Comparative Evaluation of Influence of Nd:YAG Laser (1064 nm) and 980 nm Diode Laser on Enamel around Orthodontic Brackets: An In Vitro Study
Abstract
1. Introduction
- There will be no changes to the enamel surface before and after the experimental procedure.
- There will be no difference in the percentage of chemical elements in the assessed areas before and after laser irradiation.
- The two wavelengths will not differently influence the chemical composition of the enamel.
2. Materials and Methods
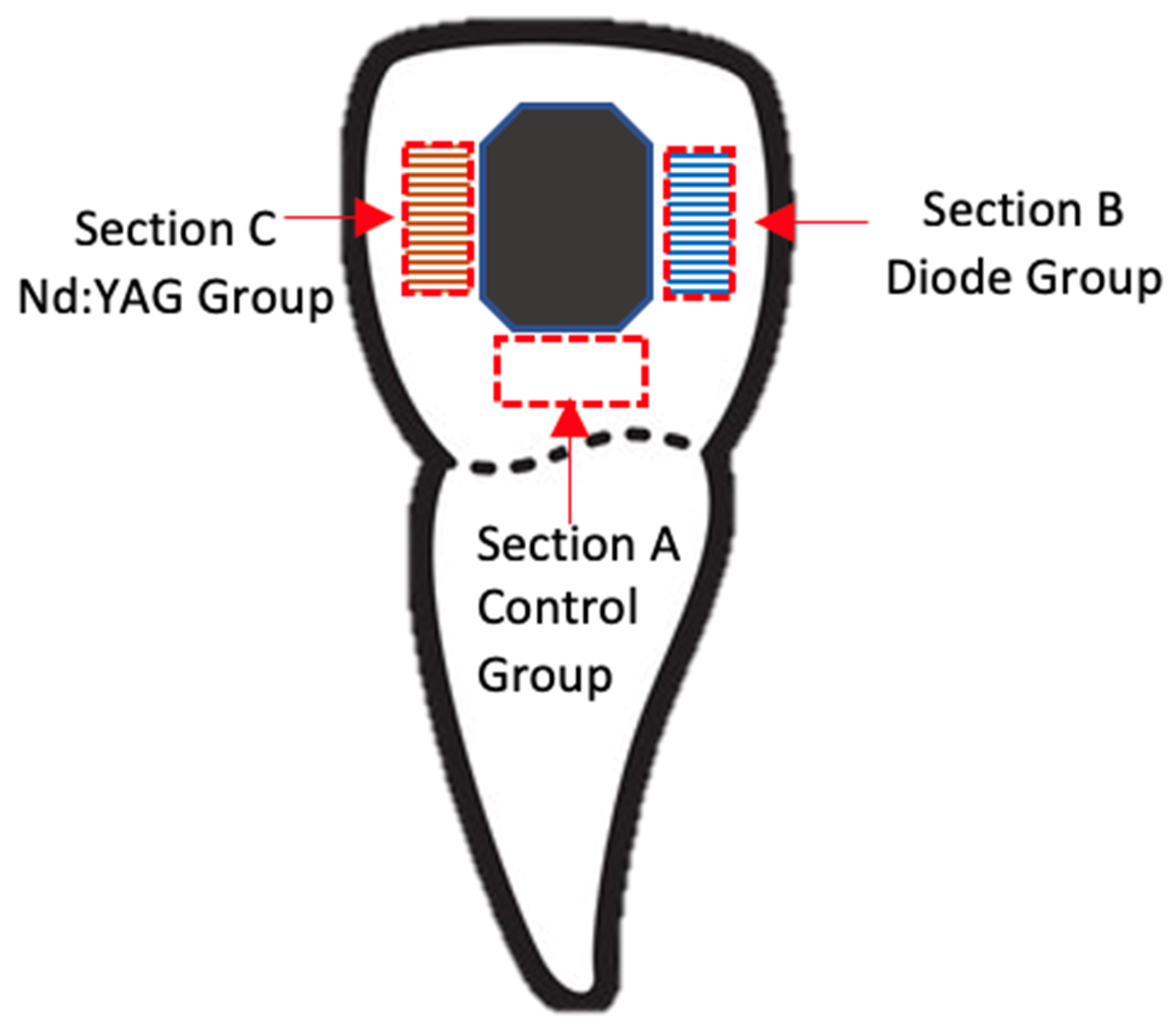
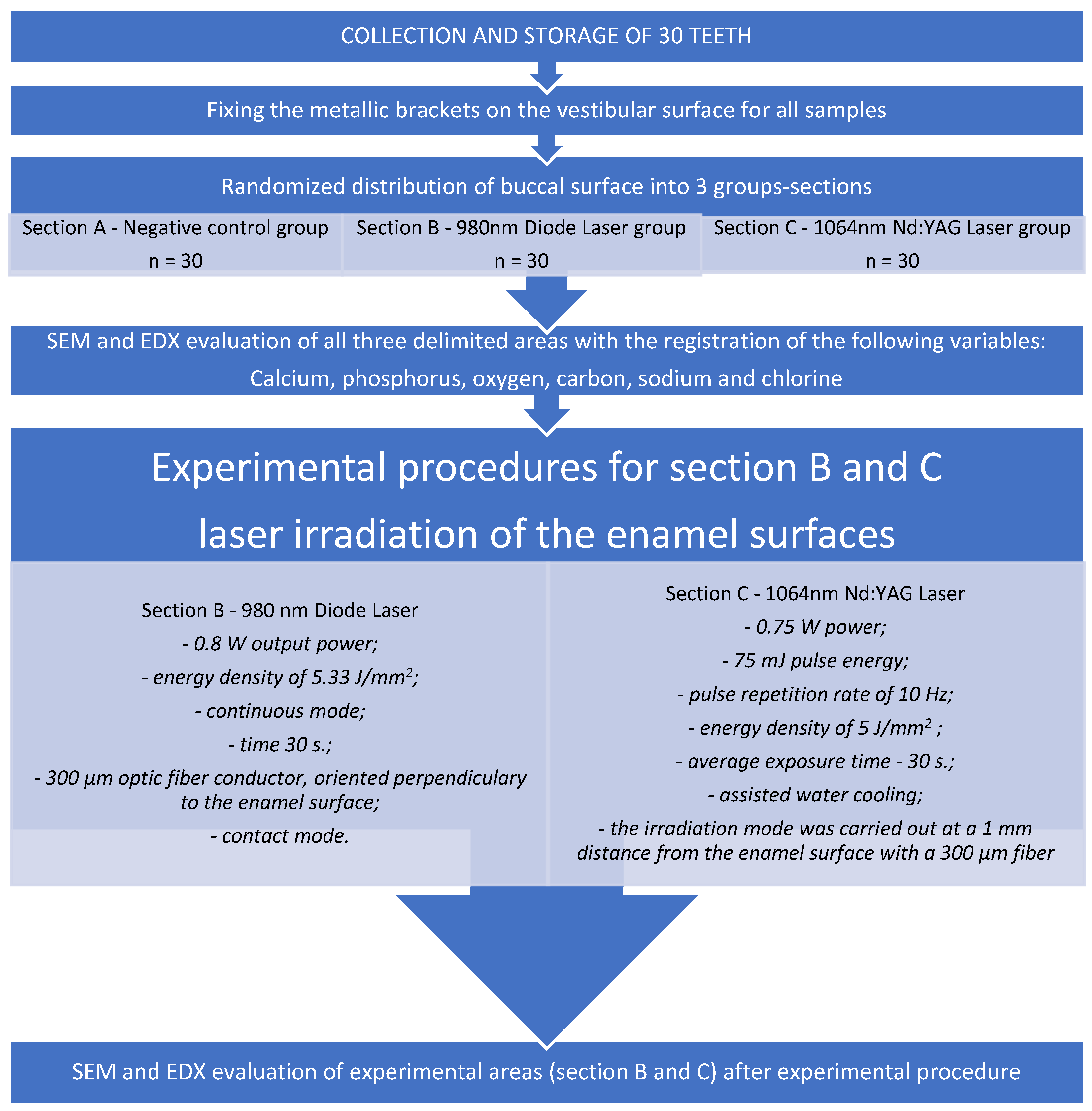
2.1. Tooth Selection and Preparation Phase
2.2. Experimental Procedures
- The 980 nm diode laser irradiation conditions were as follows. The diode laser was applied with a 0.8 W output power, an energy density of 5.33 J/mm2, and in continuous mode to Section B for 30 s. A 300 µm optic fiber conductor, as a transmission element, was attached and oriented perpendicularly to the enamel surface. The optic fiber tip was used in contact mode, and irradiation was performed by hand by the same operator who screened the enamel surface in a uniform motion in order to cover the entire selected area.
