Vascular Function Recovery Following Saturation Diving
Abstract
:1. Introduction
“Records show that since 1966 seventy-seven diving personnel tragically have lost their lives in the quest for, depending on your perspective “Black Gold” or “Devil’s excrement” in the North Sea Basin. By nationality: 53 British/Commonwealth subjects, 9 American, 7 Norwegian, 4 Dutch, 3 French, and 1 Italian. Prior to 1971/1974, applicable laws & regulations (if any) required no accurate fatal accident statistics. One can conclude that the actual combined number of deaths is higher. However, it is known that several divers received severe injuries from which they never recovered.”(https://the-norwegian.com/north-sea-diving-fatalities (accessed on 20 September 2022))
2. Methods
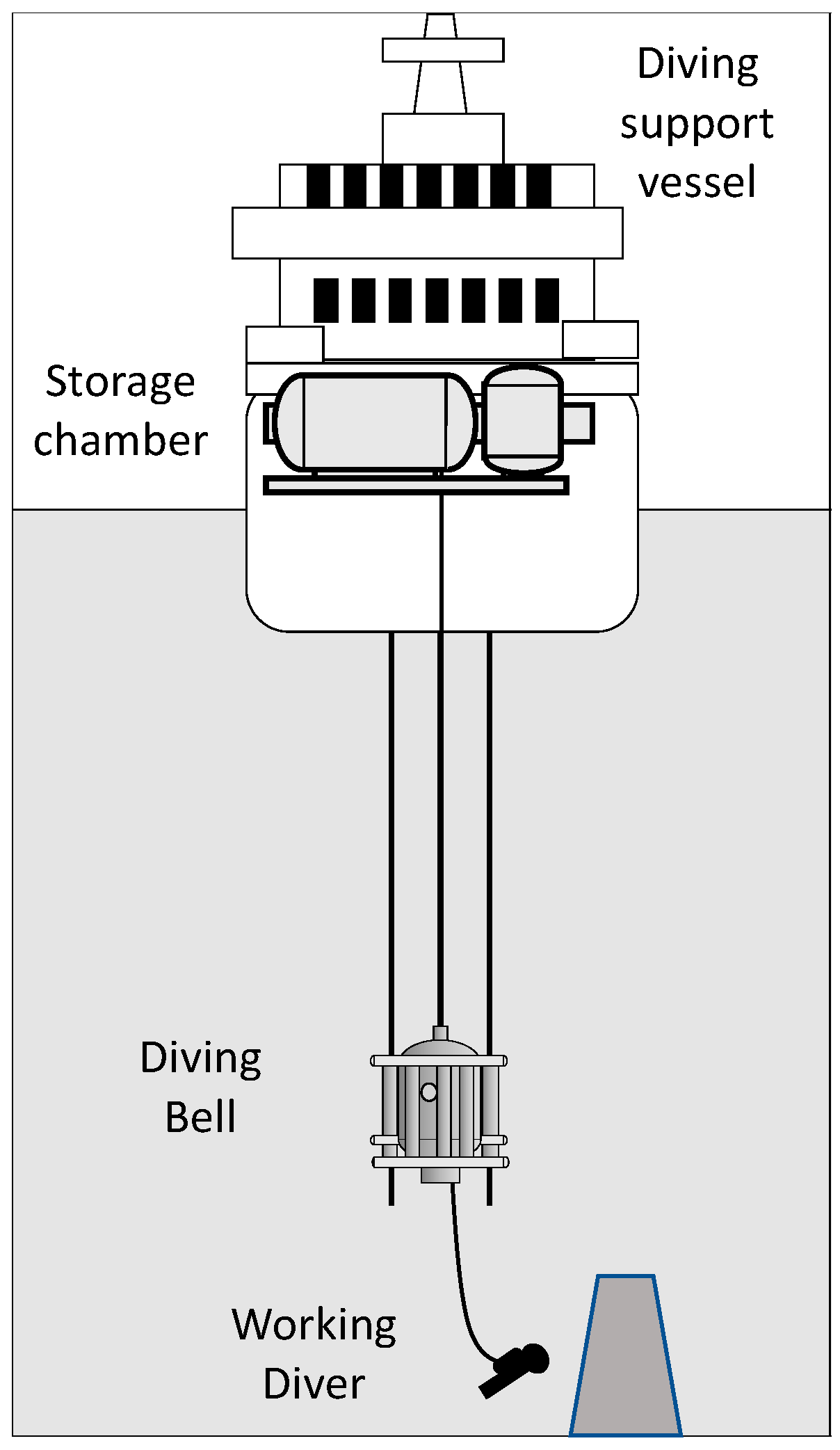
2.1. Worksites
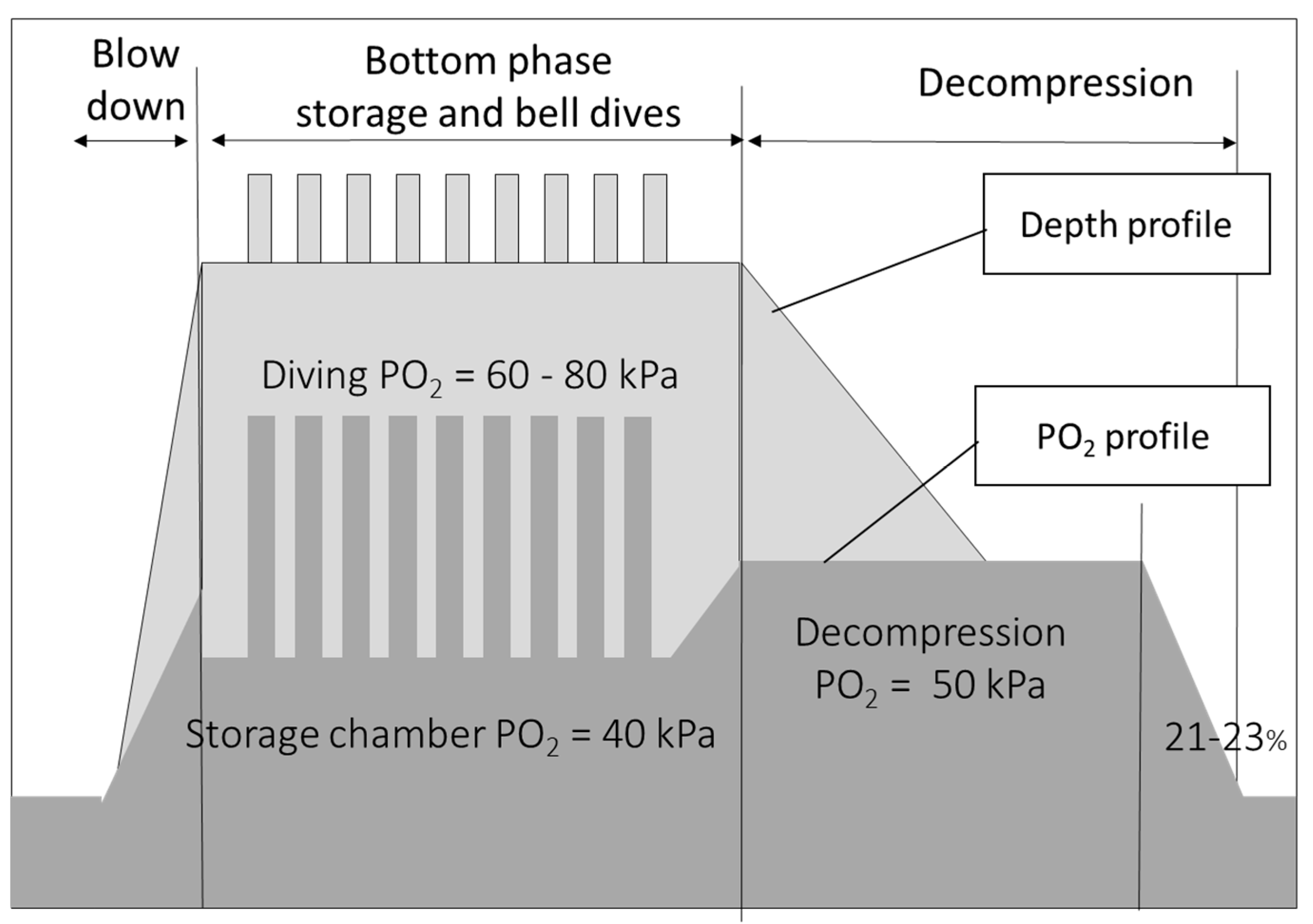
2.2. Saturation Procedures
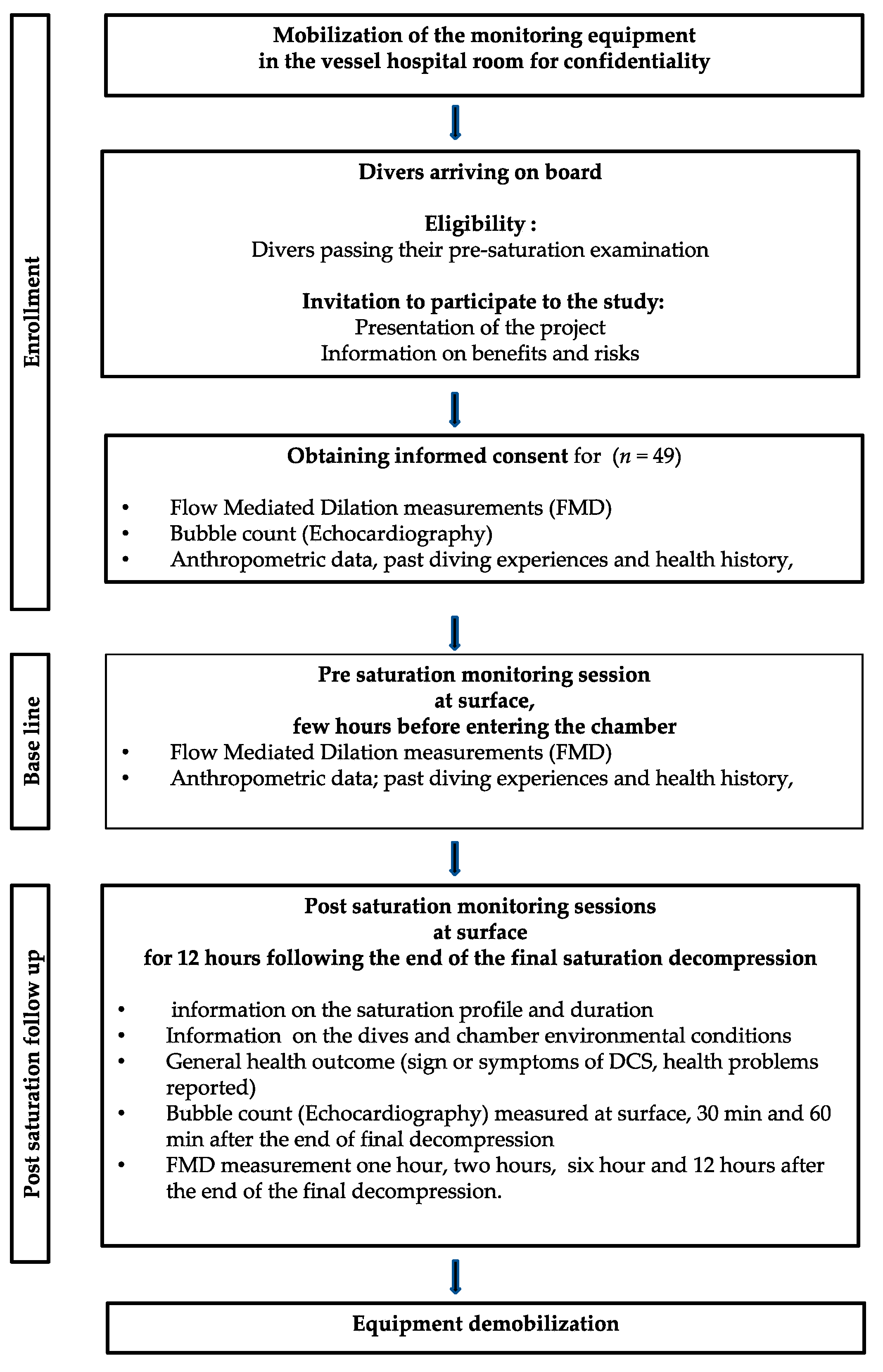
2.3. Participant Eligibility and Enrollment
2.4. Organizational Constraints
2.5. Data Acquisition
2.5.1. Flow-Mediated Dilation (FMD)
