The Influence of Maternal Factors on Neonatal Intensive Care Unit Admission and In-Hospital Mortality in Premature Newborns from Western Romania: A Population-Based Study
Abstract
:1. Introduction
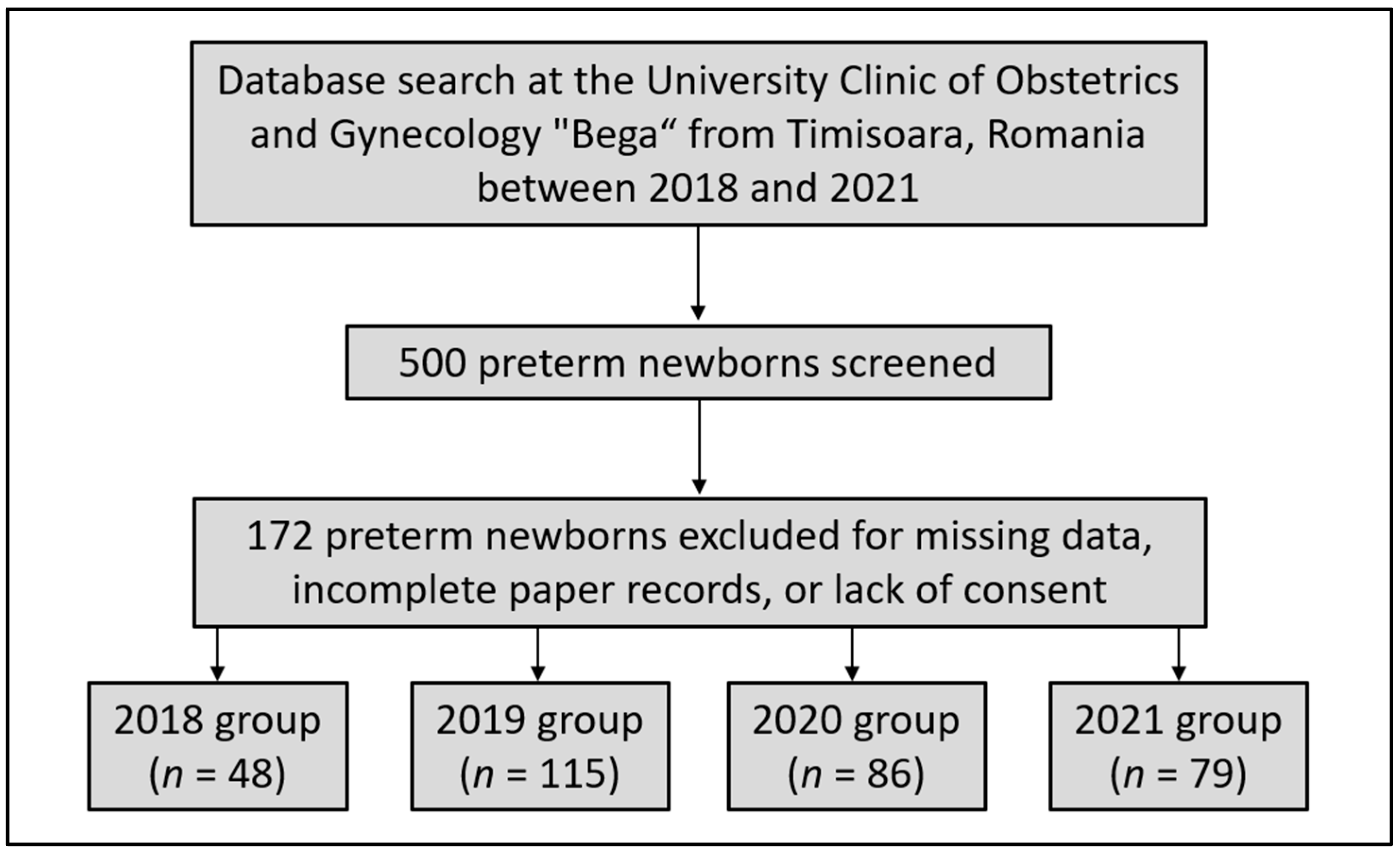
2. Materials and Methods
3. Results
3.1. Preterm Births vs. Full-Term Births
3.2. Four-Year Analysis
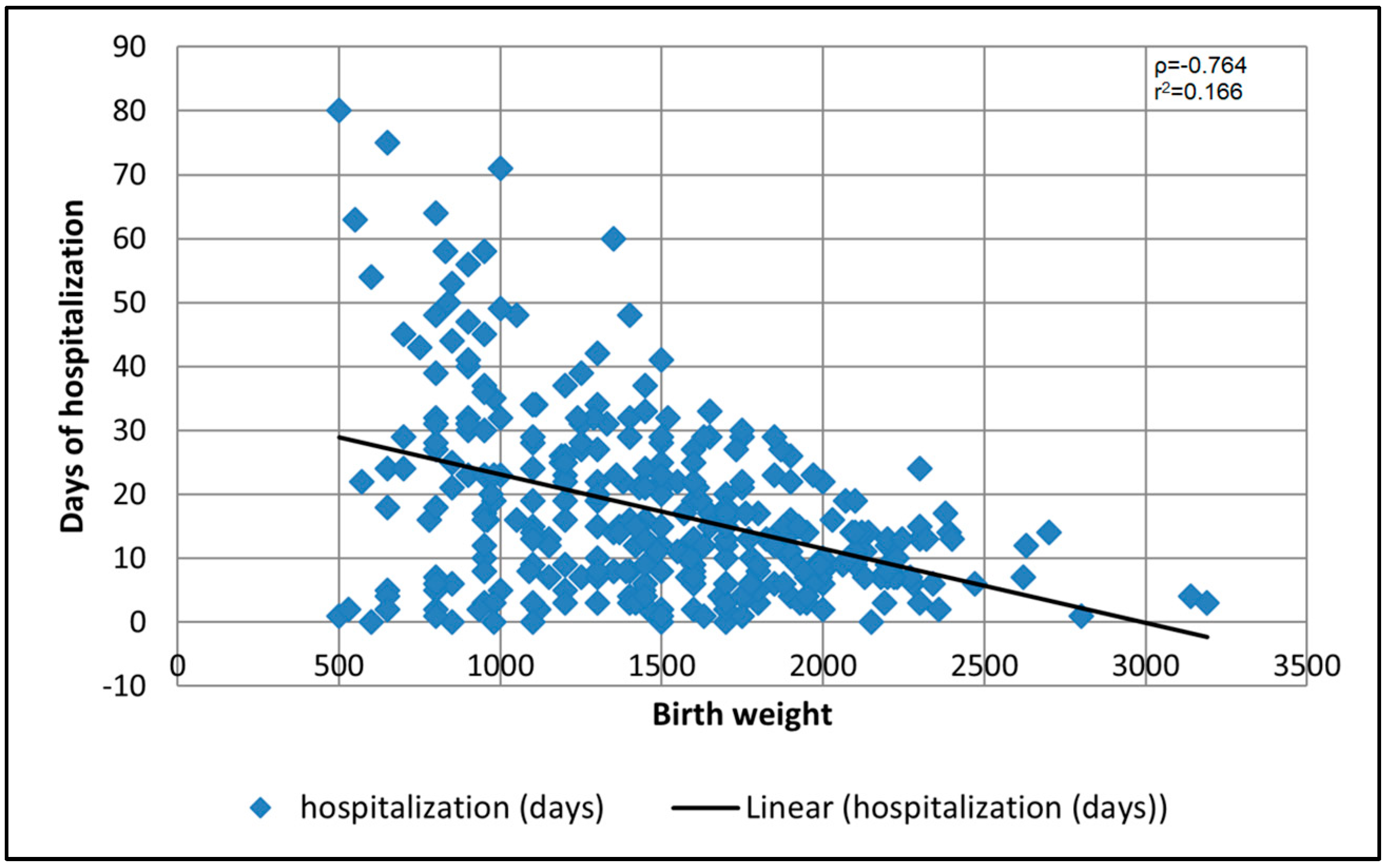
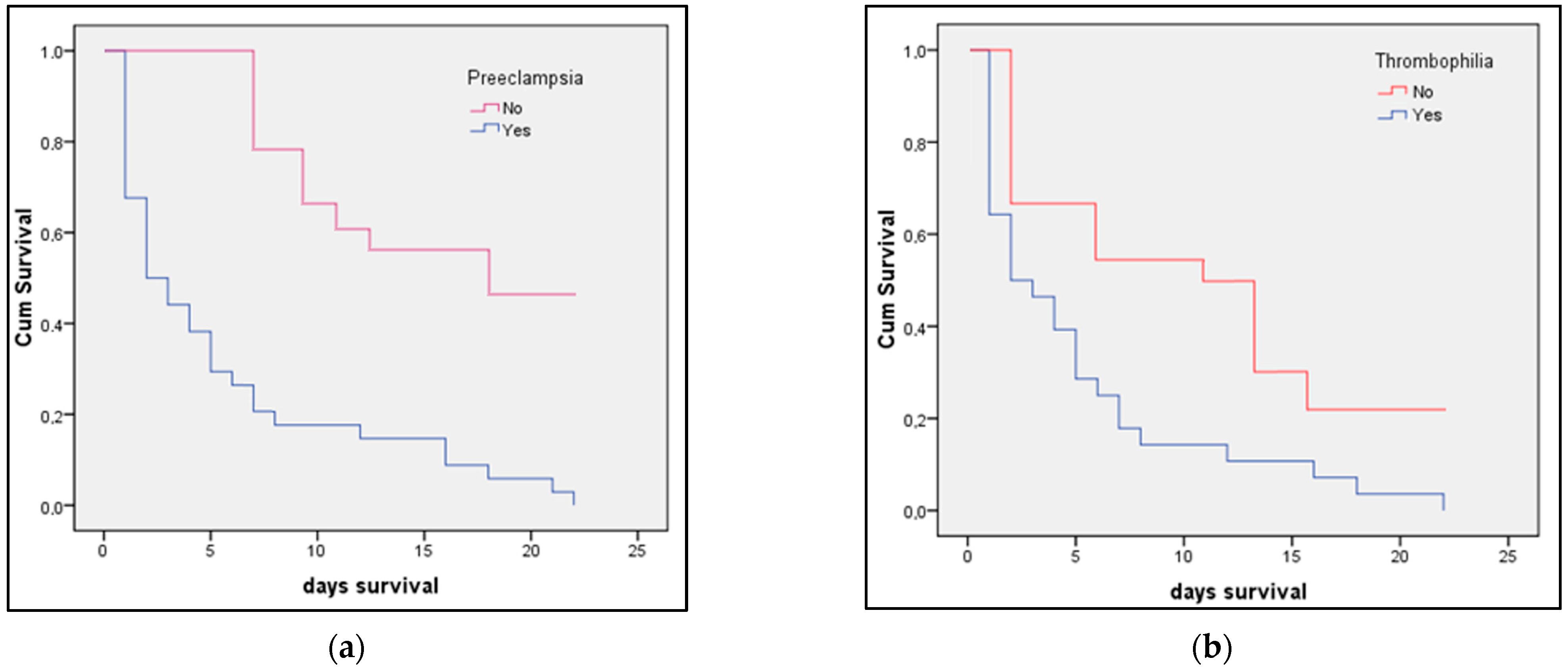
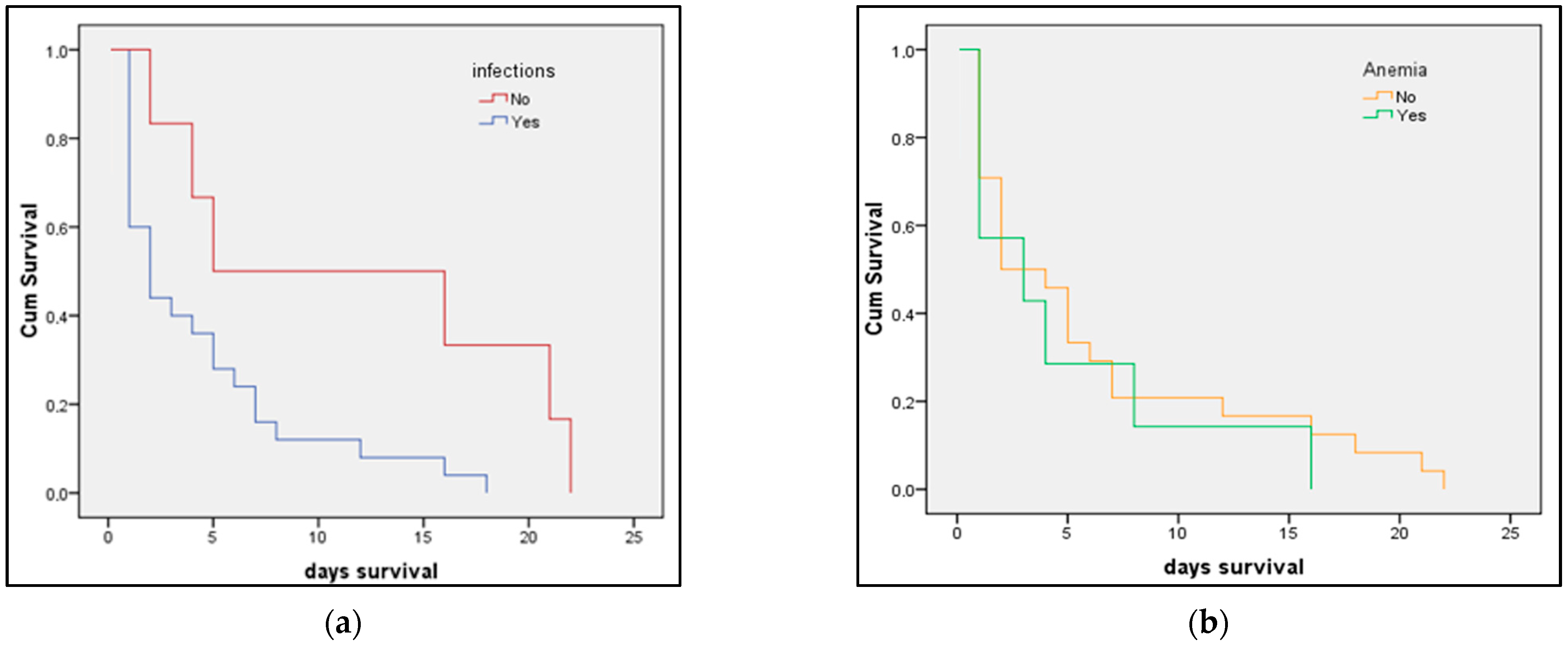
3.3. Risk Factor Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bugelli, A.; Borgès Da Silva, R.; Dowbor, L.; Sicotte, C. The Determinants of Infant Mortality in Brazil, 2010–2020: A Scoping Review. Int. J. Environ. Res. Public Health 2021, 18, 6464. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, A.; Krieger, N.; Subramanian, S.V. Learning From History About Reducing Infant Mortality: Contrasting the Centrality of Structural Interventions to Early 20th-Century Successes in the United States to Their Neglect in Current Global Initiatives. Milbank Q. 2019, 97, 285–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Sheyab, N.A.; Khader, Y.S.; Shattnawi, K.K.; Alyahya, M.S.; Batieha, A. Rate, Risk Factors, and Causes of Neonatal Deaths in Jordan: Analysis of Data From Jordan Stillbirth and Neonatal Surveillance System (JSANDS). Front. Public Health 2020, 8, 595379. [Google Scholar] [CrossRef]
- Oza, S.; Lawn, J.E.; Hogan, D.R.; Mathers, C.; Cousens, S.N. Neonatal cause-of-death estimates for the early and late neonatal periods for 194 countries: 2000–2013. Bull. World Health Organ. 2014, 93, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Baqui, A.H.; Mitra, D.K.; Begum, N.; Hurt, L.; Soremekun, S.; Edmond, K.; Kirkwood, B.; Bhandari, N.; Taneja, S.; Mazumder, S.; et al. Neonatal mortality within 24 hours of birth in six low- and lower-middle-income countries. Bull. World Health Organ. 2016, 94, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Belizán, J.M.; McClure, E.M.; Goudar, S.S.; Pasha, O.; Esamai, F.; Patel, A.; Chomba, E.; Garces, A.; Wright, L.L.; Koso-Thomas, M.; et al. Neonatal death in low- to middle-income countries: A global network study. Am. J. Perinatol. 2012, 29, 649–656. [Google Scholar] [CrossRef] [Green Version]
