–3502. [Google Scholar] [CrossRef]
- Krahel, J.A.; Baran, A.; Kamiński, T.W.; Flisiak, I. Proprotein Convertase Subtilisin/Kexin Type 9, Angiopoietin-Like Protein 8, Sortilin, and Cholesteryl Ester Transfer Protein—Friends of Foes for Psoriatic Patients at the Risk of Developing Cardiometabolic Syndrome? Int. J. Mol. Sci. 2020, 21, 3682. [Google Scholar] [CrossRef]
- Quagliarini, F.; Wang, Y.; Kozlitina, J.; Grishin, N.V.; Hyde, R.; Boerwinkle, E.; Valenzuela, D.M.; Murphy, A.J.; Cohen, J.C.; Hobbs, H.H. Atypical angiopoietin-like protein that regulates ANGPTL3. Proc. Natl. Acad. Sci. USA 2012, 109, 19751–19756. [Google Scholar] [CrossRef] [Green Version]
- Su, X. ANGPLT3 in cardio-metabolic disorders. Mol. Biol. Rep. 2021, 48, 2729–2739. [Google Scholar] [CrossRef]
- Foss-Freitas, M.C.; Akinci, B.; Neidert, A.; Bartlett, V.J.; Hurh, E.; Karwatowska-Prokopczuk, E.; Oral, E.A. Selective targeting of angiopoietin-like 3 (ANGPTL3) with vupanorsen for the treatment of patients with familial partial lipodystrophy (FPLD): Results of a proof-of-concept study. Lipids Health Dis. 2021, 20, 174. [Google Scholar] [CrossRef]
- Khadir, A.; Kavalakatt, S.; Madhu, D.; Devarajan, S.; Abubaker, J.; Al-Mulla, F.; Tiss, A. Spexin as an indicator of beneficial effects of exercise in human obesity and diabetes. Sci. Rep. 2020, 10, 10635. [Google Scholar] [CrossRef]
- Vyas, V.; Blythe, H.; Wood, E.G.; Sandhar, B.; Sarker, S.-J.; Balmforth, D.; Ambekar, S.G.; Yap, J.; Edmondson, S.J.; Di Salvo, C.; et al. Obesity and diabetes are major risk factors for epicardial adipose tissue inflammation. JCI Insight 2021, 6, e145495. [Google Scholar] [CrossRef] [PubMed]
- Türkel, I.; Memi, G.; Yazgan, B. Impact of spexin on metabolic diseases and inflammation: An updated minireview. Exp. Biol. Med. 2022. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Albeltagy, E.S.; Elbaky, N.M.A. Association of lower circulating Spexin levels with higher body mass indices and glucose metabolic profiles in adult subjects in Egypt. Hum. Nutr. Metab. 2022, 27, 200137. [Google Scholar] [CrossRef]
- Yu, M.; Wang, M.; Han, S.; Han, L.; Kan, Y.; Zhao, J.; Yu, X.; Yan, J.; Jin, Y.; Zhang, Z.; et al. Spexin ameliorates skeletal muscle insulin resistance through activation of GAL2 receptor. Eur. J. Pharmacol. 2022, 917, 174731. [Google Scholar] [CrossRef]
- Prasatthong, P.; Meephat, S.; Rattanakanokchai, S.; Bunbupha, S.; Prachaney, P.; Maneesai, P.; Pakdeechote, P. Hesperidin ameliorates signs of the metabolic syndrome and cardiac dysfunction via IRS/Akt/GLUT4 signaling pathway in a rat model of diet-induced metabolic syndrome. Eur. J. Nutr. 2021, 60, 833–848. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yang, H.; Yang, E.; Li, J.; Dong, L. Berberine Decreases Intestinal GLUT2 Translocation and Reduces Intestinal Glucose Absorption in Mice. Int. J. Mol. Sci. 2021, 23, 327. [Google Scholar] [CrossRef]
- Kasina, S.V.S.K.; Baradhi, K.M. Dipeptidyl Peptidase IV (DPP IV) Inhibitors; StatPearls: Treasure Island, FL, USA, 2021; pp. 1–5. [Google Scholar]
- Montaniel, K.R.C.; Bucher, M.; Phillips, E.A.; Li, C.; Sullivan, E.L.; Kievit, P.; Rugonyi, S.; Nathanielsz, P.W.; Maloyan, A. Dipeptidyl peptidase IV inhibition delays developmental programming of obesity and metabolic disease in male offspring of obese mothers. J. Dev. Orig. Health Dis. 2022, 1–14. [Google Scholar] [CrossRef]
- Giugliano, D.; Sportiello, L.; Capuano, A.; Maiorino, M.I.; Rossi, F.; Esposito, K. Dipeptidyl peptidase-4 inhibitors in type 2 diabetes therapy—Focus on alogliptin. Drug Des. Dev. Ther. 2013, 7, 989–1001. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Kim, I.; Cho, W.; Oh, G.T.; Park, Y.M. Vimentin Deficiency Prevents High-Fat Diet-Induced Obesity and Insulin Resistance in Mice. Diabetes Metab. J. 2021, 45, 97–108. [Google Scholar] [CrossRef]
- Okano, Y.; Takeshita, A.; Yasuma, T.; Toda, M.; Nishihama, K.; D’Alessandro, V.F.; Inoue, C.; D’Alessandro-Gabazza, C.N.; Kobayashi, T.; Yano, Y.; et al. Protective Role of Recombinant Human Thrombomodulin in Diabetes Mellitus. Cells 2021, 10, 2237. [Google Scholar] [CrossRef] [PubMed]