- The Nd:YAG laser irradiation conditions were as follows. The laser was used with a fixed wavelength of 1064 nm, a power of 0.75 W, a 75 mJ pulse energy, a pulse repetition rate of 10 Hz, an energy density of 5 J/mm2, an average exposure time of 30 s, and with assisted water cooling. The irradiation mode was carried out at a 1 mm distance from the enamel surface with a 300 µm fiber in a sweeping movement that scanned once in a horizontal direction in order to cover the entire tested area and was done by the same operator to avoid human errors.
2.3. Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray Spectroscopy (EDX) Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Msallam, F.A.; Grawish, M.E.-A.; Hafez, A.M.; Abdelnaby, Y.L. Decalcification prevention around orthodontic brackets bonded to bleached enamel using different topical agents. Prog. Orthod. 2017, 18, 15. [Google Scholar] [CrossRef] [PubMed]
- Bowen, W.H.; Burne, R.A.; Wu, H.; Koo, H. Oral Biofilms: Pathogens, Matrix, and Polymicrobial Interactions in Micro-environments. Trends Microbiol. 2018, 26, 229–242. [Google Scholar] [CrossRef]
- Borzabadi-Farahani, A.; Eslamipour, F.; Asgari, I. Association between orthodontic treatment need and caries experience. Acta Odontol. Scand. 2011, 69, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Degli Esposti, L.; Ionescu, A.C.; Brambilla, E.; Tampieri, A.; Iafisco, M. Characterization of a Toothpaste Containing Bi-oactive Hydroxyapatites and In Vitro Evaluation of Its Efficacy to Remineralize Enamel and to Occlude Dentinal Tubules. Materials 2020, 13, 2928. [Google Scholar] [CrossRef] [PubMed]
- Neel, E.A.A.; Aljabo, A.; Strange, A.; Ibrahim, S.; Coathup, M.; Young, A.M.; Bozec, L.; Mudera, V. Demineralization–remineralization dynamics in teeth and bone. Int. J. Nanomed. 2016, 11, 4743–4763. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Deng, X.; Wu, Y. Remineralizing Nanomaterials for Minimally Invasive Dentistry. In Nanotechnology in Endodontics; Springer Science and Business Media LLC: Cham, Switzerland, 2015; pp. 173–193. [Google Scholar]
- Perrini, F.; Lombardo, L.; Arreghini, A.; Medori, S.; Siciliani, G. Caries prevention during orthodontic treatment: In-vivo assessment of high-fluoride varnish to prevent white spot lesions. Am. J. Orthod. Dentofac. Orthop. 2016, 149, 238–243. [Google Scholar] [CrossRef]
- Peng, Y.; Qian, Z.; Ting, Z.; Jie, F.; Xiaomei, X.; Li, M. The effect of resin infiltration vs. fluoride varnish in enhancing enamel surface conditions after interproximal reduction. Dent. Mater. J. 2016, 35, 756–761. [Google Scholar] [CrossRef][Green Version]
- Vicente, A.; Ortiz-Ruiz, A.; Paz, B.M.G.; López, J.G.; Bravo-González, L.-A. Efficacy of fluoride varnishes for preventing enamel demineralization after interproximal enamel reduction. Qualitative and quantitative evaluation. PLoS ONE 2017, 12, e0176389. [Google Scholar] [CrossRef]