- Saleem, S.; McClure, E.M.; Goudar, S.S.; Patel, A.; Esamai, F.; Garces, A.; Chomba, E.; Althabe, F.; Moore, J.; Kodkany, B.; et al. A prospective study of maternal, fetal and neonatal deaths in low- and middle-income countries. Bull. World Health Organ. 2014, 92, 605–612. [Google Scholar] [CrossRef]
- Thwaites, C.L.; Beeching, N.J.; Newton, C.R. Maternal and neonatal tetanus. Lancet 2015, 385, 362–370. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.A.; Zahidie, A.; Rabbani, F. Interventions to reduce neonatal mortality from neonatal tetanus in low and middle income countries—A systematic review. BMC Public Health 2013, 13, 322. [Google Scholar] [CrossRef] [Green Version]
- Pop, T.L.; Burlea, M.; Falup-Pecurariu, O.; Borzan, C.; Gabor-Harosa, F.; Herdea, V.; Pop, C.F.; Rajka, D.; Ognean, M.L.; Cainap, S.S. Overview of the pediatric healthcare system in Romania. Turk Pediatri Ars. 2020, 55, 69–84. [Google Scholar] [CrossRef]
- Suciu, L.M.; Puscasiu, L.; Szabo, B.; Cucerea, M.; Ognean, M.L.; Oprea, I.; Bell, E.F. Mortality and morbidity of very preterm infants in Romania: How are we doing? Pediatr. Int. 2014, 56, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Kleinhout, M.Y.; Stevens, M.M.; Osman, K.A.; Adu-Bonsaffoh, K.; Groenendaal, F.; Zepro, N.B.; Rijken, M.J.; Browne, J.L. Evidence-based interventions to reduce mortality among preterm and low-birthweight neonates in low-income and middle-income countries: A systematic review and meta-analysis. BMJ Glob. Health 2021, 6, e003618. [Google Scholar] [CrossRef]
- Taha, Z.; Ali Hassan, A.; Wikkeling-Scott, L.; Papandreou, D. Factors Associated with Preterm Birth and Low Birth Weight in Abu Dhabi, the United Arab Emirates. Int. J. Environ. Res. Public Health 2020, 17, 1382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olack, B.; Santos, N.; Inziani, M.; Moshi, V.; Oyoo, P.; Nalwa, G.; OumaOtare, L.C.; Walker, D.; Otieno, P.A. Causes of preterm and low birth weight neonatal mortality in a rural community in Kenya: Evidence from verbal and social autopsy. BMC Pregnancy Childbirth 2021, 21, 536. [Google Scholar] [CrossRef]