2.5.2. Post Saturation Diving Decompression Vascular Gas Emboli (VGE)
3. Statistical Analysis
4. Results
4.1. Vascular Gas Emboli
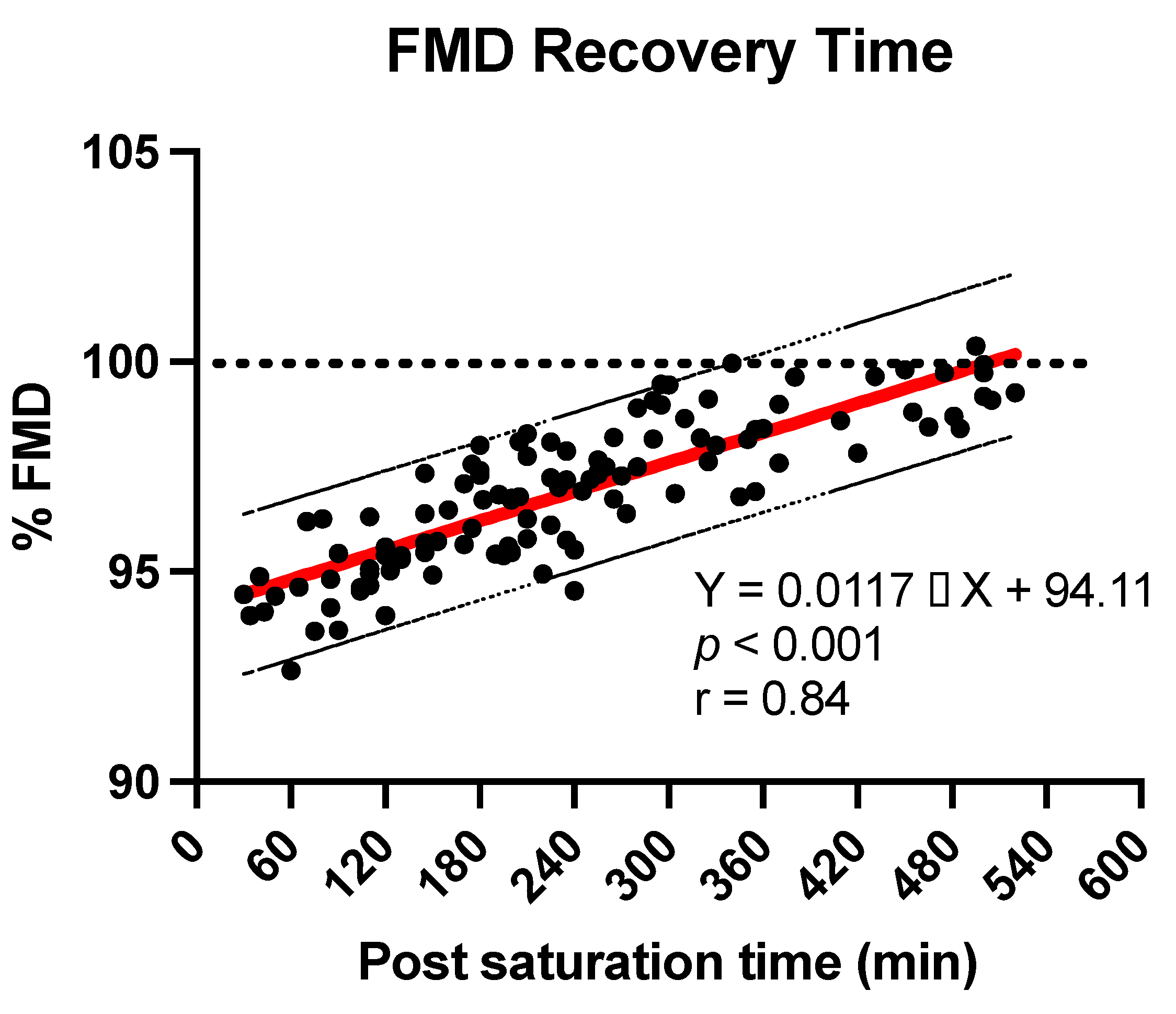
4.2. Flow Mediated Dilation
5. Discussion
6. Limitations
- -
- This study builds on established modern methods of evaluation of decompression stress including vascular function and current theories of VGE generation.