- Vicente, A.; Ruiz, A.J.O.; López, M.G.; Beneyto, Y.M.; Bravo-González, L.-A. Enamel Resistance to Demineralization After Bracket Debonding Using Fluoride Varnish. Sci. Rep. 2017, 7, 15183. [Google Scholar] [CrossRef]
- Moharam, L.-M.; Sadony, D.-M.; Nagi, S.-M. Evaluation of diode laser application on chemical analysis and surface microhardness of white spots enamel lesions with two remineralizing agents. J. Clin. Exp. Dent. 2020, 12, e271–e276. [Google Scholar] [CrossRef]
- Al-Shaker, S.M.; Nayif, M.M.; Al-Sabawi, N.A. Microhardness of Artificially Demineralized Enamel treated with Different Regimes of ACP-CPP and Fluoride Agents. Int. J. Enhan Res. Sci. Tech. Eng. 2014, 3, 102–107. [Google Scholar]
- Chen, L.; Hontsu, S.; Komasa, S.; Yamamoto, E.; Hashimoto, Y.; Matsumoto, N. Hydroxyapatite Film Coating by Er:YAG Pulsed Laser Deposition Method for the Repair of Enamel Defects. Materials 2021, 14, 7475. [Google Scholar] [CrossRef] [PubMed]
- Belcheva, A.; El Feghali, R.; Nihtianova, T.; Parker, S. Effect of the carbon dioxide 10,600-nm laser and topical fluoride gel application on enamel microstructure and microhardness after acid challenge: An in vitro study. Lasers Med Sci. 2018, 33, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Scatolin, R.S.; Colucci, V.; Lepri, T.P.; Alexandria, A.; Maia, L.C.; Galo, R.; Borsatto, M.C.; Corona, S.A.M. Er:YAG laser irradiation to control the progression of enamel erosion: An in situ study. Lasers Med Sci. 2014, 30, 1465–1473. [Google Scholar] [CrossRef]
- Jorge, A.C.T.; Cassoni, A.; de Freitas, P.M.; Reis, A.F.; Junior, A.B.; Rodrigues, J.A. Influence of Cavity Preparation with Er,Cr:YSGG Laser and Restorative Materials on In Situ Secondary Caries Development. Photomed. Laser Surg. 2015, 33, 98–103. [Google Scholar] [CrossRef]
- de Oliveira, R.M.; de Souza, V.M.; Esteves, C.M.; Lima-Arsati, Y.B.D.O.; Cassoni, A.; Rodrigues, J.A.; Junior, A.B. Er,Cr:YSGG Laser Energy Delivery: Pulse and Power Effects on Enamel Surface and Erosive Resistance. Photomed. Laser Surg. 2017, 35, 639–646. [Google Scholar] [CrossRef]
- El Mansy, M.M.; Gheith, M.; El Yazeed, A.M.; Farag, D.B.E. Influence of Er, Cr: YSGG (2780 nm) and Nanosecond Nd: YAG Laser (1064 nm) Irradiation on Enamel Acid Resistance: Morphological and Elemental Analysis. Open Access Maced. J. Med Sci. 2019, 7, 1828–1833. [Google Scholar] [CrossRef]
- Stangler, L.P.; Romano, F.L.; Shirozaki, M.U.; Galo, R.; Afonso, A.M.; Borsatto, M.C.; Matsumoto, M.A.N. Microhardness of enamel adjacent to orthodontic brac- kets after CO2 laser irradiation and fluoride application. Braz. Dent. J. 2013, 24, 508–512. [Google Scholar] [CrossRef][Green Version]
- Poosti, M.; Ahrari, F.; Moosavi, H.; Najjaran, H. The effect of fractional CO2 laser irradiation on remineralization of enamel white spot lesions. Lasers Med Sci. 2014, 29, 1349–1355. [Google Scholar] [CrossRef]
- Souza-Gabriel, A.E.; Turssi, C.P.; Colucci, V.; Tenuta, L.M.A.; Serra, M.C.; Corona, S.A.M. In situ study of the anticariogenic potential of fluoride varnish combined with CO2 laser on enamel. Arch. Oral Biol. 2015, 60, 804–810. [Google Scholar] [CrossRef]