- Rahman, A.; Rahman, M.; Pervin, J.; Razzaque, A.; Aktar, S.; Ahmed, J.U.; Selling, K.E.; Svefors, P.; El Arifeen, S.; Persson, L.Å. Time trends and sociodemographic determinants of preterm births in pregnancy cohorts in Matlab, Bangladesh, 1990-2014. BMJ Glob. Health 2019, 4, e001462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dongarwar, D.; Tahseen, D.; Wang, L.; Aliyu, M.H.; Salihu, H.M. Temporal trends in preterm birth phenotypes by plurality: Black–White disparity over half a century. J. Perinatol. 2021, 41, 204–211. [Google Scholar] [CrossRef]
- Hekimoğlu, B.; Acar, F.A. Effects of COVID-19 pandemic period on neonatal mortality and morbidity. Pediatr. Neonatol. 2022, 63, 78–83. [Google Scholar] [CrossRef]
- Villar, J.; Ariff, S.; Gunier, R.B.; Thiruvengadam, R.; Rauch, S.; Kholin, A.; Roggero, P.; Prefumo, F.; Vale, M.S.D.; Cardona-Perez, J.A.; et al. Maternal and Neonatal Morbidity and Mortality Among Pregnant Women with and without COVID-19 Infection: The INTERCOVID Multinational Cohort Study. JAMA Pediatrics 2021, 175, 817–826. [Google Scholar] [CrossRef]
- Khowaja, W.H.; Leghari, A.L.; Hussain, A.S.; Ariff, S.; Khan, I.A. Frequency and Early Complications of Late Preterm Infants: A Descriptive Analysis from Two Secondary-care Hospitals of Karachi. Cureus 2019, 11, e5789. [Google Scholar] [CrossRef] [Green Version]
- Nour, N.M. Premature delivery and the millennium development goal. Rev. Obstet. Gynecol. 2012, 5, 100–105. [Google Scholar]
- Soleimani, F.; Zaheri, F.; Abdi, F. Long-term neurodevelopmental outcomes after preterm birth. Iran. Red Crescent Med. J. 2014, 16, e17965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blencowe, H.; Lee, A.C.; Cousens, S.; Bahalim, A.; Narwal, R.; Zhong, N.; Chou, D.; Say, L.; Modi, N.; Katz, J.; et al. Preterm birth–associated neurodevelopmental impairment estimates at regional and global levels for 2010. Pediatr. Res. 2013, 74, 17–34. [Google Scholar] [CrossRef] [Green Version]
- Younes, S.; Samara, M.; Al-Jurf, R.; Nasrallah, G.; Al-Obaidly, S.; Salama, H.; Olukade, T.; Hammuda, S.; Ismail, M.; Abdoh, G.; et al. Incidence, Risk Factors, and Outcomes of Preterm and Early Term Births: A Population-Based Register Study. Int. J. Environ. Res. Public Health 2021, 18, 5865. [Google Scholar] [CrossRef] [PubMed]
- Bouvier, D.; Forest, J.-C.; Blanchon, L.; Bujold, E.; Pereira, B.; Bernard, N.; Gallot, D.; Sapin, V.; Giguère, Y. Risk Factors and Outcomes of Preterm Premature Rupture of Membranes in a Cohort of 6968 Pregnant Women Prospectively Recruited. J. Clin. Med. 2019, 8, 1987. [Google Scholar] [CrossRef] [Green Version]