- Kamiński, T.W.; Pawlak, K.; Karbowska, M.; Myśliwiec, M.; Pawlak, D. Indoxyl sulfate—The uremic toxin linking hemostatic system disturbances with the prevalence of cardiovascular disease in patients with chronic kidney disease. BMC Nephrol. 2017, 18, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez, A.D.; Patel, K.; Diego, V.P.; Almeida, M.A.; Blangero, J.; Powell, J.S.; Howard, T.E. On the Role of Hemostasis Variables in Cardiometabolic Outcomes. Blood 2021, 138, 4266. [Google Scholar] [CrossRef]
- Hörber, S.; Lehmann, R.; Stefan, N.; Machann, J.; Birkenfeld, A.L.; Wagner, R.; Heni, M.; Häring, H.-U.; Fritsche, A.; Peter, A. Hemostatic alterations linked to body fat distribution, fatty liver, and insulin resistance. Mol. Metab. 2021, 53, 101262. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Weber, N.; Cohn, D.; Hollmann, M.; DeVries, J.; Hermanides, J.; Preckel, B. Effects of Hyperglycemia and Diabetes Mellitus on Coagulation and Hemostasis. J. Clin. Med. 2021, 10, 2419. [Google Scholar] [CrossRef]
- Abels, M.; Riva, M.; Shcherbina, L.; Fischer, A.-H.T.; Banke, E.; Degerman, E.; Lindqvist, A.; Wierup, N. Overexpressed beta cell CART increases insulin secretion in mouse models of insulin resistance and diabetes. Peptides 2022, 151, 170747. [Google Scholar] [CrossRef]
- Abels, M.; Riva, M.; Bennet, H.; Ahlqvist, E.; Dyachok, O.; Nagaraj, V.; Shcherbina, L.; Fred, R.; Poon, W.; Sörhede-Winzell, M.; et al. CART is overexpressed in human type 2 diabetic islets and inhibits glucagon secretion and increases insulin secretion. Diabetologia 2016, 59, 1928–1937. [Google Scholar] [CrossRef]
- Lau, J.; Farzi, A.; Qi, Y.; Heilbronn, R.; Mietzsch, M.; Herzog, H. CART neurons in the arcuate nucleus and lateral hypothalamic area exert differential controls on energy homeostasis. Mol. Metab. 2018, 7, 102–118. [Google Scholar] [CrossRef]
- Yazdanimoghaddam, F.; Aghaei, M.; Ghasemi, M.; Soltani, N.; Rezazadeh, H.; Zadhoush, F. Beneficial effects of MgSO4 on TFAM, UPC3 and FNDC5 mRNA expressions in skeletal muscle of type 2 diabetic rats: A possible mechanism to improve insulin resistance. Mol. Biol. Rep. 2022, 49, 2795–2803. [Google Scholar] [CrossRef]
- Askari, M.; Mozaffari, H.; Jafari, A.; Ghanbari, M.; Mofrad, M.D. The effects of magnesium supplementation on obesity measures in adults: A systematic review and dose-response meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2020, 61, 2921–2937. [Google Scholar] [CrossRef]
- Kim, S.; Park, E.; Park, J.-H. Effects of Calcium Fortified Beverage Intake on Insulin Sensitivity and Antioxidant Metabolism in Healthy Elderly. Clin. Nutr. Res. 2021, 10, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Wang, W.; Fang, C.; Ni, C.; Zhou, J.; Wang, X.; Zhang, L.; Xu, X.; Cao, R.; Lang, H.; et al. Vitamin K2 Alleviates Insulin Resistance in Skeletal Muscle by Improving Mitochondrial FunctionViaSIRT1 Signaling. Antioxid. Redox Signal. 2021, 34, 99–117. [Google Scholar] [CrossRef] [PubMed]
- Wahba, N.S.; Ghareib, S.A.; Abdel-Ghany, R.H.; Abdel-Aal, M.; Alsemeh, A.E. Renoprotective effects of vitamin D3 supplementation in a rat model of metabolic syndrome. Eur. J. Nutr. 2021, 60, 299–316. [Google Scholar] [CrossRef] [PubMed]
- Manickam, R.; Tur, J.; Badole, S.L.; Chapalamadugu, K.C.; Sinha, P.; Wang, Z.; Russ, D.W.; Brotto, M.; Tipparaju, S.M. Nampt activator P7C3 ameliorates diabetes and improves skeletal muscle function modulating cell metabolism and lipid mediators. J. Cachex Sarcopenia Muscle 2022. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Liu, X.; Feng, C.; Zhou, Z.; Liu, S. Nicotinamide phosphoribosyltransferase (Nampt) of hybrid crucian carp protects intestinal barrier and enhances host immune defense against bacterial infection. Dev. Comp. Immunol. 2022, 128, 104314. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.F.; Mathur, A.; Pandey, V.K.; Kakkar, P. Endoplasmic reticulum stress-dependent activation of TRB3-FoxO1 signaling pathway exacerbates hyperglycemic nephrotoxicity: Protection accorded by Naringenin. Eur. J. Pharmacol. 2022, 917, 174745. [Google Scholar] [CrossRef]
- Patti, A.M.; Giglio, R.V.; Papanas, N.; Serban, D.; Stoian, A.P.; Pafili, K.; Al Rasadi, K.; Rajagopalan, K.; Rizvi, A.A.; Ciaccio, M.; et al. Experimental and Emerging Free Fatty Acid Receptor Agonists for the Treatment of Type 2 Diabetes. Medicina 2022, 58, 109. [Google Scholar] [CrossRef]
- Kamiński, T.; Michałowska, M.; Pawlak, D. Aryl hydrocarbon receptor (AhR) and its endogenous agonist—Indoxyl sulfate in chronic kidney disease. Postepy Hig. Med. Dosw. 2017, 71, 624–632. [Google Scholar] [CrossRef]
