Use of High-Resolution Ultrasound in Characterizing the Surface Topography of a Breast Implant
Abstract
:1. Introduction
2. Materials and Methods
3. Study Population
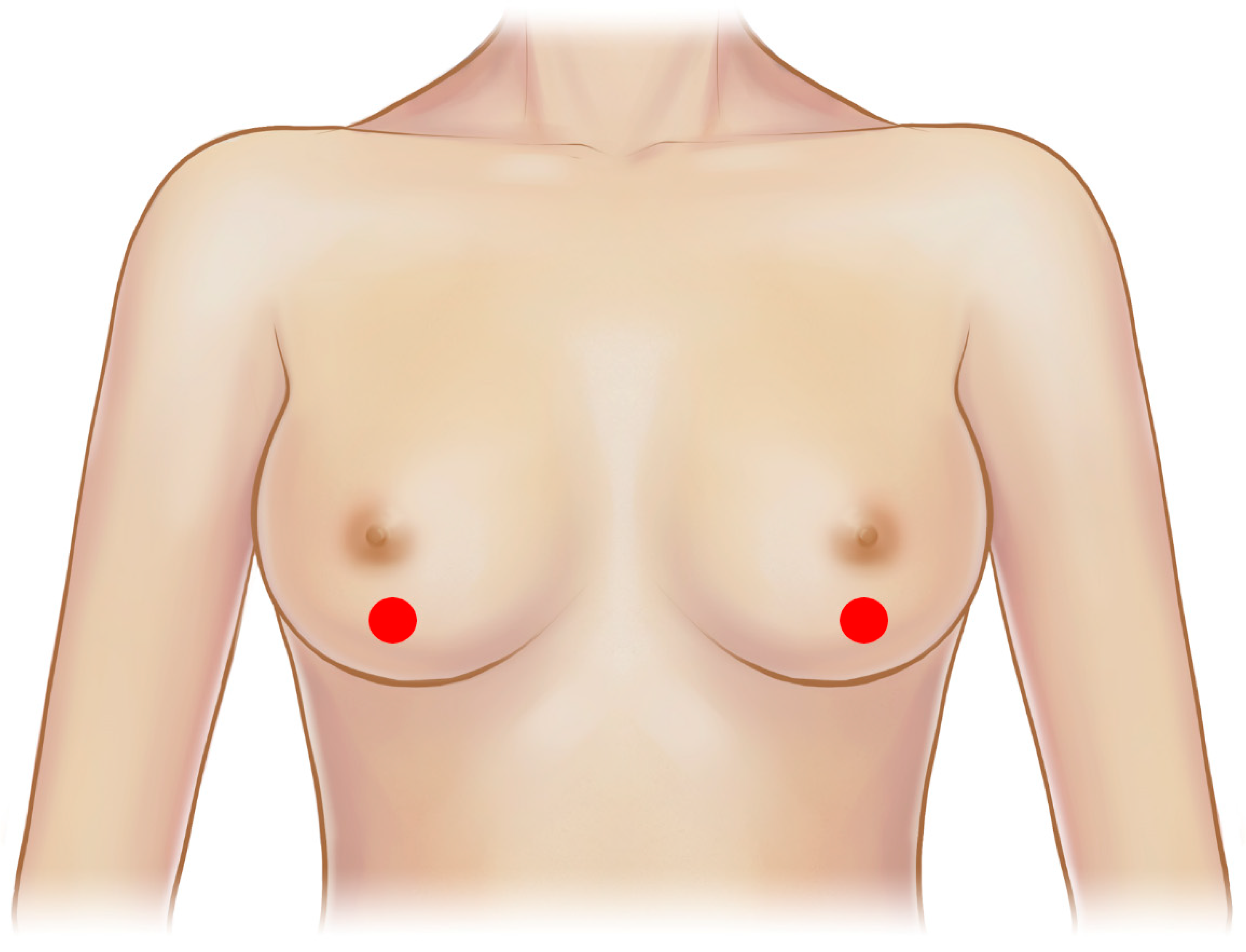
4. Breast Implant Ultrasonography Protocol
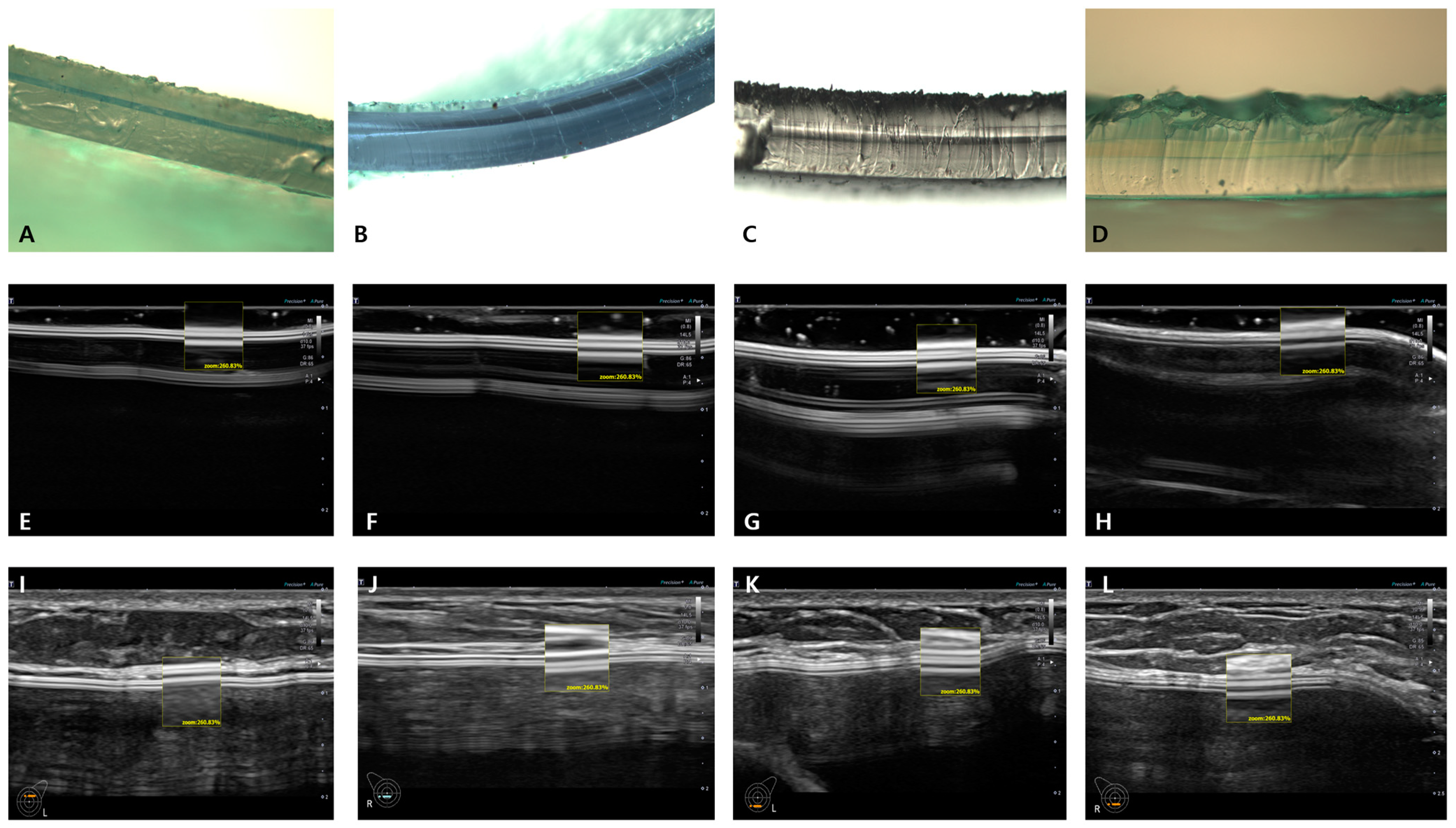
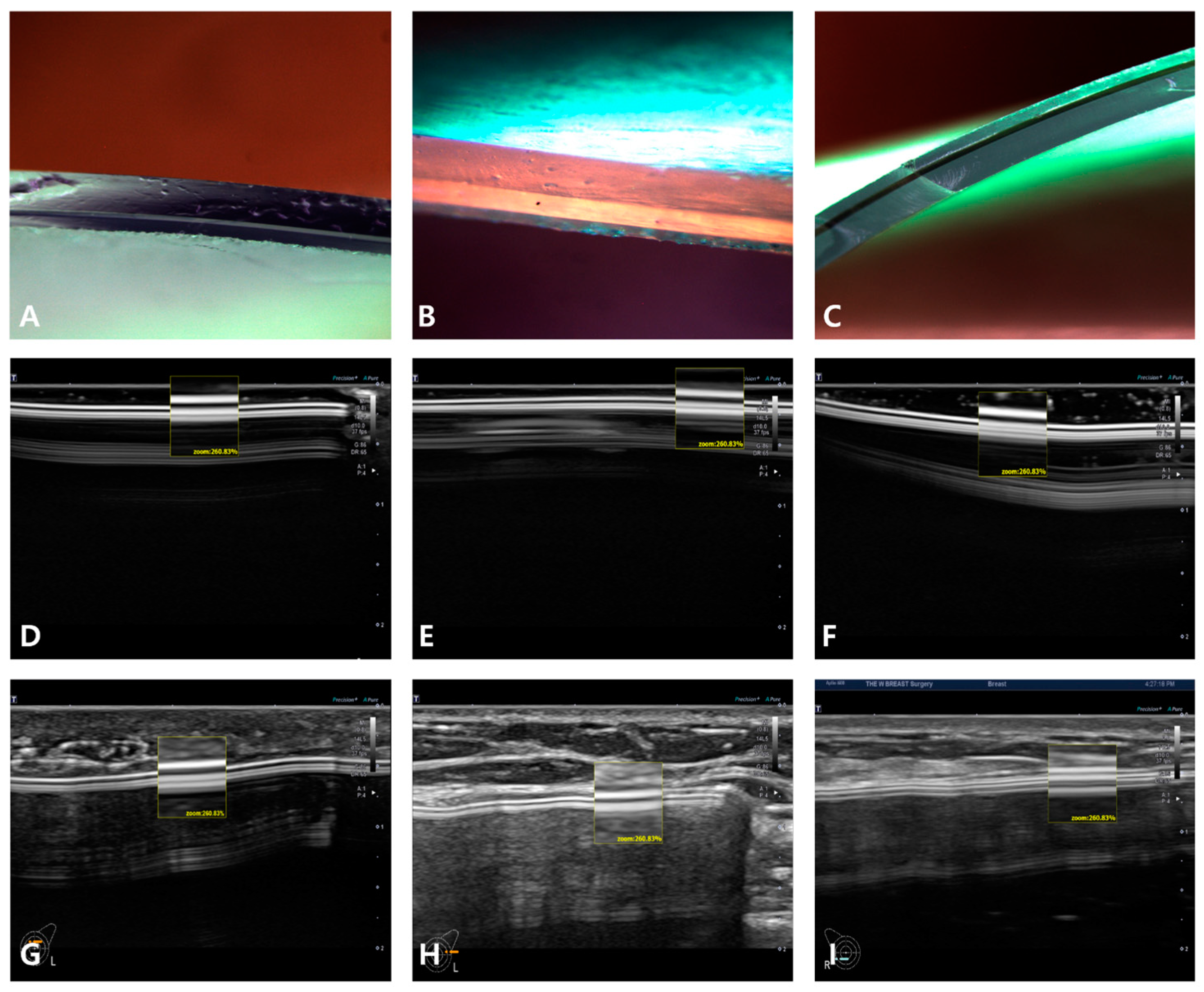
5. Breast Implant Shell Topography
6. Image Analysis
7. Results
8. Discussion
9. Limitations
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Deva, A.K.; Cuss, A.; Magnusson, M.; Cooter, R. The “Game of Implants”: A Perspective on the Crisis-Prone History of Breast Implants. Aesthet. Surg. J. 2019, 39, S55–S65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barr, S.; Bayat, A. Breast implant surface development: Perspectives on development and manufacture. Aesthet. Surg. J. 2011, 31, 56–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atlan, M.; Nuti, G.; Wang, H.; Decker, S.; Perry, T. Breast implant surface texture impacts host tissue response. J. Mech. Behav. Biomed. Mater. 2018, 88, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Barnsley, G.P.; Sigurdson, L.J.; Barnsley, S.E. Textured surface breast implants in the prevention of capsular contracture among breast augmentation patients: A meta-analysis of randomized controlled trials. Plast. Reconstr. Surg. 2006, 117, 2182–2190. [Google Scholar] [CrossRef] [PubMed]