- Ahrari, F.; Mohammadipour, H.-S.; Hajimomenian, L.; Fallah-Rastegar, A. The effect of diode laser irradiation associated with photoabsorbing agents containing remineralizing materials on microhardness, morphology and chemical structure of early enamel caries. J. Clin. Exp. Dent. 2018, 10, e955–e962. [Google Scholar] [PubMed]
- Bahrololoomi, Z.; Lotfian, M. Effect of Diode Laser Irradiation Combined with Topical Fluoride on Enamel Microhardness of Primary Teeth. J. Dent. Tehran Univ. Med Sci. 2015, 12, 85–89. [Google Scholar]
- Heravi, F.; Ahrari, F.; Mahdavi, M.; Basafa, S. Comparative evaluation of the effect of Er:YAG laser and low level laser irradiation combined with CPP-ACPF cream on treatment of enamel caries. J. Clin. Exp. Dent. 2014, 6, e121–e126. [Google Scholar] [CrossRef] [PubMed]
- Borzabadi-Farahani, A. The Adjunctive Soft-Tissue Diode Laser in Orthodontics. Compend. Contin. Educ. Dent. 2017, 38, e18–e31. [Google Scholar] [PubMed]
- Nermin, M.; Yussif, M.S.C.; Ali, M.; Saafan, S.; Mehani, S. Post-Cure Irradiation of Pit and Fissure Sealant by Diode Laser Part II, Dental Laser Application; Department National Institute of Laser Enhanced Sciences, Cairo University: Giza, Egypt, 2013. [Google Scholar]
- Da Silva Barbosa, P.; da Ana, P.A.; Poiate, I.A.; Zezell, D.M.; de Sant’ Anna, G.R. Dental enamel irradiated with a low- intensity infrared laser and photoabsorbing cream: A study of microhardness, surface, and pulp temperature. Photomed. Laser Surg. 2013, 31, 439–446. [Google Scholar] [CrossRef]
- Moghadam, N.C.Z.; Seraj, B.; Chiniforush, N.; Ghadimi, S. Effects of Laser and Fluoride on the Prevention of Enamel Demineralization: An In Vitro Study. J. Lasers Med Sci. 2018, 9, 177–182. [Google Scholar] [CrossRef]
- Díaz-Monroy, J.M.; Contreras-Bulnes, R.; Olea-Mejia, O.F.; García-Fabila, M.M.; Rodriguez-Vilchis, L.E.; Sánchez-Flores, I.; Centeno-Pedraza, C. Chemical Changes Associated with Increased Acid Resistance of Er:YAG Laser Irradiated Enamel. Sci. World J. 2014, 2014, 1–6. [Google Scholar] [CrossRef]
- Corrêa-Afonso, A.M.; Bachmann, L.; de Almeida, C.G.; Corona, S.; Borsatto, M.C. FTIR and SEM analysis of CO2 laser irradiated human enamel. Arch. Oral Biol. 2012, 57, 1153–1158. [Google Scholar] [CrossRef]
- Rodríguez-Vilchis, L.E.; Contreras-Bulnes, R.; Mejìa, O.F.O.L.; Sánchez-Flores, I.; Centeno-Pedraza, C. Morpho- logical and structural changes on human dental enamel after Er:YAG laser irradiation: AFM, SEM, and EDS evaluation. Photomed. Laser Surg. 2011, 29, 493–500. [Google Scholar] [CrossRef]
- Suhaimi, F.M.; Alam, N.Z.Z.; Ariffin, S.M.; Razak, N.A.A.; Razab, M.K.A.A. The Effect of Fluence Variations of Nd:YAG Laser Ablation and Sample Condition on Human Tooth. Adv. Sci. Technol. Eng. Syst. J. 2018, 3, 398–406. [Google Scholar] [CrossRef]