- Fehsel, K.; Schwanke, K.; Kappel, B.; Fahimi, E.; Meisenzahl-Lechner, E.; Esser, C.; Hemmrich, K.; Haarmann-Stemmann, T.; Kojda, G.; Lange-Asschenfeldt, C. Activation of the aryl hydrocarbon receptor by clozapine induces preadipocyte differentiation and contributes to endothelial dysfunction. J. Psychopharmacol. 2022, 36, 191–201. [Google Scholar] [CrossRef]
- Huang, G.; Yang, C.; Guo, S.; Huang, M.; Deng, L.; Huang, Y.; Chen, P.; Chen, F.; Huang, X. Adipocyte-specific deletion of PIP5K1c reduces diet-induced obesity and insulin resistance by increasing energy expenditure. Lipids Health Dis. 2022, 21, 6. [Google Scholar] [CrossRef]
- Porciello, N.; Kunkl, M.; Viola, A.; Tuosto, L. Phosphatidylinositol 4-Phosphate 5-Kinases in the Regulation of T Cell Activation. Front. Immunol. 2016, 7, 186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Li, J.; Liu, L.; Song, G. Insulin sensitivity in the aged heart is improved by down-regulation of KAT7 in vivo and in vitro. Cell Cycle 2022, 21, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Haas, M.J.; Parekh, S.; Kalidas, P.; Richter, A.; Warda, F.; Wong, N.C.W.; Tokuda, M.; Mooradian, A.D. Insulin mimetic effect of D-allulose on apolipoprotein A-I gene. J. Food Biochem. 2022, 46, e14064. [Google Scholar] [CrossRef] [PubMed]
- Yaryari, A.-M.; Mazdeh, M.; Mohammadi, M.; Haghi, A.R.; Ghiasian, M.; Mehrpooya, M. Evaluation of serum levels of asprosin and other metabolic profiles in patients with idiopathic tonic–clonic generalized epilepsy on treatment with valproic acid. Eur. J. Clin. Pharmacol. 2022, 78, 393–403. [Google Scholar] [CrossRef]
- Ladino, L.D.; Téllez-Zenteno, J.F. Epilepsy and Obesity: A Complex Interaction. Comorbidities Epilepsy 2019, 131–158. [Google Scholar] [CrossRef]
- Grapentine, S.; Singh, R.K.; Basu, P.; Sivanesan, S.; Mattos, G.; Oresajo, O.; Cheema, J.; Demeke, W.; Dolinsky, V.W.; Bakovic, M. Pcyt2 deficiency causes age-dependant development of nonalcoholic steatohepatitis and insulin resistance that could be attenuated with phosphoethanolamine. Sci. Rep. 2022, 12, 1048. [Google Scholar] [CrossRef]
- Leszczyńska, A.; Kamiński, T.W.; Misztal, T.; Pawlak, D. Quinolinic Acid Does Not Influence Coagulation Profile, nor Fibrinolytic Activity, under Physiological Conditions in Rats. Acta Pol. Pharm. Drug Res. 2019, 76, 863–871. [Google Scholar] [CrossRef]
- Tordjman, S.; Chokron, S.; Delorme, R.; Charrier, A.; Bellissant, E.; Jaafari, N.; Fougerou, C. Melatonin: Pharmacology, Functions and Therapeutic Benefits. Curr. Neuropharmacol. 2017, 15, 434–443. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Z.; Chen, Y.; Dong, Y. Kidney Damage Caused by Obesity and Its Feasible Treatment Drugs. Int. J. Mol. Sci. 2022, 23, 747. [Google Scholar] [CrossRef]
- Orfila, J.E.; Dietz, R.M.; Rodgers, K.M.; Dingman, A.; Patsos, O.P.; Cruz-Torres, I.; Grewal, H.; Strnad, F.; Schroeder, C.; Herson, P.S. Experimental pediatric stroke shows age-specific recovery of cognition and role of hippocampal Nogo-A receptor signaling. J. Cereb. Blood Flow Metab. 2020, 40, 588–599. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Y.; Zhao, D.; Zhang, S.; Chen, Y.; Chen, Y.; Feng, K.; Li, X.; Han, J.; Iwakiri, Y.; et al. Inhibition of high-fat diet–induced obesity via reduction of ER-resident protein Nogo occurs through multiple mechanisms. J. Biol. Chem. 2022, 298, 101561. [Google Scholar] [CrossRef] [PubMed]
- Meister, J.; Bone, D.B.J.; Knudsen, J.R.; Barella, L.F.; Velenosi, T.J.; Akhmedov, D.; Lee, R.J.; Cohen, A.H.; Gavrilova, O.; Cui, Y.; et al. Clenbuterol exerts antidiabetic activity through metabolic reprogramming of skeletal muscle cells. Nat. Commun. 2022, 13, 22. [Google Scholar] [CrossRef] [PubMed]
- Spiller, H.A.; James, K.J.; Bs, S.S.; Borys, D.J. A Descriptive Study of Adverse Events from Clenbuterol Misuse and Abuse for Weight Loss and Bodybuilding. Subst. Abus. 2013, 34, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Jeddi, S.; Yousefzadeh, N.; Kashfi, K.; Ghasemi, A. Role of nitric oxide in type 1 diabetes-induced osteoporosis. Biochem. Pharmacol. 2021, 197, 114888. [Google Scholar] [CrossRef]
- Two Flavones from Scutellaria Baicalensis Georgi and Their Binding Affinities to the Benzodiazepine Site of the GABAA Receptor Complex—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/12561253/ (accessed on 3 February 2022).