- Barr, S.; Hill, E.W.; Bayat, A. Functional biocompatibility testing of silicone breast implants and a novel classification system based on surface roughness. J. Mech. Behav. Biomed. Mater. 2017, 75, 75–81. [Google Scholar] [CrossRef]
- Brown, T.; Harvie, F.; Stewart, S. A Different Perspective on Breast Implant Surface Texturization and Anaplastic Large Cell Lymphoma (ALCL). Aesthet. Surg. J. 2019, 39, 56–63. [Google Scholar] [CrossRef]
- Wong, C.H.; Samuel, M.; Tan, B.K.; Song, C. Capsular contracture in subglandular breast augmentation with textured versus smooth breast implants: A systematic review. Plast. Reconstr. Surg. 2006, 118, 1224–1236. [Google Scholar] [CrossRef]
- Efanov, J.I.; Giot, J.P.; Fernandez, J.; Danino, M.A. Breast-implant texturing associated with delamination of capsular layers: A histological analysis of the double capsule phenomenon. Ann. Chir. Plast. Esthet. 2017, 62, 196–201. [Google Scholar] [CrossRef]
- Sheikh, Z.; Brooks, P.J.; Barzilay, O.; Fine, N.; Glogauer, M. Macrophages, Foreign Body Giant Cells and Their Response to Implantable Biomaterials. Materials 2015, 8, 5671–5701. [Google Scholar] [CrossRef] [Green Version]
- Ionescu, P.; Vibert, F.; Amé, S.; Mathelin, C. New Data on the Epidemiology of Breast Implant-Associated Anaplastic Large Cell Lymphoma. Eur. J. Breast Health 2021, 17, 302–307. [Google Scholar] [CrossRef]