- Quinn, J.-A.; Munoz, F.M.; Gonik, B.; Frau, L.; Cutland, C.; Mallett-Moore, T.; Kissou, A.; Wittke, F.; Das, M.; Nunes, T.; et al. Preterm birth: Case definition & guidelines for data collection, analysis, and presentation of immunisation safety data. Vaccine 2016, 34, 6047–6056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pathirana, J.; Muñoz, F.M.; Abbing-Karahagopian, V.; Bhat, N.; Harris, T.; Kapoor, A.; Keene, D.L.; Mangili, A.; Padula, M.A.; Pande, S.L.; et al. Neonatal death: Case definition & guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine 2016, 34, 6027–6037. [Google Scholar] [CrossRef] [Green Version]
- Holness, N. High-risk pregnancy. Nurs. Clin. 2018, 53, 241–251. [Google Scholar] [CrossRef]
- Woodd, S.L.; Montoya, A.; Barreix, M.; Pi, L.; Calvert, C.; Rehman, A.M.; Chou, D.; Campbell, O.M.R. Incidence of maternal peripartum infection: A systematic review and meta-analysis. PLoS Med. 2019, 16, e1002984. [Google Scholar] [CrossRef]
- Michel, M.C.; Colaizy, T.T.; Klein, J.M.; Segar, J.L.; Bell, E.F. Causes and circumstances of death in a neonatal unit over 20 years. Pediatr. Res. 2018, 83, 829–833. [Google Scholar] [CrossRef] [Green Version]
- Alanazi, A.F.R.; Naser, A.Y.; Pakan, P.; Alanazi, A.F.; Alanazi, A.A.A.; Alsairafi, Z.K.; Alsaleh, F.M. Trends of Hospital Admissions Due to Congenital Anomalies in England and Wales between 1999 and 2019: An Ecological Study. Int. J. Environ. Res. Public Health 2021, 18, 11808. [Google Scholar] [CrossRef]
- Czeizel, A.E.; Dudás, I.; Vereczkey, A.; Bánhidy, F. Folate Deficiency and Folic Acid Supplementation: The Prevention of Neural-Tube Defects and Congenital Heart Defects. Nutrients 2013, 5, 4760–4775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dempsey, T.; Nguyen, H.L.; Nguyen, H.T.; Bui, X.A.; Pham, P.T.T.; Nguyen, T.K.; Cavallin, F.; Trevisanuto, D.; Höök, S.M.; Pejovic, N.; et al. Incidence of Intrapartum-Related Events at the Largest Obstetric Hospital in Hanoi, Vietnam: A Retrospective Study. Children 2022, 9, 321. [Google Scholar] [CrossRef] [PubMed]