- Yu, M.; Han, S.; Wang, M.; Han, L.; Huang, Y.; Bo, P.; Fang, P.; Zhang, Z. Baicalin protects against insulin resistance and metabolic dysfunction through activation of GALR2/GLUT4 signaling. Phytomedicine 2022, 95, 153869. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, K.; Li, X.; Peng, D.; Falck, J.R.; Anugu, R.R.; Chiusa, M.; Stafford, J.M.; Wasserman, D.H.; Zent, R.; Luther, J.M.; et al. EET Analog Treatment Improves Insulin Signaling in a Genetic Mouse Model of Insulin Resistance. Diabetes 2022, 71, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, W.H.; Whelan, S.A.; Lee, N. Tryptophan, kynurenine pathway, and diabetic ketoacidosis in type 1 diabetes. PLoS ONE 2021, 16, e0254116. [Google Scholar] [CrossRef] [PubMed]
- Burhop, M.; Schuchardt, J.P.; Nebl, J.; Müller, M.; Lichtinghagen, R.; Hahn, A. Marine Oil from C. finmarchicus Enhances Glucose Homeostasis and Liver Insulin Resistance in Obese Prediabetic Individuals. Nutrients 2022, 14, 396. [Google Scholar] [CrossRef]
- Yari, Z.; Cheraghpour, M.; Hekmatdoost, A. Flaxseed and/or hesperidin supplementation in metabolic syndrome: An open-labeled randomized controlled trial. Eur. J. Nutr. 2020, 60, 287–298. [Google Scholar] [CrossRef]
- Abdel-Mottaleb, Y.; Ali, H.S.; El-Kherbetawy, M.K.; Elkazzaz, A.Y.; ElSayed, M.H.; Elshormilisy, A.; Eltrawy, A.H.; Abed, S.Y.; Alshahrani, A.M.; Hashish, A.A.; et al. Saponin-rich extract of Tribulus terrestris alleviates systemic inflammation and insulin resistance in dietary obese female rats: Impact on adipokine/hormonal disturbances. Biomed. Pharmacother. 2022, 147, 112639. [Google Scholar] [CrossRef]
- Al-Malki, A.L. Potential impact of auxin in modulation of insulin resistance in diabetic rats. Arch. Physiol. Biochem. 2020, 128, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.-W.; Yang, H.-K.; Jun, M.-S.; Lee, B.-C. Puerarin Attenuates Obesity-Induced Inflammation and Dyslipidemia by Regulating Macrophages and TNF-Alpha in Obese Mice. Biomedicines 2022, 10, 175. [Google Scholar] [CrossRef] [PubMed]
- Colchicine to Suppress Inflammation and Improve Insulin Resistance in Adults and Adolescents with Obesity—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT05017571 (accessed on 4 March 2022).
- Piattelli, M.; Minale, L.; Nicolaus, R. Pigments of centrospermae—V.: Betaxanthins from Mirabilis jalapa L. Phytochemistry 1965, 4, 817–823. [Google Scholar] [CrossRef]
- Terzo, S.; Attanzio, A.; Calvi, P.; Mulè, F.; Tesoriere, L.; Allegra, M.; Amato, A. Indicaxanthin from Opuntia ficus-indica Fruit Ameliorates Glucose Dysmetabolism and Counteracts Insulin Resistance in High-Fat-Diet-Fed Mice. Antioxidants 2021, 11, 80. [Google Scholar] [CrossRef]
- Prevention with Oleanolic Acid of Insulin Resistance—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT05049304 (accessed on 4 March 2022).