- Cash, T.F.; Duel, L.A.; Perkins, L.L. Women’s psychosocial outcomes of breast augmentation with silicone gel-filled implants: A 2-year prospective study. Plast. Reconstr. Surg. 2002, 109, 2112–2121; discussion 2122–2123. [Google Scholar] [CrossRef] [PubMed]
- Blombery, P.; Thompson, E.R.; Prince, H.M. Molecular Drivers of Breast Implant-Associated Anaplastic Large Cell Lymphoma. Plast. Reconstr. Surg. 2019, 143, 59S–64S. [Google Scholar] [CrossRef]
- Keech, J.A., Jr.; Creech, B.J. Anaplastic T-cell lymphoma in proximity to a saline- filled breast implant. Plast. Reconstr. Surg. 1997, 100, 554–555. [Google Scholar] [CrossRef]
- Carty, M.J.; Pribaz, J.J.; Antin, J.H.; Volpicelli, E.R.; Toomey, C.E.; Farkash, E.A.; Hochberg, E.P. A Patient Death Attributable to Implant-Related Primary Anaplastic Large Cell Lymphoma of the Breast. Plast. Reconstr. Surg. 2011, 128, 112e–118e. [Google Scholar] [CrossRef] [PubMed]
- Clemens, M.W.; Horwitz, S.M. NCCN consensus guidelines for the diagnosis and management of breast implant-associated anaplastic large cell lymphoma. Aesthet. Surg. J. 2017, 37, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, M.; Orrell, J.; Mortimer, C.; Ball, L. Chemotherapy-resistant breast implant-associated anaplastic large cell lymphoma. BMJ Case Rep. 2013, 2013, bcr2013201950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brody, G.S.; Deapen, D.; Taylor, C.R.; Pinter-Brown, L.; House-Lightner, S.R.; Andersen, J.S.; Carlson, G.; Lechner, M.G.; Epstein, A.L. Anaplastic Large Cell Lymphoma Occurring in Women with Breast Implants: Analysis of 173 Cases. Plast. Reconstr. Surg. 2015, 135, 695–705. [Google Scholar] [CrossRef]