- Elkabbany, S.M.H.; Mosleh, A.A.; Metwally, N.I. Remineralization effect of diode laser, Nanoseal®, and Zamzam water on initial enamel carious lesions induced around orthodontic brackets. J. Nat. Sci. Med. 2021, 4, 50–57. [Google Scholar] [CrossRef]
- Lara-Carrilloa, E.; Doroteo-Chimalb, C.; Lopez-Gonzaleza, S.; Kubodera-Itoa, T.; Morales-Luckiec, R.A.; Olea-Mejiac, O.F.; Medina-Solisd, C.E. Remineralization effect of low-level laser and amorphous sodium–calcium–phosphosilicate paste in teeth with fixed orthodontic appliances. Tanta Dent. J. 2016, 13, 55. [Google Scholar] [CrossRef]
- Mahmoudzadeh, M.; Alijani, S.; Soufi, L.R.; Farhadian, M.; Namdar, F.; Karami, S. Effect of CO2 Laser on the Prevention of White Spot Lesions During Fixed Orthodontic Treatment: A Randomized Clinical Trial. Turk. J. Orthod. 2019, 32, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Sundararaj, D.; Venkatachalapathy, S.; Tandon, A.; Pereira, A. Critical evaluation of incidence and prevalence of white spot lesions during fixed orthodontic appliance treatment: A meta-analysis. J. Int. Soc. Prev. Community Dent. 2015, 5, 433–439. [Google Scholar] [PubMed]
- Umana, M.; Heysselaer, D.; Tielemans, M.; Compere, P.; Zeinoun, T.; Nammour, S. Dentinal Tubules Sealing by Means of Diode Lasers (810 and 980 nm): A Preliminary In Vitro Study. Photomed. Laser Surg. 2013, 31, 307–314. [Google Scholar] [CrossRef]
- Borzabadi-Farahani, A. A Scoping Review of the Efficacy of Diode Lasers Used for Minimally Invasive Exposure of Impacted Teeth or Teeth with Delayed Eruption. Photonics 2022, 9, 265. [Google Scholar] [CrossRef]
- Gan, J.; Liu, S.; Zhou, L.; Wang, Y.; Guo, J.; Huang, C. Effect of Nd:YAG Laser Irradiation Pretreatment on the Long-Term Bond Strength of Etch-and-Rinse Adhesive to Dentin. Oper. Dent. 2017, 42, 62–72. [Google Scholar] [CrossRef]
- Borzabadi-Farahani, A.; Borzabadi, E.; Lynch, E. Nanoparticles in orthodontics, a review of antimicrobial and anti-caries applications. Acta Odontol. Scand. 2014, 72, 413–417. [Google Scholar] [CrossRef]
- Al-Jedani, S.; Al-Hadeethi, Y.; Ansari, M.S.; Razvi, M. Dental Hard Tissue Ablation with Laser Irradiation. Austin Dent. Sci. 2016, 1, 1007. [Google Scholar]
- Patil, A.R. Comparative Evaluation of Efficacy of Iontophoresis with 0.33% Sodium Fluoride Gel and Diode Laser Alone on Occlusion of Dentinal Tubules. J. Clin. Diagn. Res. 2017, 11, ZC123–ZC126. [Google Scholar] [CrossRef]
- Shahabi, S.; Fekrazad, R.; Johari, M.; Chiniforoush, N.; Rezaei, Y. FT-Raman spectroscopic characterization of enamel surfaces irradiated with Nd:YAG and Er:YAG lasers. J. Dent. Res. Dent. Clin. Dent. Prospect. 2016, 10, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Zulkifli, N.; Suhaimi, F.; Khairul, M.; Razab, A.; Jaafar, M.; Mokhtar, N. The Use of Nd: YAG Laser for Ablation of Dental Material; IACSIT Press: Singapore, 2015. [Google Scholar]