- Liu, W.; Li, Z.; Feng, C.; Hu, S.; Yang, X.; Xiao, K.; Nong, Q.; Xiao, Q.; Wu, K.; Li, X.-Q.; et al. The structures of two polysaccharides from Angelica sinensis and their effects on hepatic insulin resistance through blocking RAGE. Carbohydr. Polym. 2022, 280, 119001. [Google Scholar] [CrossRef]
- Song, H.; Shen, X.; Deng, R.; Chu, Q.; Zheng, X. Pomegranate peel anthocyanins prevent diet-induced obesity and insulin resistance in association with modulation of the gut microbiota in mice. Eur. J. Nutr. 2022. online ahead of print. [Google Scholar] [CrossRef]
- Watanabe, Y.; Nessa, N.; Toba, H.; Kobara, M.; Nakata, T. Angelica acutiloba Exerts Antihypertensive Effect and Improves Insulin Resistance in Spontaneously Hypertensive Rats Fed with a High-Fat Diet. Pharmacology 2022, 107, 188–196. [Google Scholar] [CrossRef]
- Zhou, Q.-B.; Chen, Y.; Zhang, Y.; Li, D.-D.; Wang, H.-Q.; Jia, Z.-J.; Jin, Y.; Xu, F.-Q.; Zhang, Y. Hypermethylation Effects of Yiqihuoxue Decoction in Diabetic Atherosclerosis Using Genome-Wide DNA Methylation Analyses. J. Inflamm. Res. 2022, 15, 163–176. [Google Scholar] [CrossRef]
- Zhang, C.; Han, M.; Zhang, X.; Tong, H.; Sun, X.; Sun, G. Ginsenoside Rb1 Protects Against Diabetic Cardiomyopathy by Regulating the Adipocytokine Pathway. J. Inflamm. Res. 2022, 15, 71–83. [Google Scholar] [CrossRef]
- Guo, C.; Liao, W.; Qiu, R.; Zhou, D.; Ni, W.; Yu, C.; Zeng, Y. Aurantio-obtusin improves obesity and insulin resistance induced by high-fat diet in obese mice. Phytotherapy Res. 2021, 35, 346–360. [Google Scholar] [CrossRef]
- Huang, P.; Yang, L.; Liu, Y.; Jiang, Y.; Li, Y.; Chen, Z.; Song, H.; Zheng, P. Lanzhang Granules Ameliorate Nonalcoholic Fatty Liver Disease by Regulating the PPARα Signaling Pathway. Evid.-Based Complement. Altern. Med. 2022, 2022, 1124901. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Mariscal, F.M.; Cardelo, M.P.; de la Cruz, S.; Alcala-Diaz, J.F.; Ramos, I.R.; Guler, I.; Vals-Delgado, C.; López-Moreno, A.; Luque, R.M.; Delgado-Lista, J.; et al. Reduction in Circulating Advanced Glycation End Products by Mediterranean Diet Is Associated with Increased Likelihood of Type 2 Diabetes Remission in Patients with Coronary Heart Disease: From the Cordioprev Study. Mol. Nutr. Food Res. 2021, 65, e1901290. [Google Scholar] [CrossRef] [PubMed]
- Huffman, K.M.; Parker, D.C.; Bhapkar, M.; Racette, S.B.; Martin, C.K.; Redman, L.M.; Das, S.K.; Connelly, M.A.; Pieper, C.F.; Orenduff, M.; et al. Calorie restriction improves lipid-related emerging cardiometabolic risk factors in healthy adults without obesity: Distinct influences of BMI and sex from CALERIE™ a multicentre, phase 2, randomised controlled trial. eClinicalMedicine 2022, 43, 101261. [Google Scholar] [CrossRef] [PubMed]
- Allehdan, S.; Basha, A.; Hyassat, D.; Nabhan, M.; Qasrawi, H.; Tayyem, R. Effectiveness of carbohydrate counting and Dietary Approach to Stop Hypertension dietary intervention on managing Gestational Diabetes Mellitus among pregnant women who used metformin: A randomized controlled clinical trial. Clin. Nutr. 2022, 41, 384–395. [Google Scholar] [CrossRef]
- Bjørklund, G.; Tippairote, T.; Dadar, M.; Lizcano, F.; Aaseth, J.; Borisova, O. The Roles of Dietary, Nutritional and Lifestyle Interventions in Adipose Tissue Adaptation and Obesity. Curr. Med. Chem. 2021, 28, 1683–1702. [Google Scholar] [CrossRef]
- Alvarez-Alvarez, I.; Zazpe, I.; de Rojas, J.P.; Bes-Rastrollo, M.; Ruiz-Canela, M.; Fernandez-Montero, A.; Hidalgo-Santamaría, M.; Martínez-González, M.A. Mediterranean diet, physical activity and their combined effect on all-cause mortality: The Seguimiento Universidad de Navarra (SUN) cohort. Prev. Med. 2018, 106, 45–52. [Google Scholar] [CrossRef]
- Tosatti, J.A.G.; Alves, M.T.; Gomes, K.B. The Role of the Mediterranean Dietary Pattern on Metabolic Control of Patients with Diabetes Mellitus: A Narrative Review. Adv. Exp. Med. Biol. 2020, 1307, 115–128. [Google Scholar] [CrossRef]
- Mirabelli, M.; Brunetti, A. The Rise and Fall of the Mediterranean Diet and Related Nutrients in Preventing Diabetes. Nutrients 2022, 14, 379. [Google Scholar] [CrossRef]
- Tagde, P.; Tagde, S.; Bhattacharya, T.; Tagde, P.; Akter, R.; Rahman, H. Multifaceted Effects of Intermittent Fasting in the Treatment and Prevention of Diabetes, Cancer and Obesity or Other Chronic Diseases. Curr. Diabetes Rev. 2021, 18, e131221198789. [Google Scholar] [CrossRef]
- Zhang, Y.; Xue, Y.; Zhang, D.; Liu, Y.; Xu, Z.; Gao, J.; Li, W.; Li, X. Effect of Vitamin D Supplementation on Glycemic Control in Prediabetes: A Meta-Analysis. Nutrients 2021, 13, 4464. [Google Scholar] [CrossRef]
- Hassanabadi, M.; Mohri, M.; Seifi, H.A. Effects of vitamin D3 injection in close-up period on insulin resistance and energy balance in transition dairy cows. Vet. Med. Sci. 2021. [Google Scholar] [CrossRef] [PubMed]
- Charatcharoenwitthaya, P.; Tansakul, E.; Chaiyasoot, K.; Bandidniyamanon, W.; Charatcharoenwitthaya, N. Dietary Composition and Its Association with Newly Diagnosed Nonalcoholic Fatty Liver Disease and Insulin Resistance. Nutrients 2021, 13, 4438. [Google Scholar] [CrossRef] [PubMed]
- Fiber and Insulin Sensitivity—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04714944 (accessed on 4 March 2022).