- Deva, A.K. Discussion: U.S. epidemiology of breast implant-associated anaplastic large-cell lymphoma. Plast. Reconstr. Surg. 2017, 139, 1051–1052. [Google Scholar] [CrossRef]
- Lee, S.; Jeong, J.P.; Sung, J.Y.; Choi, W.S.; Moon, D.S.; Kim, H.C.; Kim, J.H. High-Resolution Ultrasound-Assisted Assessment of Preliminary Short-term Safety Outcomes of an Implant-Based Augmentation Mammaplasty Using a Bioengineered, Cell-Friendly, Smooth-Surface Device in Korean Females. Aesthet. Surg. J. Open. Forum 2021, 4, ojab046. [Google Scholar] [CrossRef]
- Chiemi, J.A.; Kelishadi, S.S. A Rationale for Micro-textured Breast Implant Augmentation. Aesthet. Surg. J. Open. Forum 2022, 4, ojac020. [Google Scholar] [CrossRef]
- Han, S.; Kim, R.; Kim, T.S.; Park, J.H.; Kim, S.S.; Jeong, C.; Lee, J.H. A Preliminary Retrospective Study to Assess the Short-Term Safety of Traditional Smooth or Microtextured Silicone Gel-Filled Breast Implants in Korea. Medicina 2021, 57, 1370. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H. A Health Insurance Coverage for Women Receiving Post-Mastectomy Reconstruction. Available online: https://www.hidoc.co.kr/healthstory/news/C0000100917 (accessed on 13 July 2022).