- Dias-Moraes, M.C.; Castro, P.A.A.; Pereira, D.L.; Ana, P.A.; Freitas, A.Z.; Zezell, D.M. Assessment of the preventive effects of Nd:YAG laser associated with fluoride on enamel caries using optical coherence tomography and FTIR spectroscopy. PLoS ONE 2021, 16, e0254217. [Google Scholar] [CrossRef]
- Alia, S. Effect of Diode Laser Office Bleaching on Mineral Content and Surface Topography of Enamel Surface: An SEM Study. Int. J. Clin. Pediatr. Dent. 2021, 13, 481–485. [Google Scholar] [CrossRef]
- Suhaimi, F.M.; Alam, N.Z.Z.; Ariffin, S.M.; Razak, N.A.A.; Razab, M.K.A.A. Surface modifications of human tooth using Nd:YAG laser for dental applications. In Proceedings of the 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Jeju, Korea, 11–15 July 2017; pp. 4537–4540. [Google Scholar]
- Neto, W.R.; Lepri, C.P.; Romano, J.J.F.; Fernandes, F.S.; Raucci, L.M.S.D.C.; Bachmann, L.; Dibb, R.G.P. Chemical and Morphological Changes of Primary Teeth Irradiated with Nd:YAG Laser: AnEx VivoLong-Term Analysis. Photomed. Laser Surg. 2015, 33, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Al-Maliky, M.A.; Frentzen, M.; Meister, J. Laser-assisted prevention of enamel caries: A 10-year review of the literature. Lasers Med Sci. 2019, 35, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Dilber, E.; Malkoc, M.A.; Ozturk, A.N.; Ozturk, F. Effect of various laser irradiations on the mineral content of dentin. Eur. J. Dent. 2013, 7, 74–80. [Google Scholar]
- Salinovic, I.; Schauperl, Z.; Marcius, M.; Miletic, I. The Effects of Three Remineralizing Agents on the Microhardness and Chemical Composition of Demineralized Enamel. Materials 2021, 14, 6051. [Google Scholar] [CrossRef]
- Scholz, K.J.; Federlin, M.; Hiller, K.-A.; Ebensberger, H.; Ferstl, G.; Buchalla, W. EDX-analysis of fluoride precipitation on human enamel. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Bakr, M.A.; Saafan, A.M.; El Maghraby, E.M.F.; El Rouby, D.H.; Ahmed, S.F. Microhardness and Ultrastructure of De-mineralized Gamma-Irradiated Human Enamel after Diode Laser (980 nm) and Fluoride Surface Treatment. Braz. Arch. Biol. Technol. 2019, 62, 1–9. [Google Scholar] [CrossRef]
- Harazaki, M.; Hayakawa, K.; Fukui, T.; Isshiki, Y.; Powell, L.G. The Nd-YAG Laser is Useful in Prevention of Dental Caries During Orthodontic Treatment. Bull. Tokyo Dent. Coll. 2001, 42, 79–86. [Google Scholar] [CrossRef]
- Nandkumar, A.; Iyer, V.H. In vitro analysis comparing efficacy of lasers and desensitizing agents on dentin tubule oc-clusion: A scanning electron microscope study. Int. J. Laser Dent. 2014, 4, 1–7. [Google Scholar]
- Anderson, A.M.; Kao, E.; Gladwin, M.; Benli, O.; Ngan, P. The effects of argon laser irradiation on enamel decalcification: An in vivo study. Am. J. Orthod. Dentofac. Orthop. 2002, 122, 251–259. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pereira, D.L.; Freitas, A.Z.; Bachmann, L.; Benetti, C.; Zezell, D.M.; Ana, P.A. Variation on Molecular Structure, Crystallinity, and Optical Properties of Dentin Due to Nd:YAG Laser and Fluoride Aimed at Tooth Erosion Prevention. Int. J. Mol. Sci. 2018, 19, 433. [Google Scholar] [CrossRef] [PubMed]