- -
- As there is possible large inter-individual variation for VGE and FMD effects after diving, the subjects served as their own controls.
- -
- The measured effects are consistent with the theoretical rationale and do not require complicated new hypotheses.
- -
- The equipment used for these experiments is readily available and reliable, inviting other research groups to repeat the study.
- -
- The study was performed in real operational activities.
- -
- A large number of divers volunteered for the study (never a saturation diving study addressed so many participants).
- -
- The subjects were not homogenous or necessarily similar in body composition (age, weight, fat/lean mass distribution, sex).
- -
- Operational constraints sometimes altered the planning of the measurements.
- -
- Gender balance was impossible to reach.
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NO | Nitric Oxide |
| TAC | Total Antioxidant Capacity |
| FMD | Flow-Mediated Dilation |
| HR | Heart Rate |
| ROS | Reactive Oxygen Species |
| NOx | Nitric Oxide Metabolites |
| NO | Nitric Oxide |
| DSV | Diving Support Vessel |
| EB | Eftedal – Brubakk Score |
| hPa | Hecto-Pascal |
| msw | Meters of sea water |
| VGE | Vascular Gas Emboli |
| IL-6 | Interleukin-6 |
References
- Imbert, J.P.; Balestra, C.; Kiboub, F.Z.; Loennechen, O.; Eftedal, I. Commercial Divers’ Subjective Evaluation of Saturation. Front. Psychol. 2018, 9, 2774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiboub, F.Z.; Balestra, C.; Loennechen, O.; Eftedal, I. Hemoglobin and Erythropoietin after Commercial Saturation Diving. Front. Physiol. 2018, 9, 1176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berenji Ardestani, S.; Balestra, C.; Bouzinova, E.V.; Loennechen, O.; Pedersen, M. Evaluation of Divers’ Neuropsychometric Effectiveness and High-Pressure Neurological Syndrome via Computerized Test Battery Package and Questionnaires in Operational Setting. Front. Physiol. 2019, 10, 1386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wekre, S.L.; Landsverk, H.D.; Lautridou, J.; Hjelde, A.; Imbert, J.P.; Balestra, C.; Eftedal, I. Hydration status during commercial saturation diving measured by bioimpedance and urine specific gravity. Front. Physiol. 2022, 13, 971757. [Google Scholar] [CrossRef]
- Mrakic-Sposta, S.; Gussoni, M.; Vezzoli, A.; Dellanoce, C.; Comassi, M.; Giardini, G.; Bruno, R.M.; Montorsi, M.; Corciu, A.; Greco, F.; et al. Acute Effects of Triathlon Race on Oxidative Stress Biomarkers. Oxid. Med. Cell. Longev. 2020, 2020, 3062807. [Google Scholar] [CrossRef] [Green Version]
- Balestra, C.; Germonpre, P.; Rocco, M.; Biancofiore, G.; Kot, J. Diving physiopathology: The end of certainties? Food for thought. Minerva Anestesiol. 2019, 85, 1129–1137. [Google Scholar] [CrossRef] [Green Version]
- Thom, S.R.; Milovanova, T.N.; Bogush, M.; Bhopale, V.M.; Yang, M.; Bushmann, K.; Pollock, N.W.; Ljubkovic, M.; Denoble, P.; Dujic, Z. Microparticle production, neutrophil activation, and intravascular bubbles following open-water SCUBA diving. J. Appl. Physiol. 2012, 112, 1268–1278. [Google Scholar] [CrossRef]