- Kang, S.J. [Inspection and Audit of the Korean National Assembly] “Loss of Data from 13,000 Patients Receiving a Breast Implant”. Available online: http://www.hitnews.co.kr/news/articleView.html?idxno=30265 (accessed on 13 July 2022).
- Chao, A.H.; Garza, R., 3rd; Povoski, S.P. A review of the use of silicone implants in breast surgery. Expert. Rev. Med. Devices 2016, 13, 143–156. [Google Scholar] [CrossRef]
- Barr, S.; Hill, E.; Bayat, A. Current implant surface technology: An examination of their nanostructure and their influence on fibroblast alignment and biocompatibility. Eplasty 2009, 9, e22. [Google Scholar]
- Kim, J.H.; Paik, N.S.; Nam, S.Y.; Cho, Y.; Park, H.K. The emerging crisis of stakeholders in implant-based augmentation mammaplasty in Korea. J. Korean Med. Sci. 2020, 35, e103. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.M.; Chaudhry, H.E.; Shah, A.; Andrews, J. Breast Squamous Cell Carcinoma Following Breast Augmentation. Cureus 2018, 10, e3405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsen, D.L.; Keeney, G.L.; Chen, B.; Visscher, D.W.; Carter, J.M. Breast implant capsule-associated squamous cell carcinoma: A report of 2 cases. Hum. Pathol. 2017, 67, 94–100. [Google Scholar] [CrossRef]