- Ţarcă, E.; Roșu, S.T.; Cojocaru, E.; Trandafir, L.; Luca, A.; Rusu, D.; Ţarcă, V. Socio-Epidemiological Factors with Negative Impact on Infant Morbidity, Mortality Rates, and the Occurrence of Birth Defects. Healthcare 2021, 9, 384. [Google Scholar] [CrossRef] [PubMed]
- Gülmezoglu, A.M.; Lawrie, T.A.; Hezelgrave, N.; Oladapo, O.T.; Souza, J.P.; Gielen, M.; Lawn, J.E.; Bahl, R.; Althabe, F.; Colaci, D.; et al. Interventions to Reduce Maternal and Newborn Morbidity and Mortality. In Reproductive, Maternal, Newborn, and Child Health: Disease Control Priorities, 3rd ed.; Black, R.E., Laxminarayan, R., Temmerman, M., Walker, N., Eds.; The International Bank for Reconstruction and Development/The World Bank: Washington, DC, USA, 2016; Volume 2, Chapter 7. Available online: https://www.ncbi.nlm.nih.gov/books/NBK361904/ (accessed on 10 March 2022). [CrossRef]
- Naeh, A.; Maor-Sagie, E.; Hallak, M.; Gabbay-Benziv, R. Early Identification of the Maternal, Placental and Fetal Dialog in Gestational Diabetes and Its Prevention. Reprod. Med. 2022, 3, 1–14. [Google Scholar] [CrossRef]
- Cupen, K.; Barran, A.; Singh, V.; Dialsingh, I. Risk Factors Associated with Preterm Neonatal Mortality: A Case Study Using Data from Mt. Hope Women’s Hospital in Trinidad and Tobago. Children 2017, 4, 108. [Google Scholar] [CrossRef] [Green Version]
- Dagher, R.K.; Linares, D.E. A Critical Review on the Complex Interplay between Social Determinants of Health and Maternal and Infant Mortality. Children 2022, 9, 394. [Google Scholar] [CrossRef]
- Lewandowska, M. Maternal Obesity and Risk of Low Birth Weight, Fetal Growth Restriction, and Macrosomia: Multiple Analyses. Nutrients 2021, 13, 1213. [Google Scholar] [CrossRef]
- Słabuszewska-Jóźwiak, A.; Szymański, J.K.; Ciebiera, M.; Sarecka-Hujar, B.; Jakiel, G. Pediatrics Consequences of Caesarean Section—A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 8031. [Google Scholar] [CrossRef]
- Bizzego, A.; Gabrieli, G.; Bornstein, M.H.; Deater-Deckard, K.; Lansford, J.E.; Bradley, R.H.; Costa, M.; Esposito, G. Predictors of Contemporary under-5 Child Mortality in Low- and Middle-Income Countries: A Machine Learning Approach. Int. J. Environ. Res. Public Health 2021, 18, 1315. [Google Scholar] [CrossRef]