- Zou, J.; Huang, J. Effect of High-Quality Nursing on Blood Glucose Level, Psychological State, and Treatment Compliance of Patients with Gestational Diabetes Mellitus. Am. J. Transl. Res. 2021, 13, 13084. [Google Scholar] [PubMed]
- Maioli, T.U.; Borras-Nogues, E.; Torres, L.; Barbosa, S.C.; Martins, V.D.; Langella, P.; Azevedo, V.A.; Chatel, J.-M. Possible Benefits of Faecalibacterium prausnitzii for Obesity-Associated Gut Disorders. Front. Pharmacol. 2021, 12, 740636. [Google Scholar] [CrossRef]
- Li, D.; Zhu, Y.; Wang, Y.; Zou, Q.; Duan, J.; Sun-Waterhouse, D.; Sun, B. Perspectives on diacylglycerol-induced improvement of insulin sensitivity in type 2 diabetes. Food Sci. Hum. Wellness 2022, 11, 230–237. [Google Scholar] [CrossRef]
- Sheng, Y.; Xia, F.; Chen, L.; Lv, Y.; Lv, S.; Yu, J.; Liu, J.; Ding, G. Differential Responses of White Adipose Tissue and Brown Adipose Tissue to Calorie Restriction During Aging. J. Gerontol. Ser. A 2021, 76, 393–399. [Google Scholar] [CrossRef]
- Escalante-Araiza, F.; Gutiérrez-Salmeán, G. Traditional Mexican foods as functional agents in the treatment of cardiometabolic risk factors. Crit. Rev. Food Sci. Nutr. 2020, 61, 1353–1364. [Google Scholar] [CrossRef]
- The Effects of a Moderate Weight Loss on Insulin Resistance—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02193295 (accessed on 4 March 2022).
- Effects of Intermittent Fasting on Insulin Resistance, Cardiac Metabolism, and Cerebral Perfusion—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT05181267 (accessed on 4 March 2022).
- Von Loeffelholz, C.; Roth, J.; Coldewey, S.M.; Birkenfeld, A.L. The Role of Physical Activity in Nonalcoholic and Metabolic Dysfunction Associated Fatty Liver Disease. Biomedicines 2021, 9, 1853. [Google Scholar] [CrossRef]
- Parameshwar, A.; Maiya, G.A.; Kamath, S.U.; Shastry, B.A. Ravishankar Lifestyle Modification with Physical Activity Promotion on Leptin Resistance and Quality of Life in Metabolic Syndrome—A Systematic Review with Meta-Analysis. Curr. Diabetes Rev. 2021, 17, 345–355. [Google Scholar] [CrossRef]
- The Effect of 12-Week Circuit Training on Insulin Sensitivity and Endothelial Function in Women with Insulin Resistance—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04528693 (accessed on 4 March 2022).