- Zomerlei, T.A.; Samarghandi, A.; Terando, A.M. Primary squamous cell carcinoma arising from a breast implant capsule. Plast. Reconstr. Surg. Glob. Open. 2015, 3, 586. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Yadav, D.; Zakalik, D. Squamous cell carcinoma of the breast in the United States: Incidence, demographics, tumor characteristics, and survival. Breast Cancer Res. Treat. 2017, 164, 201–208. [Google Scholar] [CrossRef]
- Maxwell, G.P.; Scheflan, M.; Spear, S.; Nava, M.B.; Hedén, P. Benefits and Limitations of Macrotextured Breast Implants and Consensus Recommendations for Optimizing Their Effectiveness. Aesthet. Surg. J. 2014, 34, 876–881. [Google Scholar] [CrossRef] [Green Version]
- Munhoz, A.M.; Clemens, M.W.; Nahabedian, M.Y. Breast Implant Surfaces and Their Impact on Current Practices: Where We Are Now and Where Are We Going? Plast. Reconstr. Surg. Glob. Open 2019, 7, e2466. [Google Scholar] [CrossRef] [Green Version]
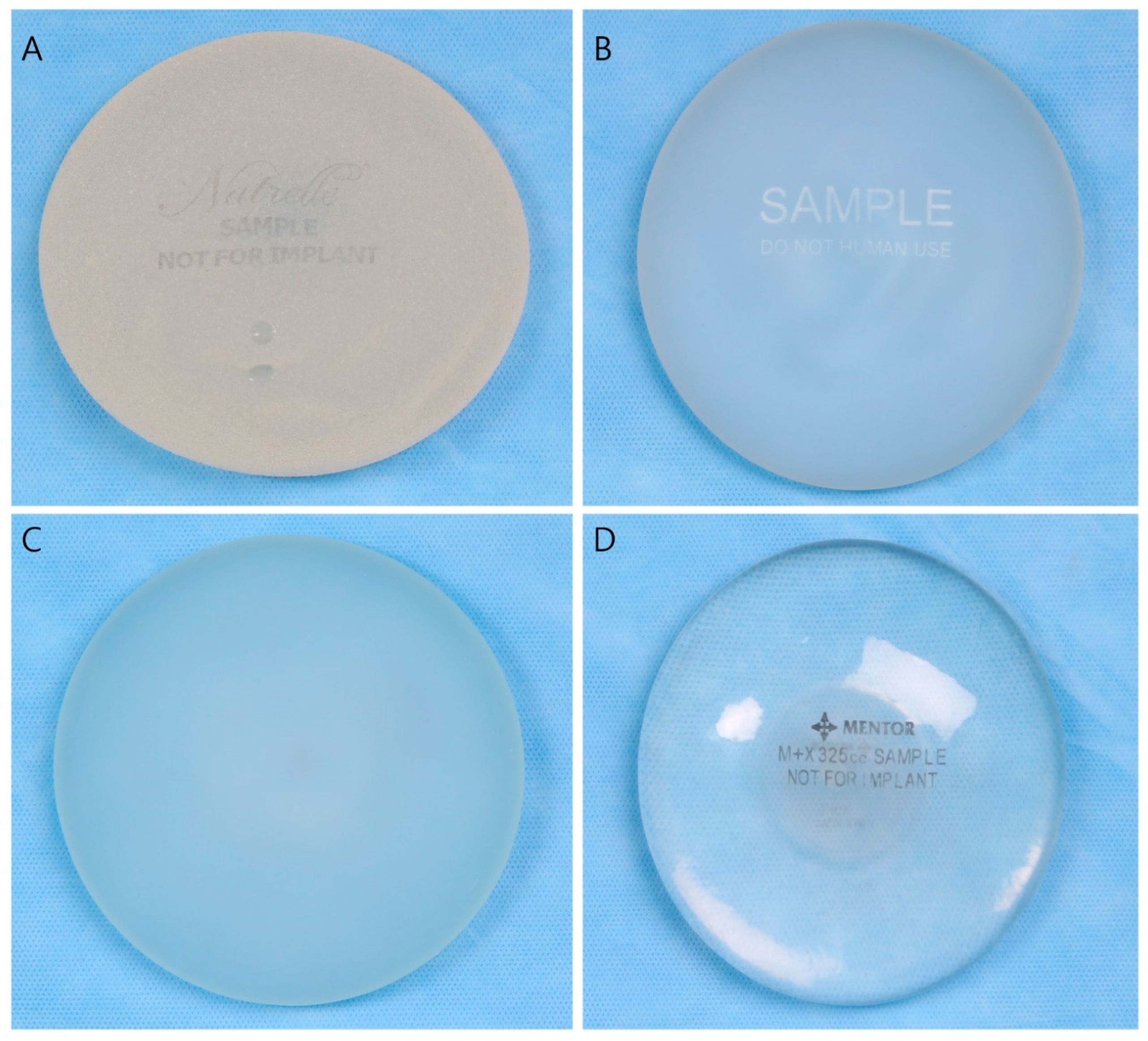
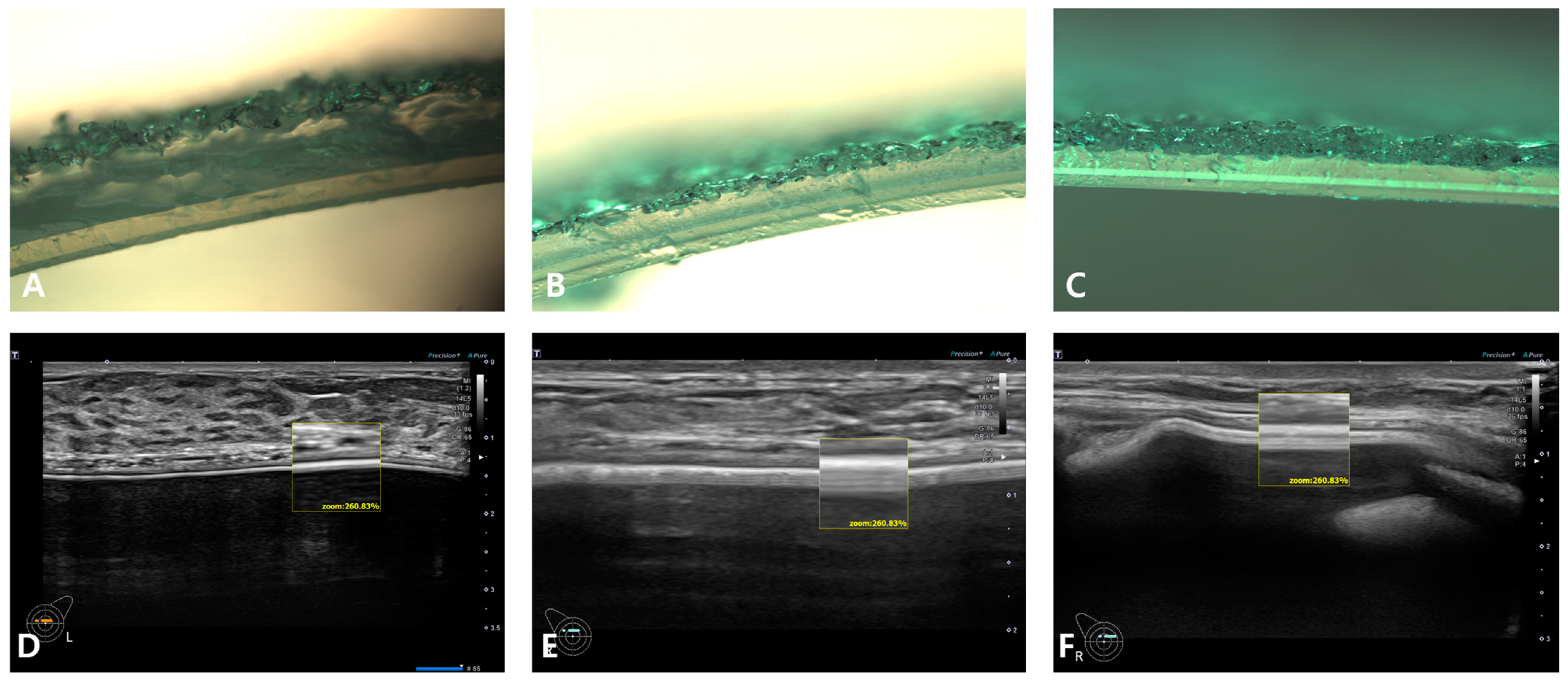
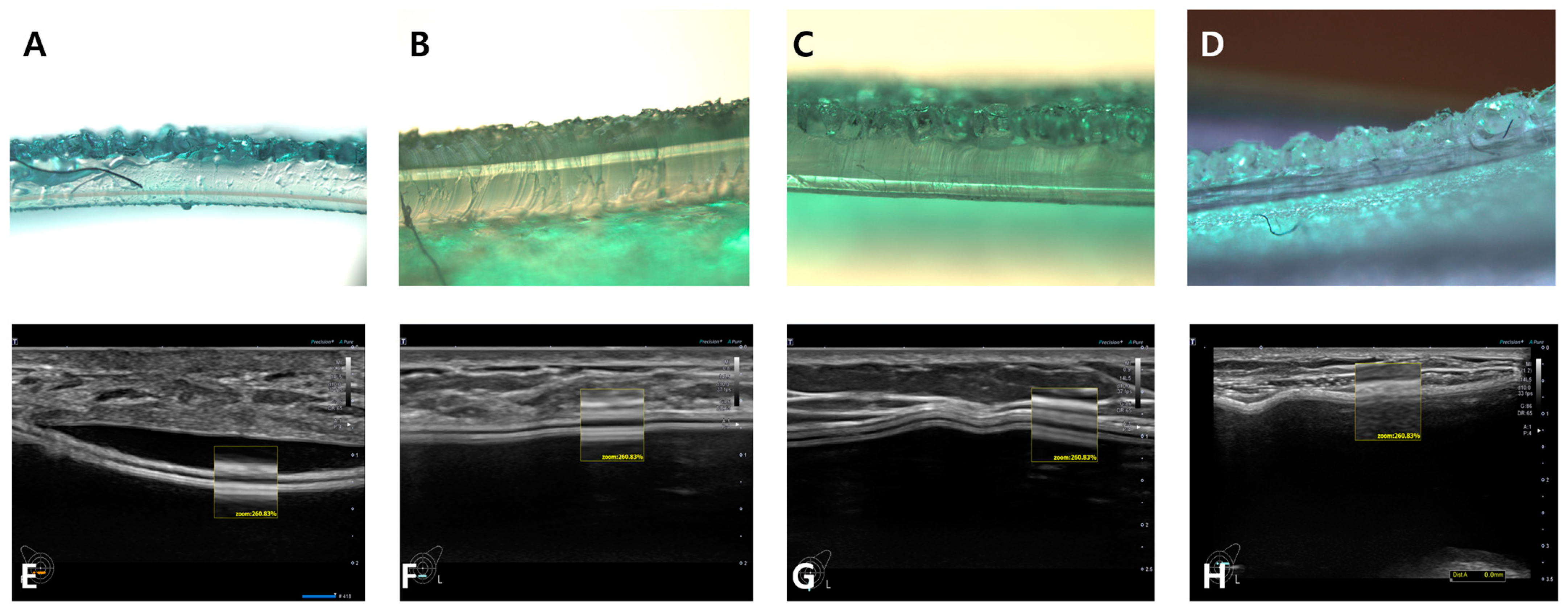
| Manufacturer | Macrotextured | Microt Extured | Nanotextured/Smooth |
|---|---|---|---|
| Allergan, Irvine, CA, USA | O | N/A | O |
| Mentor Worldwide LLC, Santa Barbara, CA, USA | O | N/A | O |
| Silimed, Rio de Janeiro, Brazil | O | N/A | N/A |
| Sebbin SAS, Boissy-l’Aillerie, France | O | O | O |
| GC Aesthetic PLC, Apt Cedex, France | O | O | N/A |
| Polytech Health & Aesthetics, Dieburg, Germany | O | N/A | N/A |