- Arieli, R. Nanobubbles Form at Active Hydrophobic Spots on the Luminal Aspect of Blood Vessels: Consequences for Decompression Illness in Diving and Possible Implications for Autoimmune Disease-An Overview. Front. Physiol. 2017, 8, 591. [Google Scholar] [CrossRef]
- Thom, S.R.; Milovanova, T.N.; Bogush, M.; Yang, M.; Bhopale, V.M.; Pollock, N.W.; Ljubkovic, M.; Denoble, P.; Madden, D.; Lozo, M.; et al. Bubbles, microparticles, and neutrophil activation: Changes with exercise level and breathing gas during open-water SCUBA diving. J. Appl. Physiol. 2013, 114, 1396–1405. [Google Scholar] [CrossRef] [Green Version]
- Tremblay, J.C.; Thom, S.R.; Yang, M.; Ainslie, P.N. Oscillatory shear stress, flow-mediated dilatation, and circulating microparticles at sea level and high altitude. Atherosclerosis 2017, 256, 115–122. [Google Scholar] [CrossRef]
- Balestra, C.; Arya, A.K.; Leveque, C.; Virgili, F.; Germonpré, P.; Lambrechts, K.; Lafère, P.; Thom, S.R. Varying Oxygen Partial Pressure Elicits Blood-Borne Microparticles Expressing Different Cell-Specific Proteins—Toward a Targeted Use of Oxygen? Int. J. Mol. Sci. 2022, 23, 7888. [Google Scholar] [CrossRef]
- Thom, S.R.; Bhopale, V.M.; Yu, K.; Huang, W.; Kane, M.A.; Margolis, D.J. Neutrophil microparticle production and inflammasome activation by hyperglycemia due to cytoskeletal instability. J. Biol. Chem. 2017, 292, 18312–18324. [Google Scholar] [CrossRef] [Green Version]
- Thom, S.R.; Yang, M.; Bhopale, V.M.; Huang, S.; Milovanova, T.N. Microparticles initiate decompression-induced neutrophil activation and subsequent vascular injuries. J. Appl. Physiol. 2011, 110, 340–351. [Google Scholar] [CrossRef]
- Mause, S.F.; Ritzel, E.; Liehn, E.A.; Hristov, M.; Bidzhekov, K.; Müller-Newen, G.; Soehnlein, O.; Weber, C. Platelet microparticles enhance the vasoregenerative potential of angiogenic early outgrowth cells after vascular injury. Circulation 2010, 122, 495–506. [Google Scholar] [CrossRef] [Green Version]
- Pyke, K.E.; Tschakovsky, M.E. The relationship between shear stress and flow-mediated dilatation: Implications for the assessment of endothelial function. J. Physiol. 2005, 568, 357–369. [Google Scholar] [CrossRef]
- Corretti, M.C.; Anderson, T.J.; Benjamin, E.J.; Celermajer, D.; Charbonneau, F.; Creager, M.A.; Deanfield, J.; Drexler, H.; Gerhard-Herman, M.; Herrington, D.; et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: A report of the International Brachial Artery Reactivity Task Force. J. Am. Coll. Cardiol. 2002, 39, 257–265. [Google Scholar] [CrossRef] [Green Version]
- Areas, G.P.T.; Mazzuco, A.; Caruso, F.R.; Jaenisch, R.B.; Cabiddu, R.; Phillips, S.A.; Arena, R.; Borghi-Silva, A. Flow-mediated dilation and heart failure: A review with implications to physical rehabilitation. Heart Fail. Rev. 2019, 24, 69–80. [Google Scholar] [CrossRef]
- Germonpre, P.; Papadopoulou, V.; Hemelryck, W.; Obeid, G.; Lafere, P.; Eckersley, R.J.; Tang, M.X.; Balestra, C. The use of portable 2D echocardiography and ‘frame-based’ bubble counting as a tool to evaluate diving decompression stress. Diving Hyperb. Med. 2014, 44, 5–13. [Google Scholar]