- Talwar, M.; Borzabadi-Farahani, A.; Lynch, E.; Borsboom, P.; Ruben, J. Remineralization of Demineralized Enamel and Dentine Using 3 Dentifrices—An InVitro Study. Dent. J. 2019, 7, 91. [Google Scholar] [CrossRef]
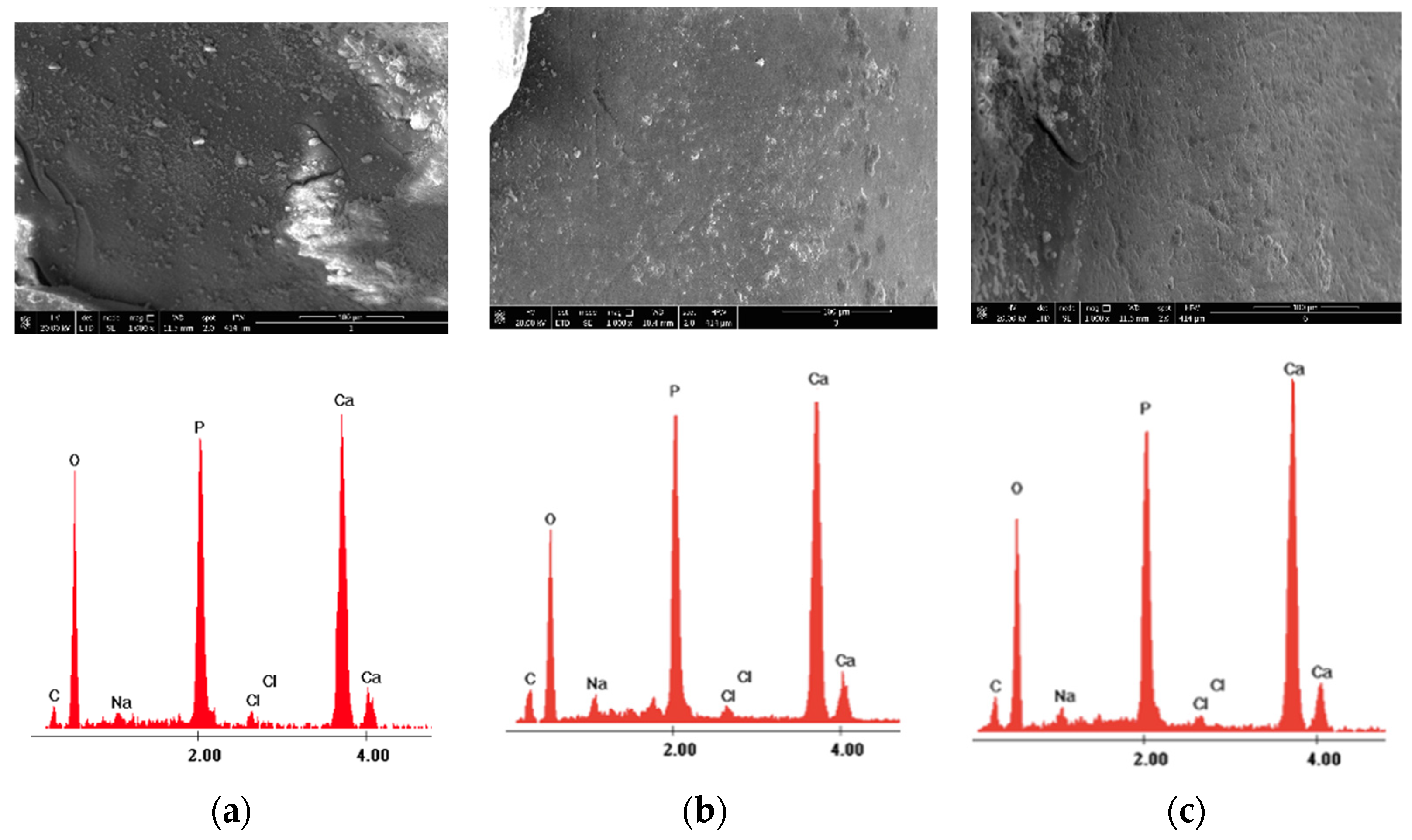
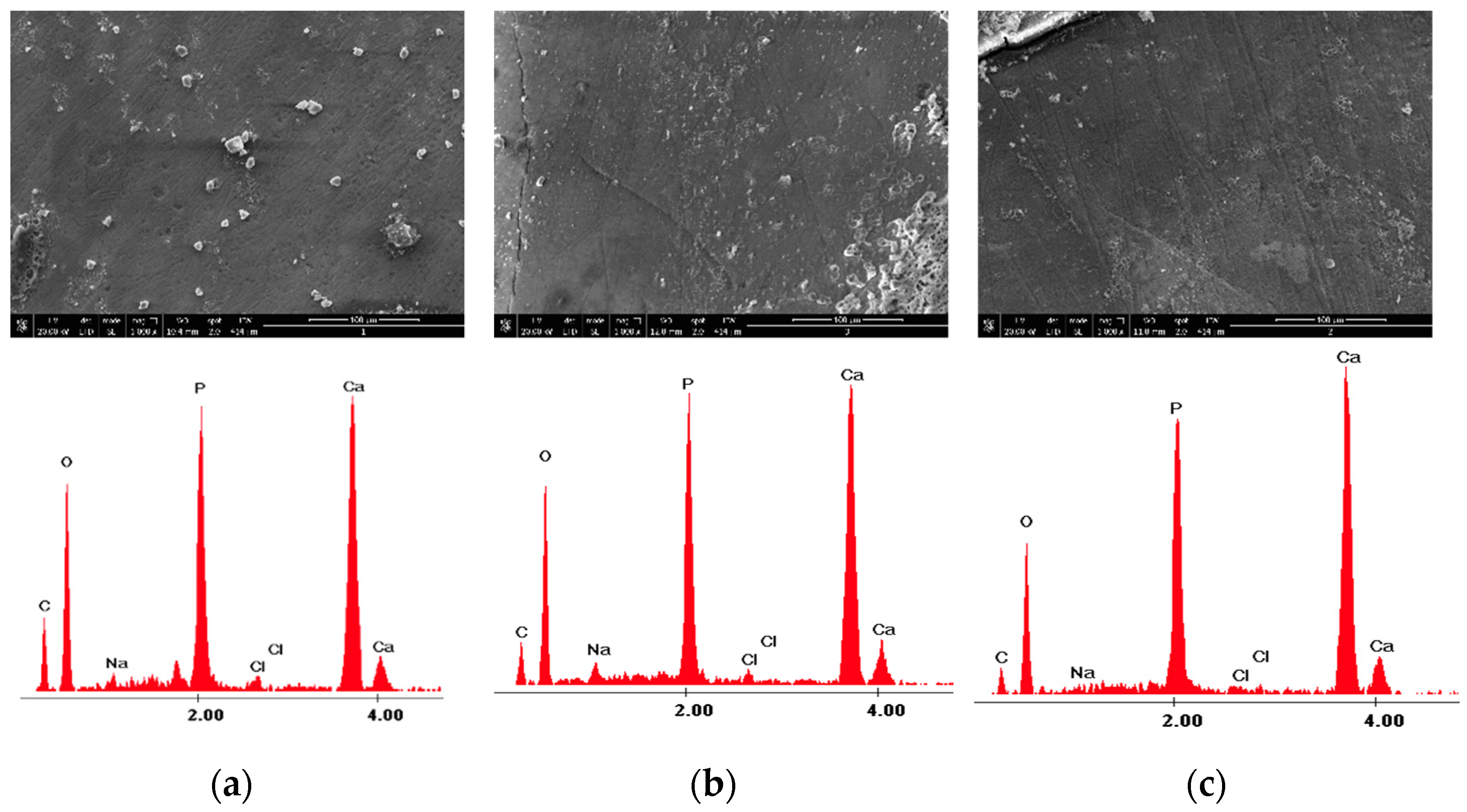
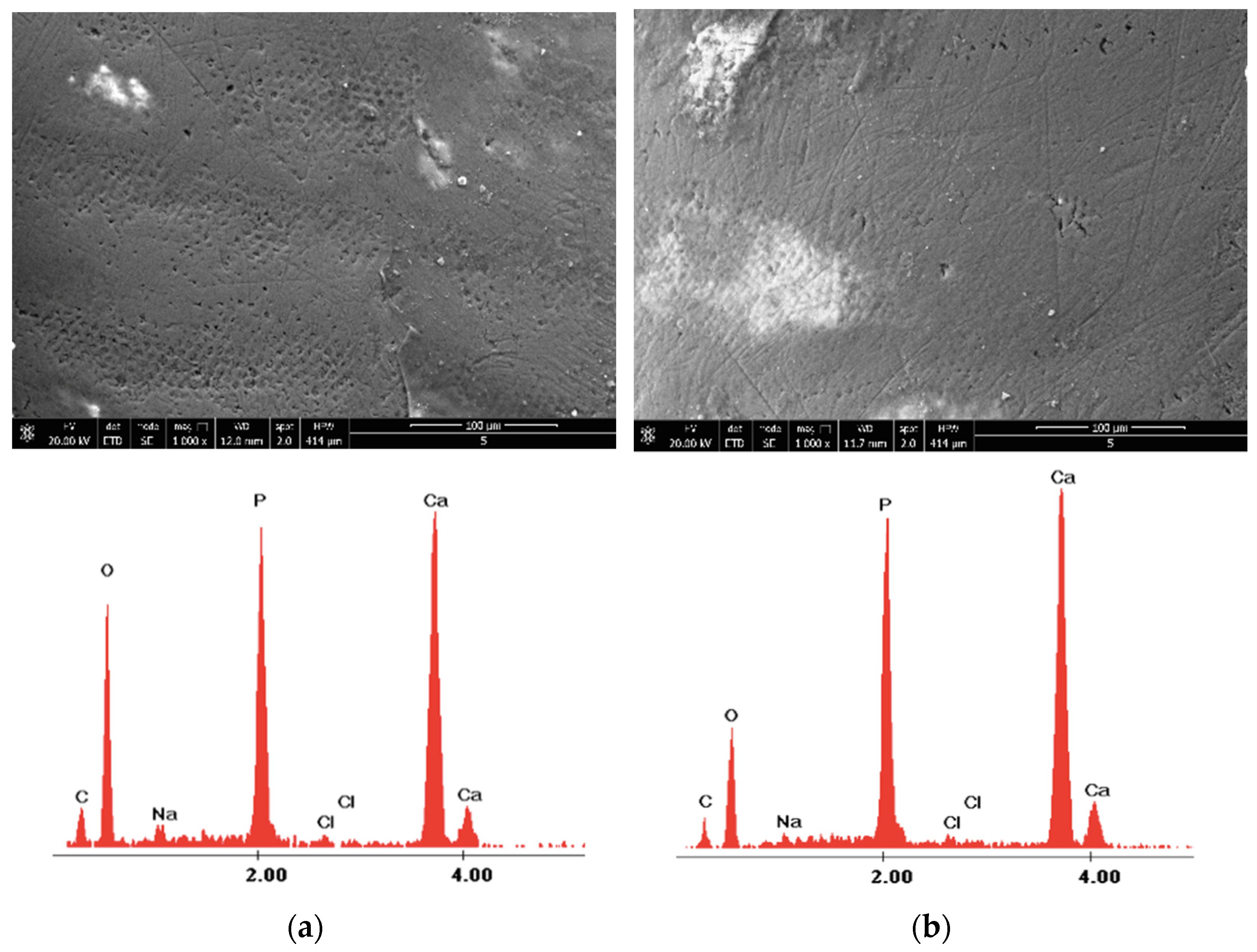
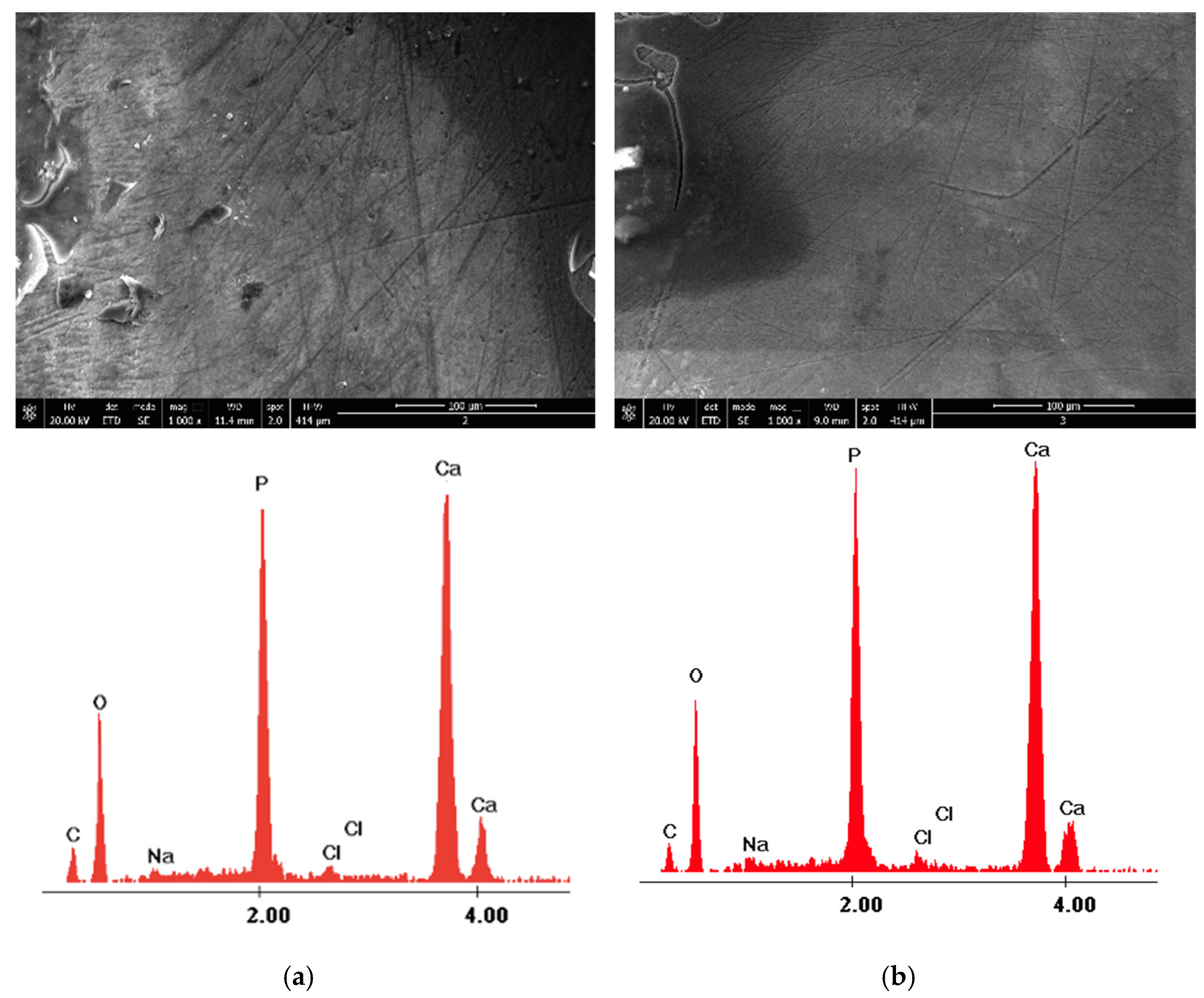
| Control Group-Section A | 980 nm Diode Laser Group-Section B—before Laser Irradiation | 980 nm Diode Laser Group-Section B—after Laser Irradiation | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | |
| Ca wt% | 21.46 ± 1.72 | 21.06 ± 3.03 | 28.24 ± 3.78 |
| P wt% | 17.37 ± 2.27 | 17.20 ± 2.18 | 16.92 ± 3.02 |
| O wt% | 48.40 ± 3.82 | 49.55 ± 3.85 | 47.02 ± 5.01 |
| C wt% | 11.42 ± 2.07 | 10.69 ± 2.73 | 6.72 ± 2.25 |
| Other elements (Na,Cl) wt% | 1.27 ± 0.67 | 1.56 ± 0.82 | 1.08 ± 0.76 |
| 1064 nm Nd:YAG Laser Group-Section C—before Laser Irradiation | 1064 nm Nd:YAG Laser Group-Section C—after Laser Irradiation | |
|---|---|---|
| Mean(SD) | Mean(SD) | |
| Ca wt% | 21.31 ± 1.75 | 33.88 ± 2.09 |
| P wt% | 17.46 ± 2.09 | 18.28 ± 2.55 |
| O wt% | 48.76 ± 2.53 | 42.89 ± 3.69 |
| C wt% | 10.85 ± 1.51 | 3.69 ± 1.11 |
| Other elements (Na,Cl) wt% | 1.63 ± 1.38 | 1.41 ± 0.80 |
| Mean (SD) | N | ||
|---|---|---|---|
| Pair 1 | Ca wt% section B (initial) | 21.06 ± 3.03 | 30 |
| Ca wt% section B (after laser) | 28.24 ± 3.78 | 30 | |
| Pair 2 | Ca wt% section C (initial) | 21.31 ± 1.75 | 30 |
| Ca wt% section C (after laser) | 33.88 ± 2.09 | 30 | |
| Pair 3 | P wt% section B (initial) | 17.20 ± 2.18 | 30 |
| P wt% section B (after laser) | 16.92 ± 3.02 | 30 | |
| Pair 4 | P wt% section C (initial) | 17.46 ± 1.97 | 30 |
| P wt% section C (after laser) | 18.28 ± 2.55 | 30 | |
| Pair 5 | O wt% section B (initial) | 49.55 ± 3.85 | 30 |
| O wt% section B (after laser) | 47.02 ± 5.01 | 30 | |
| Pair 6 | O wt% section C (initial) | 48.76 ± 2.53 | 30 |
| O wt% section C (after laser) | 42.89 ± 3.69 | 30 | |
| Pair 7 | C wt% section B (initial) | 10.69 ± 2.73 | 30 |
| C wt% section B (after laser) | 6.72 ± 2.25 | 30 | |
| Pair 8 | C wt% section C (initial) | 10.85 ± 1.51 | 30 |
| C wt% section C (after laser) | 3.69 ± 1.11 | 30 | |
| Pair 9 | Other elements wt% section B (initial) | 1.56 ± 0.82 | 30 |
| Other elements wt% section B (after laser) | 1.08 ± 0.76 | 30 | |
| Pair 10 | Other elements wt% section C (initial) | 1.63 ± 1.38 | 30 |
| Other elements wt% section C (after laser) | 1.41 ± 0.80 | 30 |
| Paired Differences | Significance | ||||
|---|---|---|---|---|---|
| Mean (SD) | 95% CI Lower—Upper | One-Sided p | Two-Sided p | ||
| Pair 1 | Ca wt% section B (initial)—Ca wt% section B (after laser) | −7.18 ± 1.88 | −7.88; −6.47 | <0.001 | <0.001 |
| Pair 2 | Ca wt% section C (initial)—Ca wt% section C (after laser) | −12.57 ± 2.29 | −13.43; −11.71 | <0.001 | <0.001 |