- Chang, J.; Namkung, J. Effects of Exercise Intervention on Mitochondrial Stress Biomarkers in Metabolic Syndrome Patients: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2021, 18, 2242. [Google Scholar] [CrossRef]
- Taheri, A.; Mirzababaei, A.; Setayesh, L.; Yarizadeh, H.; Shiraseb, F.; Imani, H.; Clark, C.C.; Mirzaei, K. The relationship between Dietary approaches to stop hypertension diet adherence and inflammatory factors and insulin resistance in overweight and obese women: A cross-sectional study. Diabetes Res. Clin. Pract. 2021, 182, 109128. [Google Scholar] [CrossRef] [PubMed]
- Adeva-Andany, M.M.; Martínez-Rodríguez, J.; González-Lucán, M.; Fernández-Fernández, C.; Castro-Quintela, E. Insulin resistance is a cardiovascular risk factor in humans. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, T.W.; Pawlak, K.; Karbowska, M.; Znorko, B.; Mor, A.L.; Mysliwiec, M.; Pawlak, D. The Impact of Antihypertensive Pharmacotherapy on Interplay between Protein-Bound Uremic Toxin (Indoxyl Sulfate) and Markers of Inflammation in Patients with Chronic Kidney Disease. Int. Urol. Nephrol. 2019, 51, 491–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazarzadeh, M.; Bidel, Z.; Canoy, D.; Copland, E.; Wamil, M.; Majert, J.; Smith Byrne, K.; Sundström, J.; Teo, K.; Davis, B.R.; et al. Blood Pressure Lowering and Risk of New-Onset Type 2 Diabetes: An Individual Participant Data Meta-Analysis. Lancet 2021, 398, 1803–1810. [Google Scholar] [CrossRef]
- Sánchez-Martínez, L.; Periago, M.J.; García-Alonso, J.; García-Conesa, M.T.; González-Barrio, R. A Systematic Review of the Cardiometabolic Benefits of Plant Products Containing Mixed Phenolics and Polyphenols in Postmenopausal Women: Insufficient Evidence for Recommendations to This Specific Population. Nutrients 2021, 13, 4276. [Google Scholar] [CrossRef]
- Papuc, C.; Goran, G.V.; Predescu, C.N.; Tudoreanu, L.; Ștefan, G. Plant polyphenols mechanisms of action on insulin resistance and against the loss of pancreatic beta cells. Crit. Rev. Food Sci. Nutr. 2020, 62, 325. [Google Scholar] [CrossRef]
- Karlafti, E.; Didangelos, T.; Benioudakis, E.; Kotzakioulafi, E.; Kaiafa, G.; Kotsis, V.; Ziakas, A.; Doumas, M.; Goulas, A.; Savopoulos, C. Effects of Moxonidine Administration on Serum Neuropeptide Y Levels in Hypertensive Individuals: A Prospective Observational Study. Endocrines 2022, 3, 43–52. [Google Scholar] [CrossRef]
- Kozono, M.; Uto, H.; Ibusuki, R.; Arima, S.; Oda, K.; Taguchi, H.; Sasaki, F.; Nasu, Y.; Hashimoto, S.; Setoyama, H.; et al. Antihypertensive Therapy Improves Insulin Resistance and Serum Levels of Interleukin-6 and-10 in Spontaneously Hypertensive Rats with Steatohepatitis. Mol. Med. Rep. 2016, 14, 5385–5394. [Google Scholar] [CrossRef] [Green Version]
- Xiao, W.Y.; Ning, N.; Tan, M.H.; Jiang, X.S.; Zhou, L.; Liu, L.; Yi, D.; Wei, P. Effects of Antihypertensive Drugs Losartan and Levamlodipine Besylate on Insulin Resistance in Patients with Essential Hypertension Combined with Isolated Impaired Fasting Glucose. Hypertens. Res. 2016, 39, 321–326. [Google Scholar] [CrossRef]
- Rane, T.; Thorat, S.T.; Kulkarni, A.R.; Avhad, A.B.; Mankar, P.; Kaila, N.K. Prevalence of Insulin Resistance among Hypertensive Patients Undergoing Antihypertensive Therapy. VIMS Health Sci. J. 2021, 8, 136–140. [Google Scholar] [CrossRef]
- Sai, N.; Kumar, S.; Vanamala, N.; Vallampalli, G.; Kumar Thatikonda, A.; Padala, R. Insulin Resistance as an Inflammatory Marker for Ischemic Stroke Severity Among Non-Diabetics: A Prospective Study. J. Neurol. Res. 2016, 6, 46–50. [Google Scholar] [CrossRef]
- Chang, Y.; Kim, C.K.; Kim, M.K.; Seo, W.-K.; Oh, K. Insulin Resistance Is Associated with Poor Functional Outcome after Acute Ischemic Stroke in Non-Diabetic Patients. Sci. Rep. 2021, 11, 1229. [Google Scholar] [CrossRef] [PubMed]
- Kernan, W.N.; Viscoli, C.M.; Furie, K.L.; Young, L.H.; Inzucchi, S.E.; Gorman, M.; Guarino, P.D.; Lovejoy, A.M.; Peduzzi, P.N.; Conwit, R.; et al. Pioglitazone after Ischemic Stroke or Transient Ischemic Attack. N. Engl. J. Med. 2016, 374, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- A Clinical Trial to Prevent the Complications of Insulin Resistance (including Type-2 Diabetes)—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT00015626 (accessed on 4 March 2022).