- Eftedal, O.; Brubakk, A.O. Agreement between trained and untrained observers in grading intravascular bubble signals in ultrasonic images. Undersea Hyperb. Med. 1997, 24, 293–299. [Google Scholar]
- Ozyigit, T.; Yavuz, C.; Egi, S.M.; Pieri, M.; Balestra, C.; Marroni, A. Clustering of recreational divers by their health conditions in a database of a citizen science project. Undersea Hyperb. Med. 2019, 46, 171–183. [Google Scholar] [CrossRef]
- Doolette, D.J. Venous gas emboli detected by two-dimensional echocardiography are an imperfect surrogate endpoint for decompression sickness. Diving Hyperb. Med. 2016, 46, 4–10. [Google Scholar] [PubMed]
- Møllerløkken, A.; Blogg, S.L.; Doolette, D.J.; Nishi, R.Y.; Pollock, N.W. Consensus guidelines for the use of ultrasound for diving research. Diving Hyperb. Med. 2016, 46, 26–32. [Google Scholar] [PubMed]
- Lambrechts, K.; Germonpré, P.; Vandenheede, J.; Delorme, M.; Lafère, P.; Balestra, C. Mini Trampoline, a New and Promising Way of SCUBA Diving Preconditioning to Reduce Vascular Gas Emboli? Int. J. Environ. Res. Public Health 2022, 19, 5410. [Google Scholar] [CrossRef] [PubMed]
- Łuczyński, D.; Lautridou, J.; Hjelde, A.; Monnoyer, R.; Eftedal, I. Hemoglobin during and following a 4-Week Commercial Saturation Dive to 200 m. Front. Physiol. 2019, 10, 1494. [Google Scholar] [CrossRef]
- Mrakic-Sposta, S.; Vezzoli, A.; D’Alessandro, F.; Paganini, M.; Dellanoce, C.; Cialoni, D.; Bosco, G. Change in Oxidative Stress Biomarkers during 30 Days in Saturation Dive: A Pilot Study. Int. J. Environ. Res. Public Health 2020, 17, 7118. [Google Scholar] [CrossRef]
- Monnoyer, R.; Haugum, K.; Lautridou, J.; Flatberg, A.; Hjelde, A.; Eftedal, I. Shifts in the Oral Microbiota during a Four-Week Commercial Saturation Dive to 200 Meters. Front. Physiol. 2021, 12, 669355. [Google Scholar] [CrossRef]
- Monnoyer, R.; Lautridou, J.; Deb, S.; Hjelde, A.; Eftedal, I. Using Salivary Biomarkers for Stress Assessment in Offshore Saturation Diving: A Pilot Study. Front. Physiol. 2021, 12, 791525. [Google Scholar] [CrossRef]
- Lambrechts, K.; Balestra, C.; Theron, M.; Henckes, A.; Galinat, H.; Mignant, F.; Belhomme, M.; Pontier, J.M.; Guerrero, F. Venous gas emboli are involved in post-dive macro, but not microvascular dysfunction. Eur. J. Appl. Physiol. 2017, 117, 335–344. [Google Scholar] [CrossRef]
- Germonpre, P.; Balestra, C. Preconditioning to Reduce Decompression Stress in Scuba Divers. Aerosp. Med. Hum. Perform. 2017, 88, 114–120. [Google Scholar] [CrossRef]
- Levenez, M.; Lambrechts, K.; Mrakic-Sposta, S.; Vezzoli, A.; Germonpré, P.; Pique, H.; Virgili, F.; Bosco, G.; Lafère, P.; Balestra, C. Full-Face Mask Use during SCUBA Diving Counters Related Oxidative Stress and Endothelial Dysfunction. Int. J. Environ. Res. Public Health 2022, 19, 965. [Google Scholar] [CrossRef]
- Arieli, R. Gas micronuclei underlying decompression bubbles may explain the influence of oxygen enriched gases during decompression on bubble formation and endothelial function in self-contained underwater breathing apparatus diving. Croat. Med. J. 2019, 60, 388. [Google Scholar] [CrossRef]