| Pair 3 | P wt% section B (initial)—P wt% section B (after laser) | 0.27 ± 1.84 | −0.41; 0.96 | 0.207 | 0.415 |
| Pair 4 | P wt% section C (initial)—P wt% section C (after laser) | −0.82 ± 1.83 | −1.50; −0.14 | 0.010 | 0.020 |
| Pair 5 | O wt% section B (initial)—O wt% section B (after laser) | 2.53 ± 3.59 | 1.18; 3.87 | <0.001 | <0.001 |
| Pair 6 | O wt% section C (initial)—O wt% section C (after laser) | 5.86 ± 2.84 | 4.80; 6.92 | <0.001 | <0.001 |
| Pair 7 | C wt% section B (initial)—C wt% section B (after laser) | 3.97 ± 1.84 | 3.28; 4.66 | <0.001 | <0.001 |
| Pair 8 | C wt% section C (initial)—C wt% section C (after laser) | 7.16 ± 1.43 | 6.62; 7.69 | <0.001 | <0.001 |
| Pair 9 | Others wt% section B (initial)—Others wt% section B (after laser) | 0.48 ± 1.17 | 0.04; 0.92 | 0.016 | 0.032 |
| Pair 10 | Others wt% section C (initial)—Others wt% section C (after laser) | 0.22 ± 1.74 | −0.43; 0.87 | 0.247 | 0.494 |
| Laser Type | N | Mean (SD) | |
|---|---|---|---|
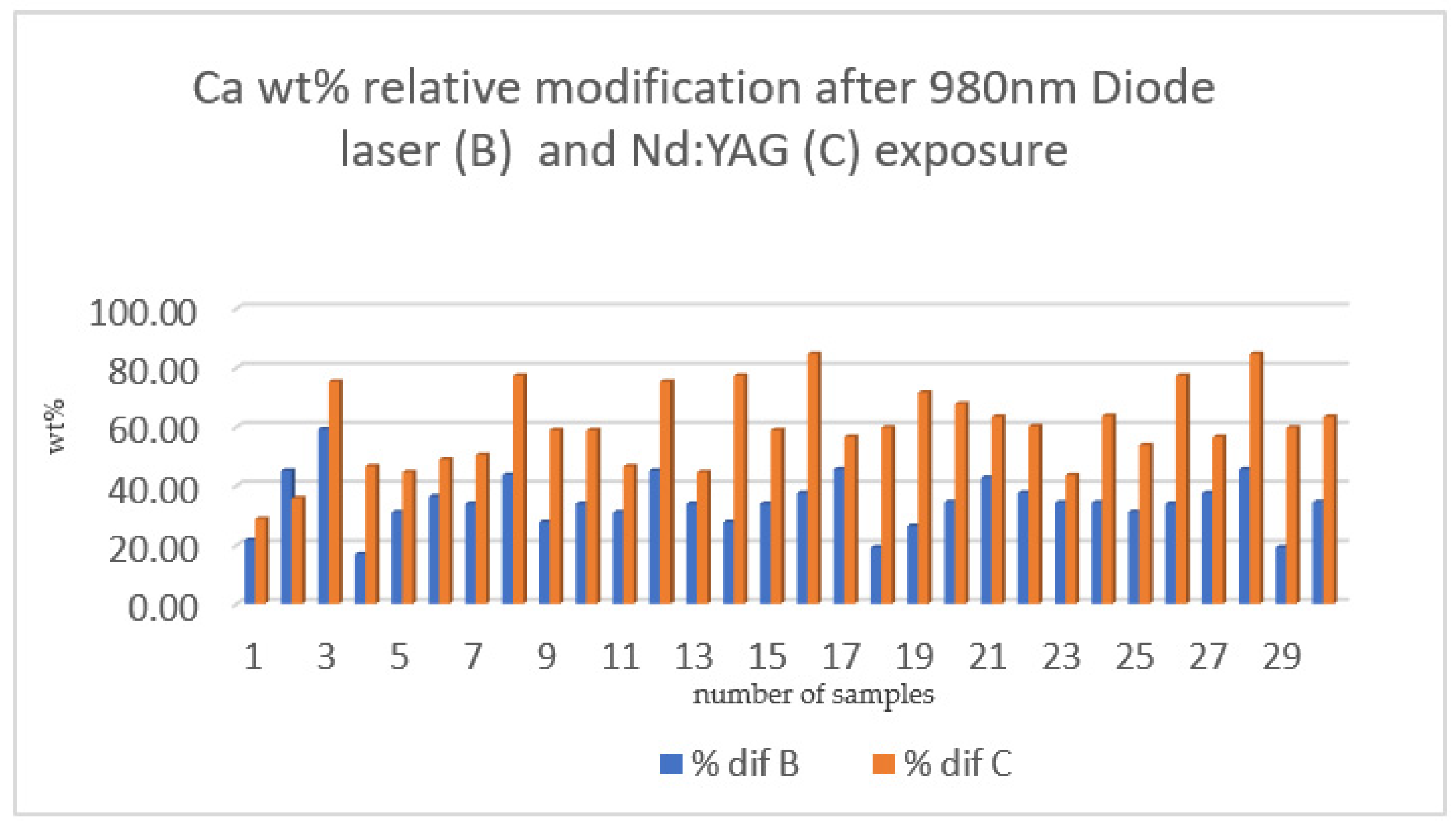
| % DIF OF SECTION B % DIF OF SECTION C related to Calcium | 980 nm diode laser | 30 | 34.49 ± 9.10 |
| Nd:YAG laser | 30 | 59.81 ± 14.06 | |
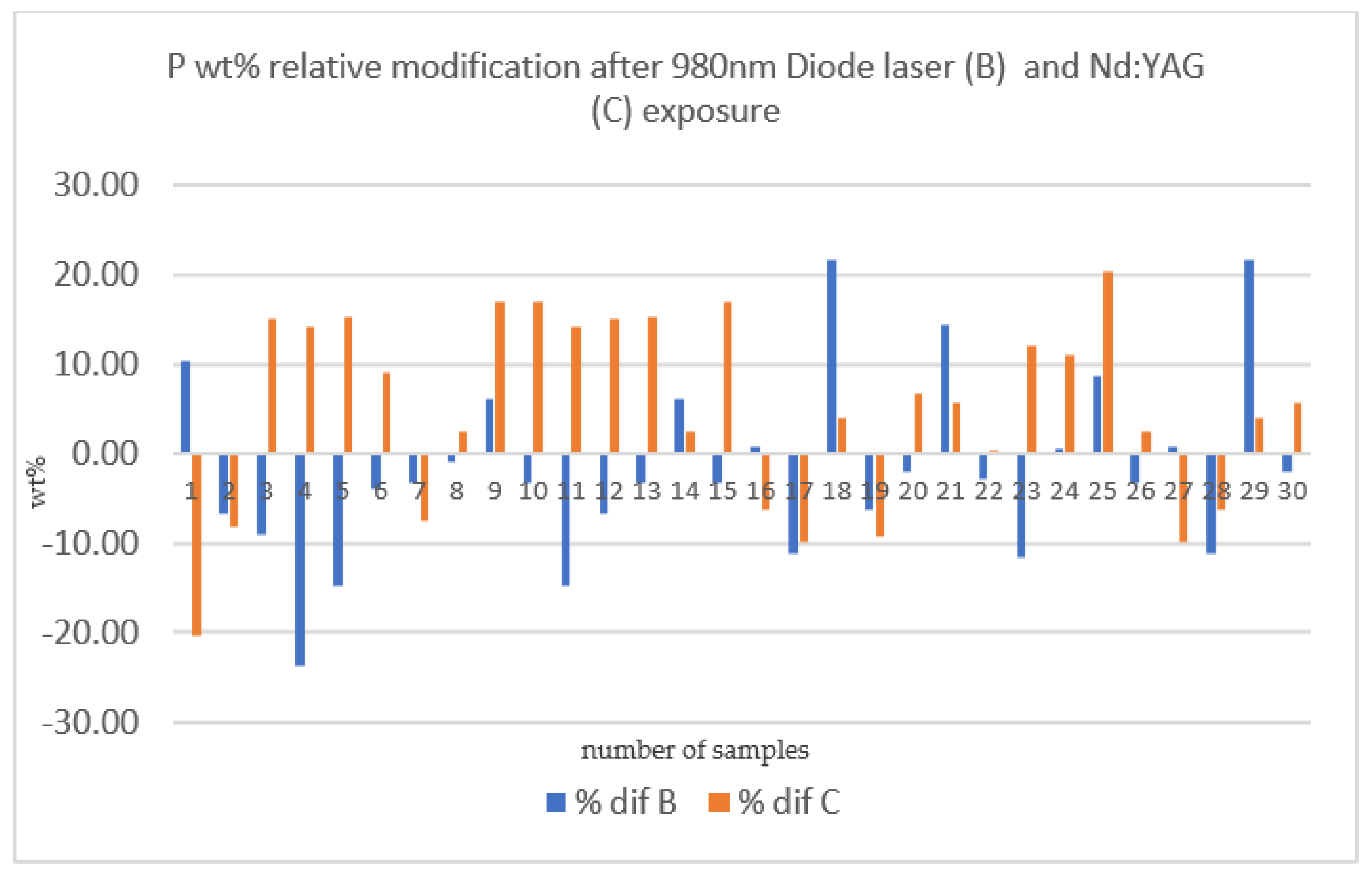
| % DIF OF SECTION B % DIF OF SECTION C related to Phosphorus | 980 nm diode laser | 30 | −1.77 ± 10.16 |
| Nd:YAG laser | 30 | 4.91 ± 10.59 | |
| t-Test for Equality of Means | Significance | |||
|---|---|---|---|---|
| df | One-Sided p | Two-Sided p | ||
|
% dif B (980 nm diode laser) % dif C (Nd:YAG) Calcium | Equal variances assumed | 58 | <0.001 | <0.001 |
| Equal variances not assumed | 49.68 | <0.001 | <0.001 | |
|
% dif B (980 nm diode laser) % dif C (Nd:YAG) Phosphorus | Equal variances assumed | 58 | 0.008 | 0.015 |
| Equal variances not assumed | 57.90 | 0.008 | 0.015 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mocuta, D.-E.; Grad, O.; Mateas, M.; Luca, R.; Carmen Todea, D. Comparative Evaluation of Influence of Nd:YAG Laser (1064 nm) and 980 nm Diode Laser on Enamel around Orthodontic Brackets: An In Vitro Study. Medicina 2022, 58, 633. https://doi.org/10.3390/medicina58050633
Mocuta D-E, Grad O, Mateas M, Luca R, Carmen Todea D. Comparative Evaluation of Influence of Nd:YAG Laser (1064 nm) and 980 nm Diode Laser on Enamel around Orthodontic Brackets: An In Vitro Study. Medicina. 2022; 58(5):633. https://doi.org/10.3390/medicina58050633
Chicago/Turabian StyleMocuta(Bojoga), Daliana-Emanuela, Oana Grad(Buriac), Marius Mateas, Ruxandra Luca, and Darinca Carmen Todea. 2022. "Comparative Evaluation of Influence of Nd:YAG Laser (1064 nm) and 980 nm Diode Laser on Enamel around Orthodontic Brackets: An In Vitro Study" Medicina 58, no. 5: 633. https://doi.org/10.3390/medicina58050633
APA StyleMocuta, D.-E., Grad, O., Mateas, M., Luca, R., & Carmen Todea, D. (2022). Comparative Evaluation of Influence of Nd:YAG Laser (1064 nm) and 980 nm Diode Laser on Enamel around Orthodontic Brackets: An In Vitro Study. Medicina, 58(5), 633. https://doi.org/10.3390/medicina58050633