- Mahumud, R.A.; Sultana, M.; Sarker, A.R. Distribution and Determinants of Low Birth Weight in Developing Countries. J. Prev. Med. Public Health 2017, 50, 18–28. [Google Scholar] [CrossRef] [Green Version]

| Preterm (n = 328) | Full-Term (n = 422) | p-Value * | |
|---|---|---|---|
| Maternal background | |||
| Age (≥35 years) | 81 (24.7%) | 115 (27.3%) | 0.429 |
| Weight at birth (>25 kg/m2) | 74 (22.6%) | 79 (18.7%) | 0.195 |
| Twin birth | 40 (12.2%) | 49 (11.6%) | 0.806 |
| Gestational age | 29.5 ± 2.4 | 37.8 ± 4.9 | <0.001 |
| Days of hospitalization ** | 5.0 (2.9–7.1) | 3.3 (1.9–6.0) | 0.001 |
| High obstetrical risk (n–%) | 267 (81.4%) | 74 (17.5%) | <0.001 |
| Maternal comorbidities | |||
| Preeclampsia | 12 (3.7%) | 6 (1.4%) | 0.047 |
| Thrombophilia | 20 (6.1%) | 13 (3.1) | 0.045 |
| Anemia | 184 (56.1%) | 123 (29.1%) | <0.001 |
| Peripartum infection | 82 (25.0%) | 24 (5.7%) | <0.001 |
| Other maternal infections | 68 (20.7%) | 28 (6.6%) | <0.001 |
| Neonatal characteristics | |||
| Gender (male) | 172 (52.4%) | 198 (46.9%) | 0.133 |
| Abnormal APGAR score | 224 (68.3%) | 77 (18.2%) | <0.001 |
| Birth weight *** (grams) | 1503 ± 494 | 2597 ± 606 | <0.001 |
| In vitro fertilization | 28 (8.5%) | 33 (7.8%) | 0.721 |
| Delivery type (C-section) | 221 (67.4%) | 147 (34.8%) | <0.001 |
| Infection after membrane rupture | 71 (21.6%) | 76 (18.0%) | 0.213 |
| Congenital abnormalities | 14 (4.3%) | 8 (1.9%) | 0.056 |
| Severe prematurity | 39 (11.9%) | - | - |
| NICU admission | 42 (12.8%) | 11 (2.6%) | <0.001 |
| Resuscitation | 38 (11.6%) | 19 (4.5%) | <0.001 |
| Days of hospitalization ** | 16.9 (14.3–19.8) | 3.7 (1.4–5.9) | <0.001 |
| Days of NICU stay ** | 8.3 (5.1–12.8) | 7.7 (4.9–11.7) | 0.084 |
| Mortality | 31 (9.5%) | 5 (1.2%) | <0.001 |
| Neonatal therapy | |||
| Surfactant | 79 (24.1%) | 44 (10.4%) | <0.001 |
| Steroids | 52 (15.9%) | 38 (9.0%) | 0.004 |
| Antibiotics | 260 (79.3%) | 194 (46.0%) | <0.001 |
| 2018 (n = 48) | 2019 (n = 115) | 2020 (n = 86) | 2021 (n = 79) | p-Value * | |
|---|---|---|---|---|---|
| Maternal background | |||||
| Age ≥ 35 years | 8 (16.7%) | 29 (25.2%) | 20 (23.3%) | 24 (30.4%) | 0.369 |
| Weight at birth (>kg/m2) | 10 (20.8%) | 22 (19.1%) | 20 (23.6%) | 18 (22.7%) | 0.447 |
| Twin birth (>25 kg/m2) | 6 (12.5%) | 14 (12.2%) | 16 (18.6%) | 4 (5.1%) | 0.070 |