- Gee, S.E.; Ma’Ayeh, M.; Kniss, D.; Landon, M.B.; Gabbe, S.G.; Rood, K.M. Glycemic Control and Aspirin Resistance in Patients Taking Low-Dose Aspirin for Pre-eclampsia Prevention. Am. J. Perinatol. 2021, 39, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Bako, H.Y.; Ibrahim, M.A.; Isah, M.S.; Ibrahim, S. Inhibition of JAK-STAT and NF-κB signalling systems could be a novel therapeutic target against insulin resistance and type 2 diabetes. Life Sci. 2019, 239, 117045. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhao, J.; Zhang, Y.; Wu, L.; Yu, Z.; He, D.; Huang, H.; Qu, W.; Luo, X. Triglyceride glucose index influences platelet reactivity in acute ischemic stroke patients. BMC Neurol. 2021, 21, 409. [Google Scholar] [CrossRef]
- Jia, W.; Jia, Q.; Zhang, Y.; Zhao, X.; Wang, Y. Association between insulin resistance and aspirin or clopidogrel resistance in Chinese patients with recent ischemic stroke/TIA. Neurol. Res. 2021, 43, 406–411. [Google Scholar] [CrossRef]
- Giallauria, F.; Strisciuglio, T.; Cuomo, G.; Di Lorenzo, A.; D’Angelo, A.; Volpicelli, M.; Izzo, R.; Manzi, M.V.; Barbato, E.; Morisco, C. Exercise Training: The Holistic Approach in Cardiovascular Prevention. High Blood Press. Cardiovasc. Prev. 2021, 28, 561–577. [Google Scholar] [CrossRef]
- Ibsen, D.B.; Levitan, E.B.; Åkesson, A.; Gigante, B.; Wolk, A. The DASH diet is associated with a lower risk of heart failure: A cohort study. Eur. J. Prev. Cardiol. 2022. online ahead of print. [Google Scholar] [CrossRef]
- Esfandiari, S.; Bahadoran, Z.; Mirmiran, P.; Tohidi, M.; Azizi, F. Adherence to the dietary approaches to stop hypertension trial (DASH) diet is inversely associated with incidence of insulin resistance in adults: The Tehran lipid and glucose study. J. Clin. Biochem. Nutr. 2017, 61, 123–129. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, S.; Ohsaki, Y.; Shimizu, M.; Nako, K.; Okamura, M.; Kabayama, S.; Tabata, K.; Tanaka, Y.; Ito, S. Electrolyzed hydrogen-rich water for oxidative stress suppression and improvement of insulin resistance: A multicenter prospective double-blind randomized control trial. Diabetol. Int. 2021, 13, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Newsholme, P.; Keane, K.N.; Carlessi, R.; Cruzat, V. Oxidative Stress Pathways in Pancreatic β-Cells and Insulin-Sensitive Cells and Tissues: Importance to Cell Metabolism, Function, and Dysfunction. Am. J. Physiol. Cell Physiol. 2019, 317, C420–C433. [Google Scholar] [CrossRef] [PubMed]
- Penno, G.; Solini, A.; Orsi, E.; Bonora, E.; Fondelli, C.; Trevisan, R.; Vedovato, M.; Cavalot, F.; Zerbini, G.; Lamacchia, O.; et al. Insulin resistance, diabetic kidney disease, and all-cause mortality in individuals with type 2 diabetes: A prospective cohort study. BMC Med. 2021, 19, 66. [Google Scholar] [CrossRef] [PubMed]
- Ladarixin as Adjunctive Therapy to Improve Insulin Sensitivity and Glucometabolic Outcomes in Type 1 Diabetes—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT05035368 (accessed on 4 March 2022).
- Nkondjock, V.R.N.; Wabo, T.M.C.; Kosgey, J.C.; Zhang, Y.; Amporfro, D.A.; Adnan, H.; Shah, I.; Li, Y. Insulin Resistance, Serum Calcium and Hypertension: A Cross-Sectional Study of a Multiracial Population, and a Similarity Assessment of Results from a Single-Race Population’s Study. Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 14, 3361–3373. [Google Scholar] [CrossRef] [PubMed]
- White, P.E.; Król, E.; Szwengiel, A.; Tubacka, M.; Szczepankiewicz, D.; Staniek, H.; Vincent, J.B.; Krejpcio, Z. Effects of Bitter Melon and a Chromium Propionate Complex on Symptoms of Insulin Resistance and Type 2 Diabetes in Rat Models. Biol. Trace Element Res. 2021, 199, 1013–1026. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolosowicz, M.; Prokopiuk, S.; Kaminski, T.W. Recent Advances in the Treatment of Insulin Resistance Targeting Molecular and Metabolic Pathways: Fighting a Losing Battle? Medicina 2022, 58, 472. https://doi.org/10.3390/medicina58040472
Wolosowicz M, Prokopiuk S, Kaminski TW. Recent Advances in the Treatment of Insulin Resistance Targeting Molecular and Metabolic Pathways: Fighting a Losing Battle? Medicina. 2022; 58(4):472. https://doi.org/10.3390/medicina58040472
Chicago/Turabian StyleWolosowicz, Marta, Slawomir Prokopiuk, and Tomasz W. Kaminski. 2022. "Recent Advances in the Treatment of Insulin Resistance Targeting Molecular and Metabolic Pathways: Fighting a Losing Battle?" Medicina 58, no. 4: 472. https://doi.org/10.3390/medicina58040472
APA StyleWolosowicz, M., Prokopiuk, S., & Kaminski, T. W. (2022). Recent Advances in the Treatment of Insulin Resistance Targeting Molecular and Metabolic Pathways: Fighting a Losing Battle? Medicina, 58(4), 472. https://doi.org/10.3390/medicina58040472