| Motiva, Establishment Labs Holdings Inc., Alajuela, Costa Rica | N/A | N/A | O |
| HansBiomed, Seoul, Republic of Korea | O | O | N/A |
| Variable | Value (Mean ± SD), (%) |
|---|---|
| Age (years old) | 35.9 ± 8.3 |
| Sex (male-to-female ratio) | 0:1901 |
| Height (cm) | 162.7 ± 4.8 |
| Weight (kg) | 53.1 ± 6.4 |
| BMI (kg/m2) | 20.0 ± 2.1 |
| Days from previous surgery | 41,870.3 ± 2169.5 |
| Less than 3 years | 570 (30.0), 521.3 ± 317.3 |
| 3~10 years | 908 (47.7), 2318.3 ± 720.3 |
| 10~20 years | 361(19.0), 4760.7 ± 928.8 |
| More than 20 years | 47 (2.5), 10,534.5 ± 7480.0 |
| Not applicable | 15 (0.8) |
| Variable | Value (%) |
|---|---|
| Purpose of surgery | |
| Esthetic | 3793 (99.8) |
| Reconstructive | 9 (0.2) |
| Type of incision | |
| Trans-axillary | 2503 (65.8) |
| IMF | 959 (25.2) |
| Peri-areolar | 277 (7.3) |
| Mastectomy scar | 3 (0.1) |
| Umbilical | 56 (1.5) |
| Other | 4 (0.1) |
| Type of pocket | |
| Subpectoral | 3524 (92.7) |
| Subglandular | 278 (7.3) |
| Fill material | |
| Silicone | 3615 (95.1) |
| Saline | 181 (4.7) |
| Dual chamber | 6 (0.2) |
| Shell type | |
| Texture | 2034 (53.5) |
| Microtexture | 73 (1.9) |
| Smooth | 1622 (42.7) |
| Not applicable due to rupture | 73 (1.9) |
| Shape type | |
| Anatomical | 1011 (26.6) |
| Round | 2642 (69.5) |
| Not applicable due to rupture | 149 (3.9) |