| Gestational age | 29.9 ± 2.3 | 29.4 ± 2.3 | 29.6 ± 2.4 | 29.7 ± 2.3 | 0.105 |
| Days of hospitalization ** | 4.3 (1.9–6.3) | 4.1 (1.9–6.0) | 6.6 (2.8–7.2) | 6.8 (2.9–7.4) | 0.003 |
| High obstetrical risk (n–%) | 40 (83.3%) | 89 (77.4%) | 81 (94.2%) | 57 (72.2%) | 0.672 |
| Maternal comorbidities | |||||
| Preeclampsia | 2 (4.2%) | 4 (3.5%) | 3 (3.5%) | 3 (3.8%) | 0.996 |
| Thrombophilia | 3 (6.3%) | 11 (9.6%) | 3 (3.5%) | 3 (3.8%) | 0.244 |
| Anemia | 21 (43.8%) | 75 (65.2%) | 43 (50.0%) | 45 (57.0%) | 0.042 |
| Peripartum infection | 14 (29.2%) | 24 (20.9%) | 25 (29.1%) | 19 (24.1%) | 0.515 |
| Other maternal infections | 14 (29.2%) | 25 (21.7%) | 18 (20.9%) | 11 (13.9%) | 0.223 |
| Neonatal characteristics | |||||
| Gender (male) | 19 (39.6%) | 65 (56.5%) | 46 (53.5%) | 42 (53.2%) | 0.261 |
| Abnormal APGAR score | |||||
| Birth weight ***(grams) | 1521 ± 517 | 1416 ± 449 | 1473 ± 537 | 1480 ± 496 | 0.246 |
| In vitro fertilization | 3 (6.3%) | 10 (8.7%) | 12 (14.0%) | 3 (3.8%) | 0.120 |
| Delivery type (C-section) | 34 (70.8%) | 79 (68.7%) | 50 (58.1%) | 58 (73.4%) | 0.172 |
| Infection after membrane rupture | 10 (20.8%) | 24 (20.9%) | 19 (22.1%) | 19 (24.0%) | 0.956 |
| Congenital abnormalities | 2 (4.2%) | 6 (5.2%) | 3 (3.5%) | 3 (3.8%) | 0.934 |
| Severe prematurity | 6 (12.5%) | 10 (8.7%) | 13 (15.1%) | 10 (12.6%) | 0.567 |
| NICU admission | 3 (6.2%) | 13 (11.3%) | 13 (15.1%) | 9 (11.4%) | 0.495 |
| Resuscitation | 0 (0.0%) | 15 (13.0%) | 14 (16.3%) | 9 (11.4%) | 0.038 |
| Days of hospitalization ** | 15.9 (11.5–20.3) | 20.3 (17.4–23.3) | 16.7 (14.0–19.5) | 17.5 (15.9–19.0) | 0.053 |
| Days of NICU stay ** | 8.1 (5.3–12.1) | 9.0 (5.8–13.4) | 8.6 (5.5–12.3) | 8.5 (5.2–12.4) | 0.496 |
| Mortality | 5 (10.4%) | 10 (8.7%) | 11 (12.8%) | 5 (6.3%) | 0.542 |
| Neonatal therapy | |||||
| Surfactant | 5 (10.4%) | 25 (21.7%) | 24 (27.9%) | 25 (31.6%) | 0.038 |
| Steroids | 0 (0.0%) | 3 (2.6%) | 20 (23.3%) | 29 (36.7%) | <0.001 |
| Antibiotics | 24 (50.0%) | 79 (68.7%) | 81 (94.2%) | 76 (96.2%) | <0.001 |
| Risk Factors | NICU Admission (OR–95% CI) | p-Value | Mortality (OR–95% CI) | p-Value |
|---|---|---|---|---|
| Age ≥ 35 years | 1.59 (1.21–2.37) | 0.012 | 1.40 (1.06–1.99) | 0.041 |
| Weight at birth | 1.02 (0.84–1.23) | 0.294 | 0.93 (0.82–1.06) | 0.192 |