- Blatteau, J.E.; Boussuges, A.; Gempp, E.; Pontier, J.M.; Castagna, O.; Robinet, C.; Galland, F.M.; Bourdon, L. Haemodynamic changes induced by submaximal exercise before a dive and its consequences on bubble formation. Br. J. Sport. Med. 2007, 41, 375–379. [Google Scholar] [CrossRef]
- Arieli, Y.; Katsenelson, K.; Arieli, R. Bubble reduction after decompression in the prawn Palaemon elegans by pretreatment with hyperbaric oxygen. Undersea Hyperb. Med. 2007, 34, 369–378. [Google Scholar]
- Blatteau, J.E.; Souraud, J.B.; Gempp, E.; Boussuges, A. Gas nuclei, their origin, and their role in bubble formation. Aviat. Space Environ. Med. 2006, 77, 1068–1076. [Google Scholar]
- Gempp, E.; Blatteau, J.E. Preconditioning methods and mechanisms for preventing the risk of decompression sickness in scuba divers: A review. Res. Sport. Med. 2010, 18, 205–218. [Google Scholar] [CrossRef]
- Wang, Q.; Mazur, A.; Guerrero, F.; Lambrechts, K.; Buzzacott, P.; Belhomme, M.; Theron, M. Antioxidants, endothelial dysfunction, and DCS: In vitro and in vivo study. J. Appl. Physiol. 2015, 119, 1355–1362. [Google Scholar] [CrossRef]
- Lambrechts, K.; Pontier, J.M.; Balestra, C.; Mazur, A.; Wang, Q.; Buzzacott, P.; Theron, M.; Mansourati, J.; Guerrero, F. Effect of a single, open-sea, air scuba dive on human micro- and macrovascular function. Eur. J. Appl. Physiol. 2013, 113, 2637–2645. [Google Scholar] [CrossRef]
- Germonpre, P.; Van der Eecken, P.; Van Renterghem, E.; Germonpre, F.L.; Balestra, C. First impressions: Use of the Azoth Systems O’Dive subclavian bubble monitor on a liveaboard dive vessel. Diving Hyperb. Med. 2020, 50, 405–412. [Google Scholar] [CrossRef]

| Mean ± SD | |
|---|---|
| Age | 45.7 ± 7.32 |
| Height | 180.4 ± 7.2 cm |
| Weight | 86.4 ± 11.5 kg |
| BMI | 26.5 ± 2.4 |
| Mean ± SD | |
|---|---|
| Experience as a commercial air diver | 21.3 ± 8.3 years |
| Experience as a saturation diver | 14.7 ± 8.1 years |
| Antioxidant Supplements | Yes | No | Sometimes |
|---|---|---|---|
| During normal surface life | 58% | 38% | 4% |
| During saturation | 59% | 29% | 12% |
| Type of Physical Activity | Percentage |
|---|---|
| Outdoor, intense like running, surfing, cycling, climbing, biking, kitesurf | 72.9% |
| Outdoor, moderate like golf, hiking | 6.8% |
| Indoor, intense: swimming, hockey, boxing, gym | 13.6% |
| Moderate, or no sport | 5% |
| Unclassified (i.e., working as a farmer) | 1.7% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imbert, J.-P.; Egi, S.-M.; Balestra, C. Vascular Function Recovery Following Saturation Diving. Medicina 2022, 58, 1476. https://doi.org/10.3390/medicina58101476
Imbert J-P, Egi S-M, Balestra C. Vascular Function Recovery Following Saturation Diving. Medicina. 2022; 58(10):1476. https://doi.org/10.3390/medicina58101476
Chicago/Turabian StyleImbert, Jean-Pierre, Salih-Murat Egi, and Costantino Balestra. 2022. "Vascular Function Recovery Following Saturation Diving" Medicina 58, no. 10: 1476. https://doi.org/10.3390/medicina58101476
APA StyleImbert, J. -P., Egi, S. -M., & Balestra, C. (2022). Vascular Function Recovery Following Saturation Diving. Medicina, 58(10), 1476. https://doi.org/10.3390/medicina58101476