| Manufacturer | |
| Sebbin | 175 (4.6) |
| HansBiomed | 24 (0.6) |
| Motiva | 14 (0.4) |
| Eurosilicone | 12 (0.3) |
| Allergan | 1079 (28.4) |
| Mentor | 236 (6.2) |
| Polytech | 265 (7.0) |
| Silimed | 96 (2.5) |
| Other | 0 (0.0) |
| Unknown due to–roundness | 1764 (46.4) |
| –Rupture | 137 (3.6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-H.; Park, D.-W.; Song, K.-Y.; Lim, H.-G.; Jeong, J.-P.; Kim, J.-H. Use of High-Resolution Ultrasound in Characterizing the Surface Topography of a Breast Implant. Medicina 2023, 59, 1092. https://doi.org/10.3390/medicina59061092
Kim Y-H, Park D-W, Song K-Y, Lim H-G, Jeong J-P, Kim J-H. Use of High-Resolution Ultrasound in Characterizing the Surface Topography of a Breast Implant. Medicina. 2023; 59(6):1092. https://doi.org/10.3390/medicina59061092
Chicago/Turabian StyleKim, Yang-Hee, Dong-Wook Park, Keun-Yeong Song, Hyung-Guhn Lim, Jeong-Pil Jeong, and Jae-Hong Kim. 2023. "Use of High-Resolution Ultrasound in Characterizing the Surface Topography of a Breast Implant" Medicina 59, no. 6: 1092. https://doi.org/10.3390/medicina59061092
APA StyleKim, Y.-H., Park, D.-W., Song, K.-Y., Lim, H.-G., Jeong, J.-P., & Kim, J.-H. (2023). Use of High-Resolution Ultrasound in Characterizing the Surface Topography of a Breast Implant. Medicina, 59(6), 1092. https://doi.org/10.3390/medicina59061092