| Twin births | 1.14 (1.02–1.36) | 0.047 | 1.02 (0.82–1.25) | 0.298 |
| Low gestational age | 1.66 (1.23–2.11) | 0.001 | 1.27 (1.02–1.88) | 0.017 |
| High obstetrical risk (n–%) | 1.19 (0.91–1.32) | 0.402 | 1.01 (0.88–1.33) | 0.194 |
| Preeclampsia | 2.33 (1.86–3.18) | 0.001 | 1.24 (1.09–1.76) | 0.037 |
| Thrombophilia | 1.15 (0.97–1.43) | 0.316 | 0.98 (0.75–1.04) | 0.339 |
| Peripartum infection | 2.25 (1.46–3.23) | 0.001 | 1.93 (1.16–2.83) | 0.009 |
| Anemia | 1.12 (0.93–1.32) | 0.422 | 1.05 (0.83–1.41) | 0.510 |
| Other maternal infections | 1.07 (0.92–1.40) | 0.294 | 1.01 (0.84–1.29) | 0.318 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilyes, S.-G.; Chiriac, V.D.; Gluhovschi, A.; Mihaela, V.; Dahma, G.; Mocanu, A.G.; Neamtu, R.; Silaghi, C.; Radu, D.; Bernad, E.; et al. The Influence of Maternal Factors on Neonatal Intensive Care Unit Admission and In-Hospital Mortality in Premature Newborns from Western Romania: A Population-Based Study. Medicina 2022, 58, 709. https://doi.org/10.3390/medicina58060709
Ilyes S-G, Chiriac VD, Gluhovschi A, Mihaela V, Dahma G, Mocanu AG, Neamtu R, Silaghi C, Radu D, Bernad E, et al. The Influence of Maternal Factors on Neonatal Intensive Care Unit Admission and In-Hospital Mortality in Premature Newborns from Western Romania: A Population-Based Study. Medicina. 2022; 58(6):709. https://doi.org/10.3390/medicina58060709
Chicago/Turabian StyleIlyes, Stelian-Gabriel, Veronica Daniela Chiriac, Adrian Gluhovschi, Valcovici Mihaela, George Dahma, Adelina Geanina Mocanu, Radu Neamtu, Carmen Silaghi, Daniela Radu, Elena Bernad, and et al. 2022. "The Influence of Maternal Factors on Neonatal Intensive Care Unit Admission and In-Hospital Mortality in Premature Newborns from Western Romania: A Population-Based Study" Medicina 58, no. 6: 709. https://doi.org/10.3390/medicina58060709
APA StyleIlyes, S. -G., Chiriac, V. D., Gluhovschi, A., Mihaela, V., Dahma, G., Mocanu, A. G., Neamtu, R., Silaghi, C., Radu, D., Bernad, E., & Craina, M. (2022). The Influence of Maternal Factors on Neonatal Intensive Care Unit Admission and In-Hospital Mortality in Premature Newborns from Western Romania: A Population-Based Study. Medicina, 58(6), 709. https://doi.org/10.3390/medicina58060709







